Abstract
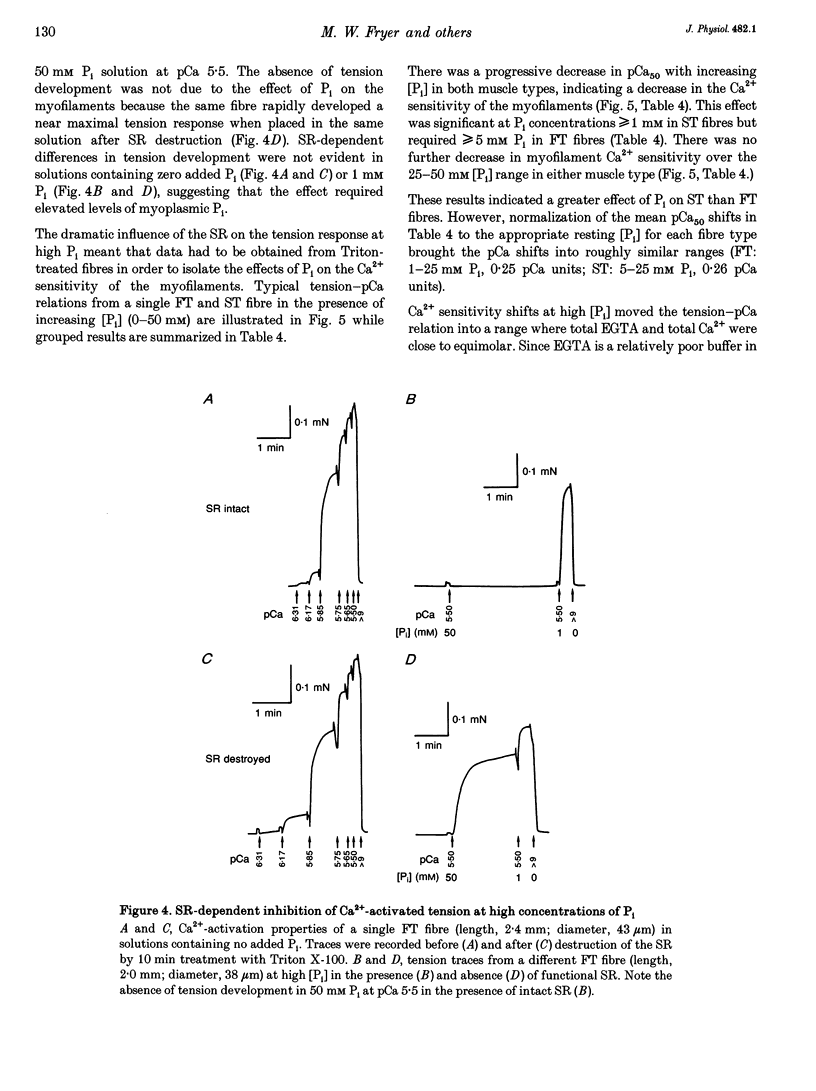
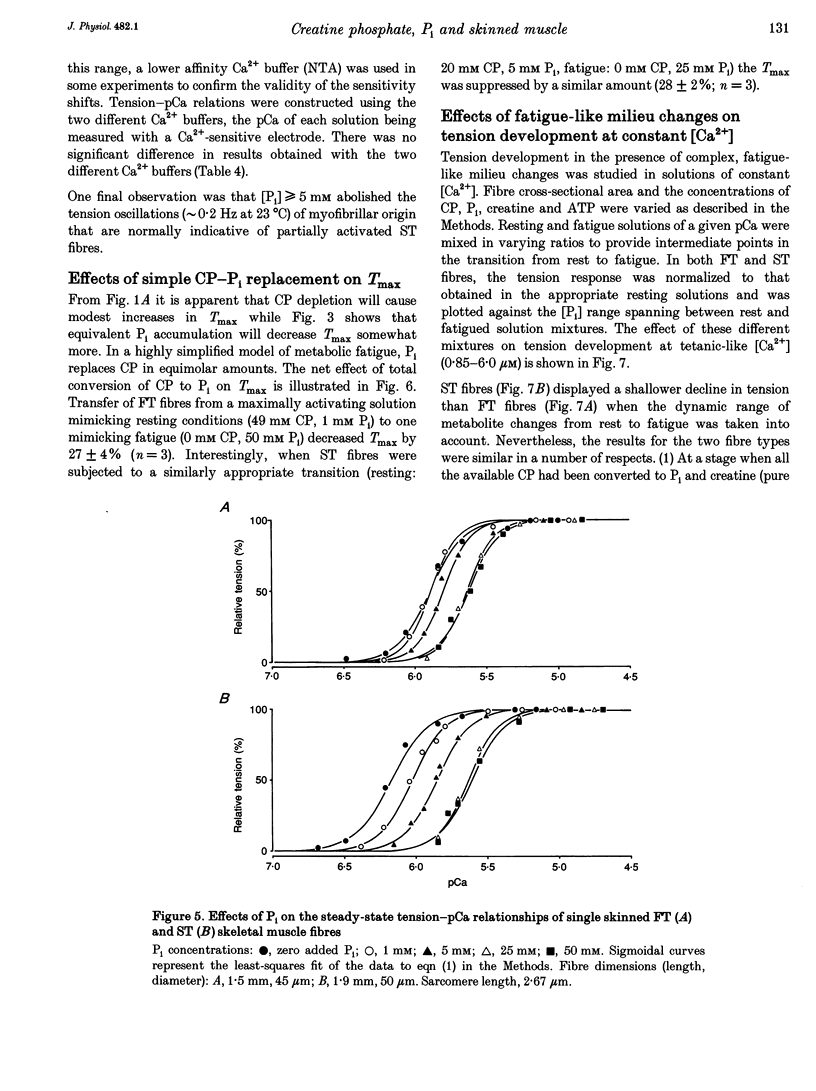
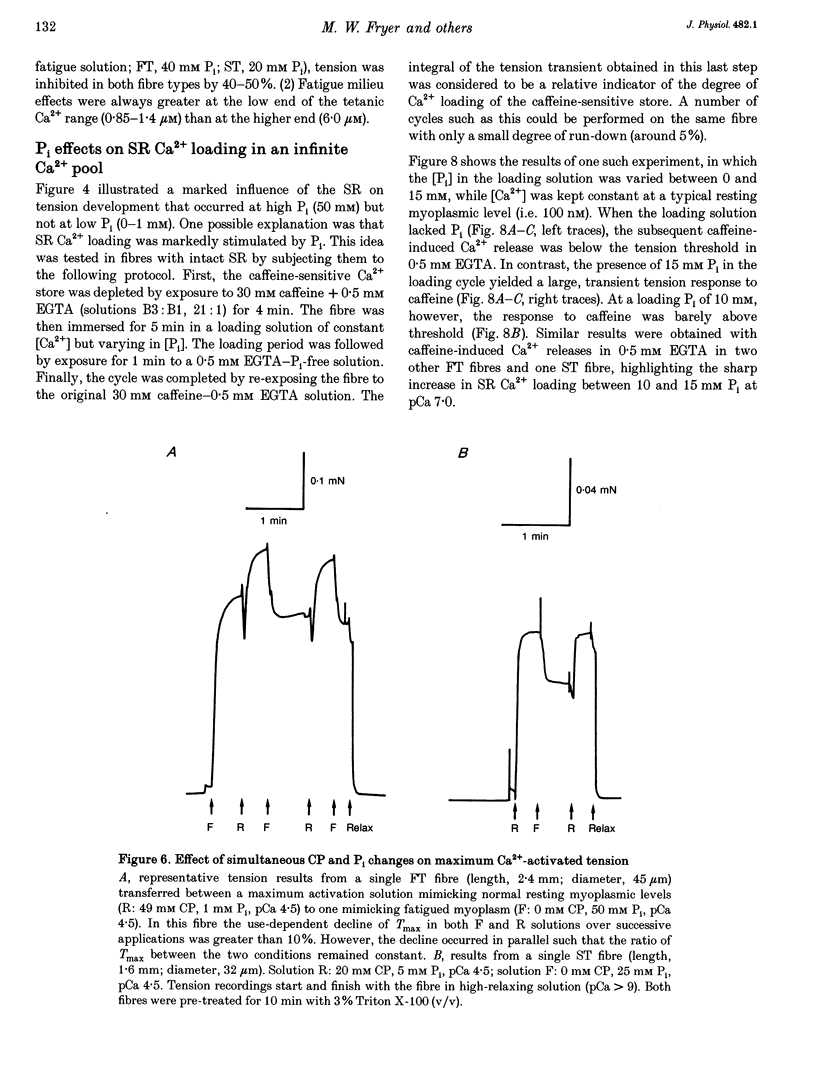
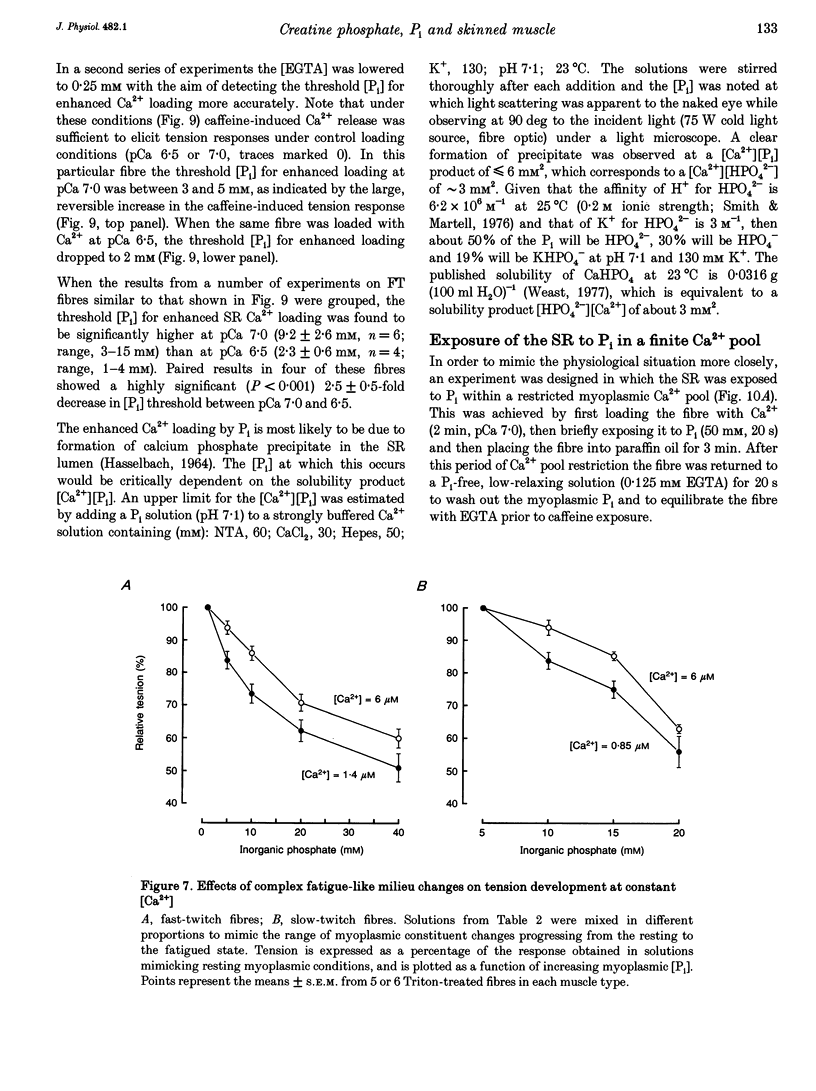
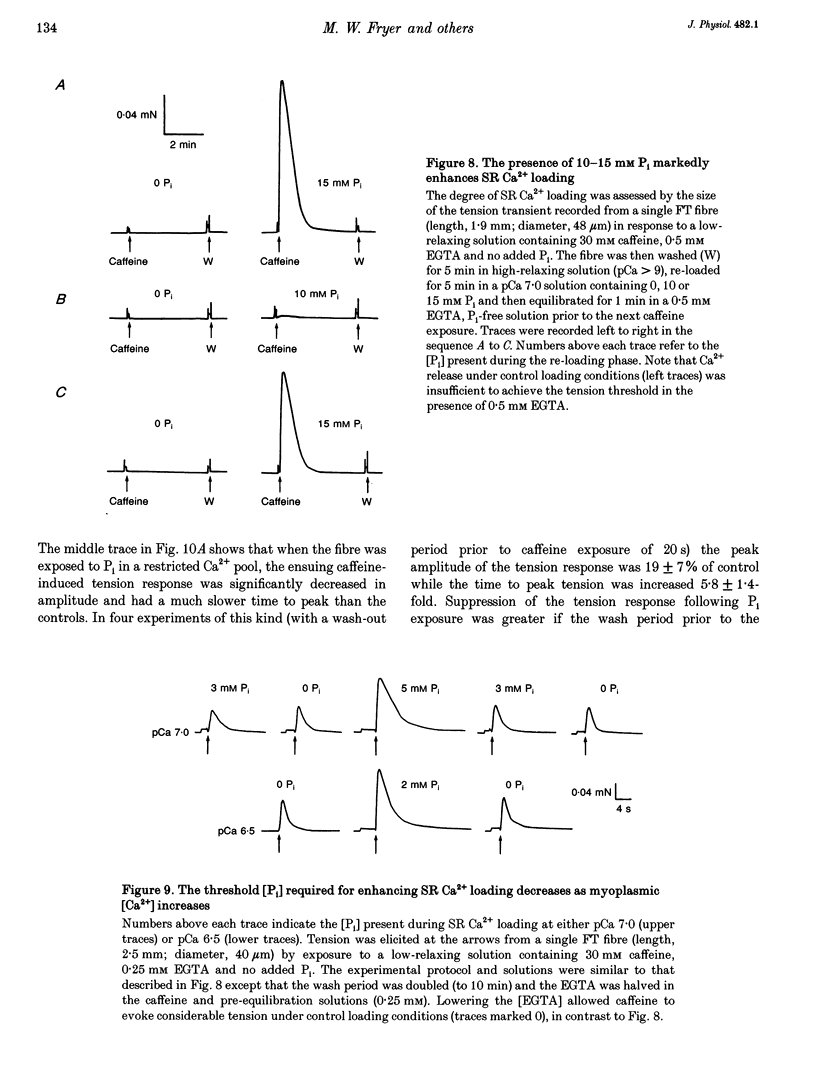
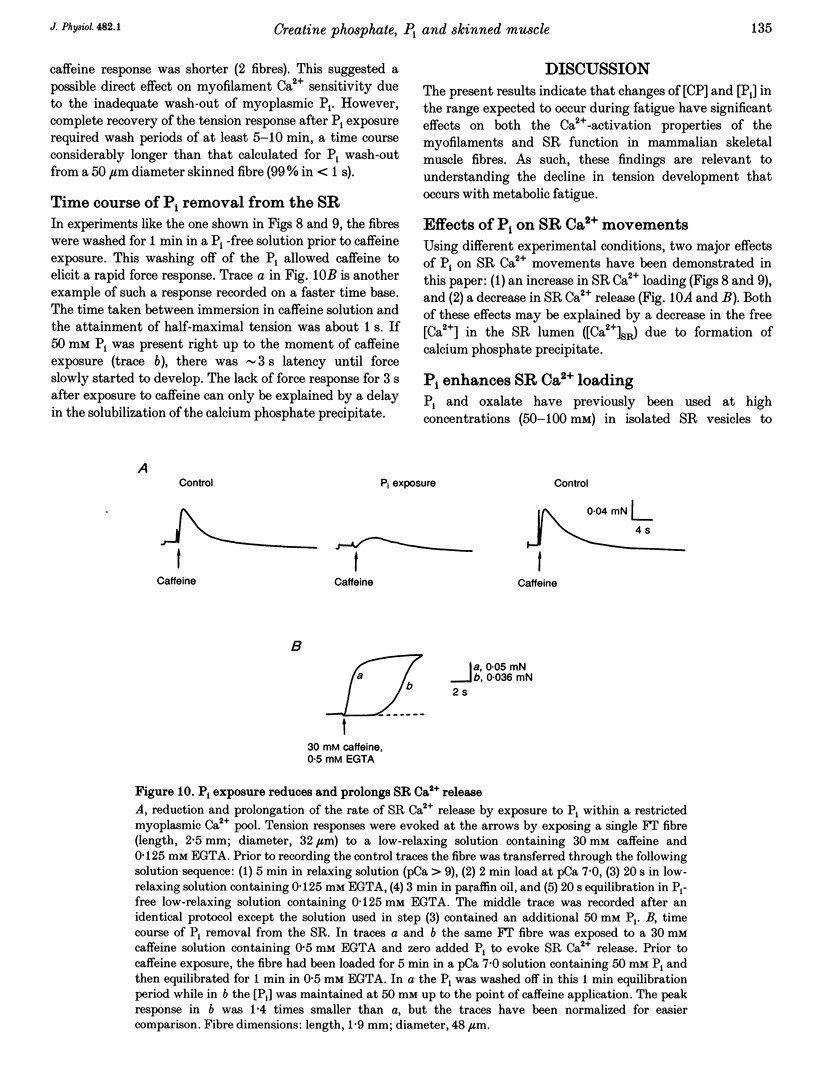
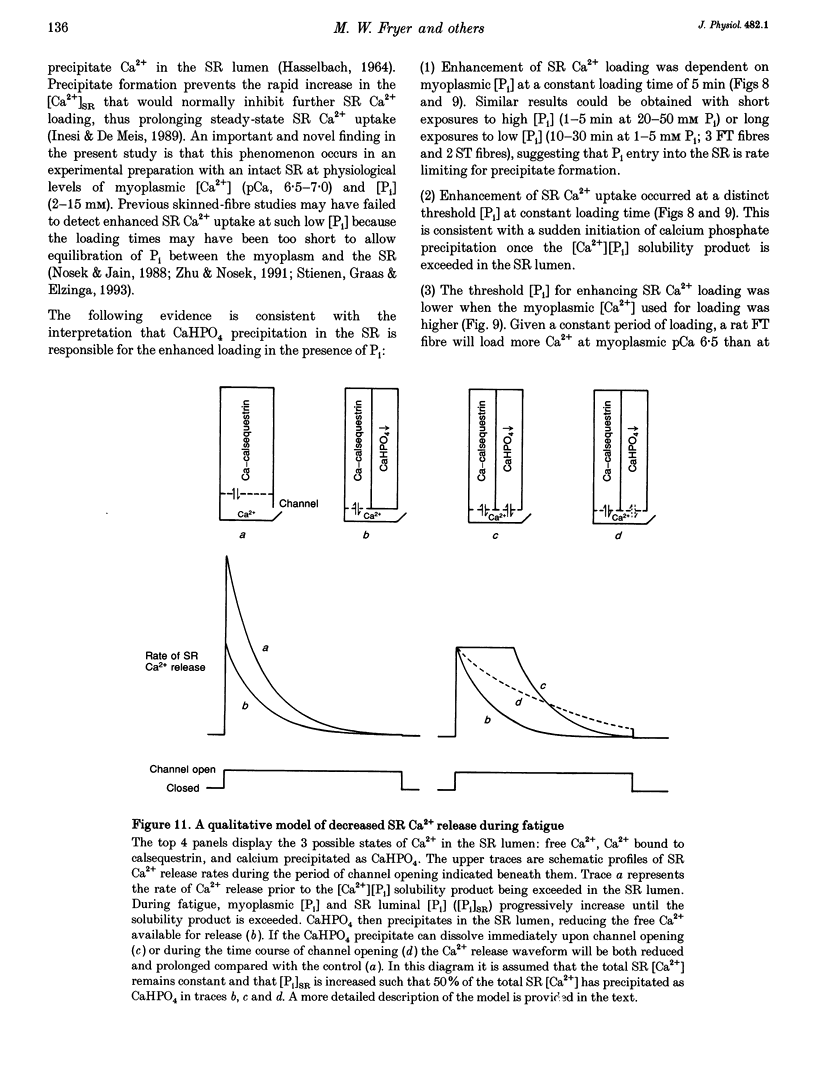
1. Mechanically skinned fast-twitch (FT) and slow-twitch (ST) muscle fibres of the rat were used to investigate the effects of fatigue-like changes in creatine phosphate (CP) and inorganic phosphate (P(i)) concentration on Ca(2+)-activation properties of the myofilaments as well as Ca2+ movements into and out of the sarcoplasmic reticulum (SR). 2. Decreasing CP from 50 mM to zero in FT fibres increased maximum Ca(2+)-activated tension (Tmax) by 16 +/- 2% and shifted the mid-point of the tension-pCa relation (pCa50) to the left by 0.28 +/- 0.03 pCa units. In ST fibres, a decrease of CP from 25 mM to zero increased Tmax by 9 +/- 1% and increased the pCa50 by 0.16 +/- 0.01 pCa units. The effect of CP on Tmax was suppressed in both fibre types by prior treatment with 0.3 mM FDNB (1-fluoro-2,4-dinitrobenzene), suggesting that these effects may occur via changes in creatine kinase activity. 3. Increases of P(i) in the range 0-50 mM reduced the pCa50 and Tmax in both fibre types. These effects were more pronounced in ST fibres than in FT fibres in absolute terms. However, normalization of the results to resting P(i) levels appropriate to both fibre types (1 mM for FT and 5 mM for ST fibres) revealed similar decreases in Tmax (approximately 39% at 25 mM P(i) and approximately 48% at 50 mM P(i)) and pCa50 (0.25 pCa units at 25-50 mM P(i)). The depressant action of P(i) on both parameters was considerably reduced when the rise in P(i) was accompanied by an equivalent reduction in [CP]. 4. Tension development in the presence of complex, fatigue-like milieu changes (40 mM P(i) for FT; 20 mM P(i) for ST) was decreased by 35-40% at a constant myoplasmic [Ca2+] of 6 microM in both fibre types. 5. SR Ca2+ loading at a myoplasmic [Ca2+] of 100 nM was found to increase abruptly when the [P(i)] during loading was increased to near 9 mM. At a myoplasmic [Ca2+] of 300 nM, the threshold P(i) for this effect dropped to approximately 3 mM. 6. Tension responses evoked by caffeine in the absence of P(i) were smaller and slower to peak if fibres were exposed to P(i) in a restricted myoplasmic Ca2+ pool after SR Ca2+ loading. This indicated that myoplasmic P(i) can decrease and prolong the rate of Ca2+ release from the SR.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altringham J. D., Johnston I. A. Effects of phosphate on the contractile properties of fast and slow muscle fibres from an Antarctic fish. J Physiol. 1985 Nov;368:491–500. doi: 10.1113/jphysiol.1985.sp015871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt P. W., Cox R. N., Kawai M., Robinson T. Effect of cross-bridge kinetics on apparent Ca2+ sensitivity. J Gen Physiol. 1982 Jun;79(6):997–1016. doi: 10.1085/jgp.79.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Chifflet S., Torriglia A., Chiesa R., Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal Biochem. 1988 Jan;168(1):1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. J Physiol. 1980 Feb;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edström L., Hultman E., Sahlin K., Sjöholm H. The contents of high-energy phosphates in different fibre types in skeletal muscles from rat, guinea-pig and man. J Physiol. 1982 Nov;332:47–58. doi: 10.1113/jphysiol.1982.sp014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G., Williams D. A. Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. J Physiol. 1986 Jan;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E., Nosek T. M. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989 May;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Somlyo A. V., McClellan G., Shuman H., Borrero L. M., Somlyo A. P. Composition of vacuoles and sarcoplasmic reticulum in fatigued muscle: electron probe analysis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1329–1333. doi: 10.1073/pnas.75.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güth K., Potter J. D. Effect of rigor and cycling cross-bridges on the structure of troponin C and on the Ca2+ affinity of the Ca2+-specific regulatory sites in skinned rabbit psoas fibers. J Biol Chem. 1987 Oct 5;262(28):13627–13635. [PubMed] [Google Scholar]
- Hibberd M. G., Dantzig J. A., Trentham D. R., Goldman Y. E. Phosphate release and force generation in skeletal muscle fibers. Science. 1985 Jun 14;228(4705):1317–1319. doi: 10.1126/science.3159090. [DOI] [PubMed] [Google Scholar]
- Inesi G., de Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem. 1989 Apr 5;264(10):5929–5936. [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushmerick M. J., Moerland T. S., Wiseman R. W. Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7521–7525. doi: 10.1073/pnas.89.16.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G., Stienen G. J. Effects of osmolality and ionic strength on the mechanism of Ca2+ release in skinned skeletal muscle fibres of the toad. J Physiol. 1993 May;464:629–648. doi: 10.1113/jphysiol.1993.sp019655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Goldman Y. E., Simmons R. M. Changes in the lateral filament spacing of skinned muscle fibres when cross-bridges attach. J Mol Biol. 1984 Feb 15;173(1):15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Brown T. R., Kushmerick M. J. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985 Mar;248(3 Pt 1):C279–C287. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Homsher E. Kinetics of force generation and phosphate release in skinned rabbit soleus muscle fibers. Am J Physiol. 1992 May;262(5 Pt 1):C1239–C1245. doi: 10.1152/ajpcell.1992.262.5.C1239. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Biol Chem. 1990 Nov 25;265(33):20234–20240. [PubMed] [Google Scholar]
- Moisescu D. G., Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol. 1978 Feb;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek T. M., Leal-Cardoso J. H., McLaughlin M., Godt R. E. Inhibitory influence of phosphate and arsenate on contraction of skinned skeletal and cardiac muscle. Am J Physiol. 1990 Dec;259(6 Pt 1):C933–C939. doi: 10.1152/ajpcell.1990.259.6.C933. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Steele D. S. Inorganic phosphate decreases the Ca2+ content of the sarcoplasmic reticulum in saponin-treated rat cardiac trabeculae. J Physiol. 1992 Dec;458:457–473. doi: 10.1113/jphysiol.1992.sp019427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen G. J., van Graas I. A., Elzinga G. Uptake and caffeine-induced release of calcium in fast muscle fibers of Xenopus laevis: effects of MgATP and P(i). Am J Physiol. 1993 Sep;265(3 Pt 1):C650–C657. doi: 10.1152/ajpcell.1993.265.3.C650. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992 Apr;449:49–71. doi: 10.1113/jphysiol.1992.sp019074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991 Sep;98(3):615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Lee J. A., Lännergren J., Allen D. G. Cellular mechanisms of fatigue in skeletal muscle. Am J Physiol. 1991 Aug;261(2 Pt 1):C195–C209. doi: 10.1152/ajpcell.1991.261.2.C195. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Seo Y., Nishikawa H., Morimoto T. Application of pulsed-gradient 31P NMR on frog muscle to measure the diffusion rates of phosphorus compounds in cells. Biophys J. 1982 May;38(2):209–211. doi: 10.1016/S0006-3495(82)84549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Nosek T. M. Intracellular milieu changes associated with hypoxia impair sarcoplasmic reticulum Ca2+ transport in cardiac muscle. Am J Physiol. 1991 Sep;261(3 Pt 2):H620–H626. doi: 10.1152/ajpheart.1991.261.3.H620. [DOI] [PubMed] [Google Scholar]


