Abstract
Background
Diabetic heart disease may eventually lead to heart failure, a leading cause of mortality in diabetic individuals. The lack of effective treatments for diabetes-induced heart failure may result from a failure to address the underlying pathological processes, including chronic, low-grade inflammation. Previous studies have reported that lipoxin A4 (LXA4), known to promote resolution of inflammation, attenuates diabetes-induced atherosclerosis, but its impact on diabetic hearts has not been sought. Thus, we aimed to determine whether LXA4 therapeutic treatment attenuates diabetes-induced cardiac pathology.
Methods
Six-week-old male apolipoprotein E-deficient (ApoE−/−) mice were followed for 16 weeks after injection of streptozotocin (STZ, 55 mg/kg/day, i.p. for 5 days) to induce type-1 diabetes (T1DM). Treatment with LXA4 (5 μg/kg, i.p.) or vehicle (0.02% ethanol, i.p.) was administered twice weekly for the final 6 weeks. One week before endpoint, echocardiography was performed within a subset of mice from each group. At the end of the study, mice were euthanized with sodium pentobarbital (100 mg/kg i.p.) and hearts were collected for ex vivo analysis, including histological assessment, gene expression profiling by real-time PCR and protein level measurement by western blot.
Results
As expected diabetic mice showed a significant elevation in plasma glycated hemoglobin (HbA1c) and glucose levels, along with reduced body weight. Vehicle-treated diabetic mice exhibited increased cardiac inflammation, macrophage content, and an elevated ratio of M1-like to M2-like macrophage markers. In addition, myocardial fibrosis, cardiomyocytes apoptosis and hypertrophy (at the genetic level) were evident, with echocardiography revealing early signs of left ventricular (LV) diastolic dysfunction. Treatment with LXA4 ameliorated diabetes-induced cardiac inflammation, pro-inflammatory macrophage polarization and cardiac remodeling (especially myocardial fibrosis and cardiomyocytes apoptosis), with ultimate improvement in cardiac function. Of note, this improvement was independent of glucose control.
Conclusions
These findings demonstrated that LXA4 treatment attenuated the extent of cardiac inflammation in diabetic hearts, resulting in limited cardiac remodeling and improved LV diastolic function. This supports further exploration of LXA4-based therapy for the management of diabetic heart disease. The recent development of stable LXA4 mimetics holds potential as a novel strategy to treat cardiac dysfunction in diabetes, paving the way for innovative and more effective therapeutic strategies.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02501-x.
Keywords: Diabetic cardiomyopathy, Cardiac inflammation, Resolution of inflammation, Cardiac fibrosis, Specialized pro-resolving lipid mediators, Lipoxin A4
Introduction
Cardiovascular pathologies, such as atherosclerosis, myocardial infarction (MI), cardiomyopathy and heart failure, are leading causes of morbidity and mortality in individuals with diabetes, a major global health concern [1–3]. The diabetic heart is characterized by LV diastolic dysfunction due to cardiomyocyte hypertrophy, apoptosis, increased inflammation, and myocardial fibrosis [4, 5]. The mechanism of cardiac pathophysiology in diabetes is complex and still not fully understood. However, it includes but is not limited to, upregulated lipid oxidation, increased intramyocardial triglyceride deposition, and decreased glucose utilization, leading to higher levels of oxidative stress, mitochondrial dysfunction, and inflammation [2].
Studies of diabetic animal models and human disease have demonstrated that diabetes is associated with persistent systemic inflammation due to increased secretion of pro-inflammatory cytokines, such as the tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-18 [6–8]. This supports the targeting of inflammation as a plausible therapeutic approach. As a self-protective reaction, inflammation aims to limit infection or injury and promote tissue healing. The temporal phases of successful inflammatory responses are characterized by distinct phases, including initiation and resolution. The initiation phase of an acute inflammatory response includes the release of pro-inflammatory eicosanoids [9, 10]. During the resolution phase, arachidonic acid-derived lipids undergo class switching to produce a class of specialized pro-resolving lipid mediators (SPMs), including lipoxins (LXs), resolvins, protectins, and maresins, which make an important contribution to restoring cellular homeostasis [11, 12]. Failure to resolve inflammation effectively leads to excessive tissue damage and ultimately progression to chronic low-grade inflammation, which underlies many common cardiometabolic disorders [13, 14].
Four naturally occurring LXs have been identified, i.e. LXA4, LXB4, aspirin-triggered LXA4 and aspirin-triggered LXB4 [15]. Studies have shown that LXs inhibit the activation nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) pathway in human leukocytes and reduce the release of several pro-inflammatory cytokines [16]. Brennan and colleagues also demonstrated that LXA4 protects against diabetic kidney disease and diabetes-associated atherosclerosis [17, 18]. The stable delivery of LXs has also been well studied. Halade and his colleagues demonstrated that the administration of aspirin-triggered LXA4 via liposomes promotes cardiac repair and healing, with a particular focus on modulating macrophage and fibroblast activity [19]. Based on both in vitro studies and in vivo models of MI in mice, their findings highlight the therapeutic potential of aspirin-triggered LXA4 in promoting post-MI healing processes through its effects on key immune and stromal cell populations. Given the growing evidence of the protective role of LXs against inflammation, promoting resolution and repair, and the inherent immunosuppression and other complications associated with anti-inflammatory, there is growing interest in developing novel therapies to promote the “resolution of inflammation” to effectively treat diabetic cardiac dysfunction.
To date, the therapeutic potential of LXA4, or its mimetics, on impaired cardiac phenotype and function in diabetes has not been fully explored. This study aims to test the hypothesis that treatment with the endogenous specialized pro-resolving lipid mediator, LXA4, attenuates cardiac inflammation, with concomitant protection against diabetes-induced cardiac remodeling and cardiac dysfunction, in mice with established T1DM.
Methods
Animals and experimental design
The use of mice was in accordance with protocols approved by the Alfred Medical Research and Education Precinct (AMREP) Animal Ethics Committee (E/1755/2017/B) and performed in accordance with the National Health and Medical Research Council Australia guidelines. All the animals were cared for and housed in the AMREP Precinct Animal Centre under a 12 h light/dark cycle on a standard mouse chow diet (unlimited access to water and food) (Barastoc; Ridley Agriproducts, St. Arnaud, Victoria, Australia; 20% protein, 5% fat, 6% fiber, 0.5% sodium, 0.38% ω-3 fatty acid, and 1.52% ω-6 fatty acid). The animal use flowchart was compiled by using the template from Consolidated Standards of Animal Experiment Report (CONSAERT) [20]. Age-matched, 6-week-old ApoE−/− male mice (C57BL/6 background) were randomly assigned to either the diabetic or non-diabetic (ND) cohort at the start of the study. The diabetic cohort received i.p. injections of STZ (Sigma-Aldrich, St Louis, USA) for five consecutive days (55 mg/kg/day in 0.1 M citric acid vehicle). The ND cohort received the citric acid vehicle (Sigma-Aldrich, vehicle control for STZ) by i.p. injection (0.5 M). Blood glucose levels and body weight were monitored weekly after STZ injections until the end of the study. Each cohort (diabetic and ND) was randomly divided into two groups, allocated to receive either treatment vehicle (0.02% ethanol) or LXA4 (5 μg/kg, in 0.02% ethanol; Merck, Calbiochem, Darmstadt, Germany) twice weekly by i.p. injection [18]. Mice received LXA4 or treatment vehicle from week 11 to week 16 (Supplemental Figure S1 and S2).
At study endpoint, to confirm the diabetic phenotype, the following parameters were used: mice in the diabetic cohort with HbA1c levels ≥ 10% and glucose levels ≥ 25 mmol/L were included in the study. For mice where the glucose levels were ≤ 26 mmol/L, if their HbA1c levels were ≥ 10%, they were also included in the study (HbA1c levels reflects long-term glucose control [21]).
Echocardiography
LV function was assessed using echocardiography one week before the endpoint. Mice were anaesthetized using a ketamine/xylazine/atropine cocktail (KXA; 80/8/0.96 mg/kg i.p.). Four imaging modes [B-mode, M-mode, pulsed wave (PW) Doppler and tissue Doppler] were utilized (images obtained in that order) using a Vevo2100 imaging system and MS 550D transducer (FUJIFILM VisualSonics, Toronto, Canada). All images were analyzed by using Vevo Lab (v5.7.1) and validated by an experienced investigator (blinded) from the Preclinical Cardiology Microsurgery and Imaging Platform, Baker Heart Institute [22].
Histology
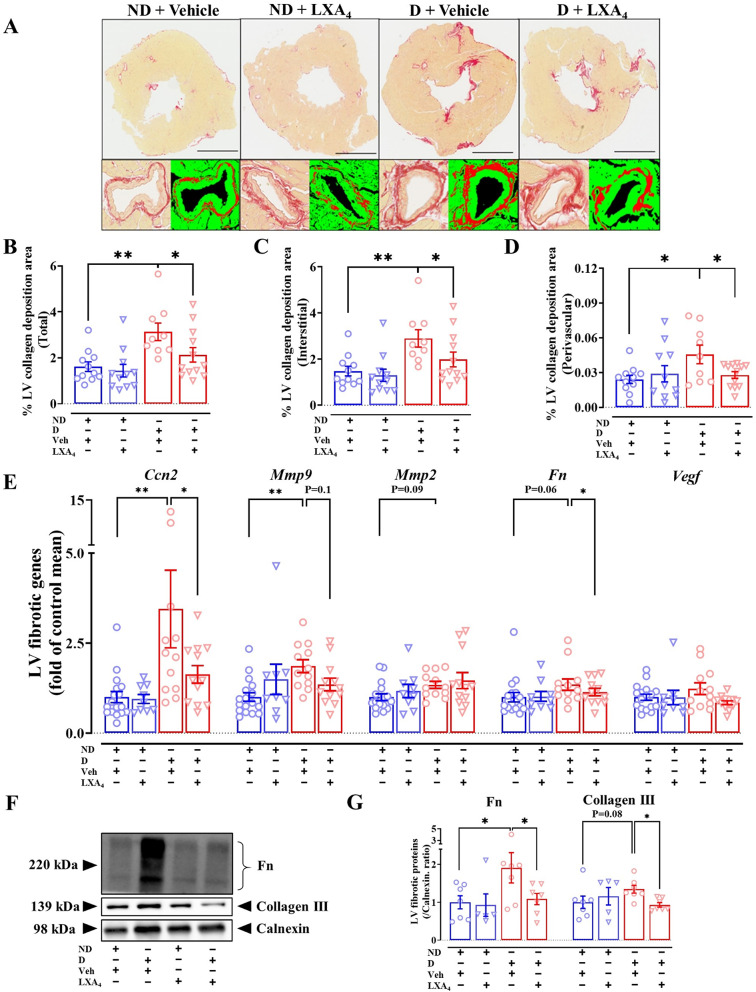
The uppermost portion of the LV was embedded in paraffin and sections were cut (4 μm) using a Leica 2135 microtome (Leica Microsystems, Wetzlar, Hessen, Germany) and stained and scanned by the Monash University Histology Platform using an Aperio Slide scanner (Leica Microsystem, Wetzlar, Hessen, Germany). One set of sections was stained with picrosirius red to determine myocardial fibrosis (cardiac collagen content). The images (whole LV) were analyzed using a custom macro using the Fiji distribution of ImageJ (1.54f; National Institute of Health, Bethesda, Maryland, USA). In brief, the macro allowed the user to outline the regions of interest (ROI, which are the vessels in the left ventricle) for each sample. It then employed a color deconvolution and threshold function in ImageJ to automatically detect and measure the area stained by picrosirius red (Fig. 4A). The ratio of the positive stained area (highlighted in red) to the total tissue area (highlighted in green) represents the percentage of total LV collagen deposition area. The ratio of the positively stained ROI area to the total tissue area represents the percentage of collagen deposition in the perivascular region, while the ratio of the remaining positively stained area to the total tissue area represents the percentage of collagen deposition in the interstitial area. One set of sections was stained with hematoxylin and eosin (H&E) to assess cardiomyocyte size (cross-sectional area and width). The images (whole LV, 200 cardiomyocytes/slide) were analyzed using ImageJ software. One set of LV sections were stained with CardioTACS™ in situ Apoptosis Detection Kit (R&D Systems Inc, Minneapolis, Minnesota, United States) [23]. The image was taken using Leica DMi8 microscope (Leica Microsystem, Wetzlar, Hessen, Germany). TUNEL-positive nuclei and total nuclei were counted by ImageJ software.
Fig. 4.
Effects of lipoxin A4 on cardiac fibrosis in diabetic mice. A Representative image of the left ventricle with picrosirius red staining. Scale bar = 1 mm. B–D Quantification of the entire left ventricle, interstitial and perivascular collagen deposition in the indicated groups. E mRNA levels of fibrotic markers. F Representative LV immunoblot of fibronectin, collagen III and calnexin. G LV protein expression of fibronectin and collagen III. Data are presented as mean ± SEM. Two-way ANOVA followed by Fisher’s LSD post hoc test was used to compare the effects of phenotype and treatment. *P < 0.05, **P < 0.01. Ccn2, connective tissue growth factor; Mmp9, matrix metallopeptidase 9; Mmp2, metallopeptidase 2; Fn, fibronectin; Vegf, vascular endothelial growth factor
The middle portion of the LV was frozen in Tissue-Tek Optimal Cutting Temperature compound and sectioned at 6 μm using a Microm H525 cryostat (Thermo Fisher Scientific™, Walldorf, Baden-Württemberg, Germany). Immunofluorescence staining was used to assess the number of macrophages in the myocardium. The sections were fixed with 4% paraformaldehyde (Sigma-Aldrich, St. Louis, Missouri, USA). Subsequently, 5% normal goat serum was added for blocking. Slides were incubated with either rat anti-mouse CD68+ (1:200, Bio-Rad, Hercules, California, USA), rat anti-mouse iNOS (1:100, Thermo Fisher Scientific™, Melbourne, Victoria, Australia), or rabbit anti-mouse CD206+ (1:100, Thermo Fisher Scientific™, Melbourne, Victoria, Australia), and then placed into a humidified chamber and incubated at 4 °C overnight. Next day, the slides were applied with Alexa Fluor 546 goat anti-rat IgG (1:1000, Invitrogen, Carlsbad, California, USA) or Alexa Fluor 488 goat anti-rabbit IgG (1:1000, Invitrogen, Carlsbad, California, USA). 4′,6-diamidino-2-phenylindole (DAPI; 1:1000; Invitrogen, Melbourne, Victoria, Australia) was then applied for nuclei staining. Sudan Black was added to decrease the autofluorescence, as previously described [24]. Slides were stored at 4 °C until imaged. The image (whole LV) was taken using a Nikon A1R confocal microscope (Nikon, Tokyo, Japan), with DAPI and FIC channels under 20 × magnification. 10 representative images per LV were averaged.
Gene expression
The LV RNA extraction was performed by using a GenElute™ Mammalian Total RNA Miniprep Kit (Sigma-Aldrich, St. Louis, Missouri, USA), according to the manufacturer’s instructions. cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit, following the manufacturer’s instructions (Thermo Fisher Scientific™, Melbourne, Victoria, Australia). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to assess genes of interest (e.g. myocardial fibrotic genes, inflammatory genes, apoptotic genes) by using SYBR® Green Real-Time PCR Mix (Applied Biosystems, Melbourne, Victoria, Australia), and primers generated from mouse-specific sequences (Supplemental Table S1). The expression of the target gene was calculated by using the comparative 2−ΔΔCt method. The housekeeper gene (β-actin) was used to normalize the relative expression of target genes as described [24].
Western blotting
As previously described, one portion of LV (~ 30 mg) was extracted using RIPA buffer [25]. A BCA protein assay kit (Sigma-Aldrich) determined the protein concentration. Diluted protein lysates (30 μg) were loaded onto 7.5% SDS-PAGE gels and transferred to a polyvinylidene difluoride membrane (Immobilon-FL, Millipore). Membranes were incubated with primary antibodies (1:1000 dilution, anti-5 lipoxygenase, Thermo Fisher; 1:1000 dilution, anti-15 lipoxygenase antibody, Abcam; 1:1000 dilution, anti-calnexin C-terminal Rabbit Polyclonal, Abcam; 1:1000 dilution, anti-collagen III antibody, Abcam; 1:200 dilution, anti-fibronectin antibody, Santa Cruz) overnight at 4 °C. The next day, the membranes were incubated with secondary antibodies (1:3000 in skim milk) for 1 h at room temperature. Images were captured using a ChemiDoc imaging system (Bio-Rad), and quantified by Image Lab software (Bio-Rad).
Statistical analysis
The data are presented as mean ± SEM. The experimental groups (ND + vehicle, ND + LXA4, diabetic + vehicle, and diabetic + LXA4) were compared using two-way ANOVA with a Fishers LSD post hoc test and a p value of < 0.05 was considered significant. Graphs were generated and analyses were performed using GraphPad Prism® software version 8 (GraphPad Software, California, USA).
Results
Streptozotocin induces type 1 diabetes in ApoE−/− mice
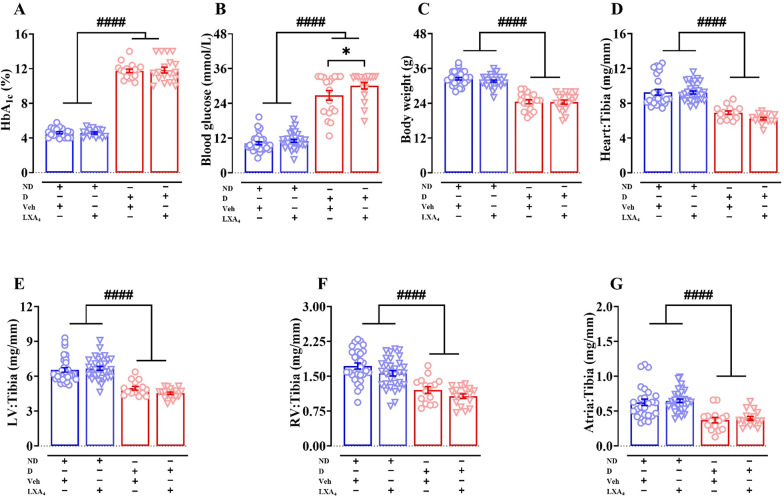
This study used STZ to induce type 1 diabetes in ApoE−/− mice. We followed mice for 16 weeks, in order for cardiac pathology to become established. At the study endpoint, the HbA1c levels and blood glucose levels were significantly elevated in diabetic mice compared with their control (p(phenotype) < 0.0001, Fig. 1A and B). The LXA4-treated diabetic mice exhibited slightly (but significantly) higher blood glucose levels compared to diabetic mice (30.1 ± 1.1 mmol/L vs. 26.8 ± 1.6 mmol/L, Fig. 1B). However, treatment with LXA4 had no impact on HbA1c levels in either diabetic or control groups (Fig. 1A). Diabetic mice exhibited a lower final body weight compared to ND mice (p(phenotype) < 0.0001, Fig. 1C). Administration of LXA4 therapy for 6 weeks had no impact on body weight (Fig. 1C). Tibia length did not alter between groups (Table 1). Relative to tibia length, the whole heart weight, LV weight, right ventricle (RV) weight and atrial weight were significantly reduced in diabetic mice (Fig. 1D–G). Taken together, these data indicate that this model (STZ treated mice studied on an ApoE−/− background) is consistent with a type 1 diabetes phenotype and that LXA4 treatment had a minimum effect on the diabetic phenotype.
Fig. 1.
Metabolic parameters at study endpoint. A Endpoint HbA1c levels. B Endpoint blood glucose levels. C Endpoint body weight. D–G Cardiac weight relative to tibia length. Data are presented as means ± SEM. Two-way ANOVA followed by Fisher’s LSD post hoc test was used to compare the effects of phenotype and treatment. *p < 0.05, ####p < 0.0001. HbA1c, glycated haemoglobin; LV, left ventricle; RV, right ventricle; ND, non-diabetic; D, diabetic; Veh, vehicle
Table 1.
Systemic characteristics at endpoint
| Systemic characteristics | Non-diabetic | Diabetic | ||
|---|---|---|---|---|
| Vehicle | Lipoxin A4 | Vehicle | Lipoxin A4 | |
| Heart (mg) | 163.0 ± 5.8 (n = 28) | 159.5 ± 3.2 (n = 31) | 116.5 ± 2.5## (n = 18) | 105.9 ± 2.7$ (n = 16) |
| Left ventricle (mg) | 115.2 ± 3.5 (n = 32) | 115.5 ± 3.0 (n = 31) | 85.2 ± 2.2## (n = 18) | 77.3 ± 1.9$ (n = 18) |
| Right ventricle (mg) | 30.7 ± 1.3 (n = 31) | 27.0 ± 1.0# (n = 31) | 19.5 ± 1.1## (n = 18) | 18.2 ± 0.8$ (n = 18) |
| Atria (mg) | 11.0 ± 0.8 (n = 28) | 11.2 ± 0.5 (n = 31) | 6.3 ± 0.5## (n = 18) | 6.8 ± 0.5$ (n = 16) |
| Tibia length (mm) | 17.3 ± 0.1 (n = 32) | 17.3 ± 0.1 (n = 31) | 16.7 ± 0.1 (n = 18) | 16.9 ± 0.1 (n = 18) |
#P < 0.05, ##P < 0.0001 vs non-diabetic + vehicle; $P < 0.0001 vs non-diabetic + lipoxin A4 (2-way ANOVA, Fisher’s post hoc for multiple comparisons)
Lipoxin A4 limits macrophage polarization in LV
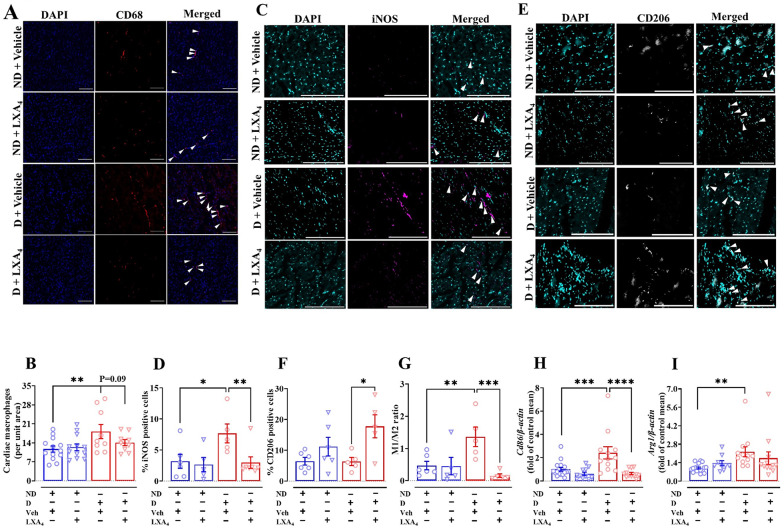
Macrophages are the key contributors to both the initiation and the resolution of inflammation. There was a greater accumulation of CD68-positive (a marker of pan macrophages) in the LV in diabetic mice compared with the ND mice (Fig. 2A and B), which was not impacted by LXA4 administration (Fig. 2B). We then investigated whether LXA4 promotes macrophage polarization which is critical for the resolution of inflammation. We found that the mRNA expression of Cd86 (a marker of M1-like macrophages, Fig. 2H) and arginase 1 (mArg1, a marker of M2-like macrophages, Fig. 2I) were upregulated in the LV of vehicle-treated diabetic mice. Notably, although no changes were detected in the mRNA expression of mArg1 in LXA4-treated diabetic mice, the expression of mCd86 significantly decreased in LXA4-treated diabetic mice compared with vehicle-treated diabetic mice (Fig. 2H and I). To confirm these observations, immunofluorescence staining was used to further demonstrate that the percentage of iNOS-positive cells (a marker of M1-like macrophages) was higher in vehicle-treated diabetic mice. Further, LXA4 significantly reduced the abundance of iNOS-positive cells in mouse LV tissues (Fig. 2C and D). The presence of CD206-positive cells (a marker of M2-like macrophages) was not different in vehicle-treated diabetic mice. However, treatment of mice LXA4 exhibited a higher percentage of CD206-positive cells (Fig. 2E and F). Furthermore, the M1/M2 ratio was higher in vehicle-treated diabetic mice compared to ND mice, and that this ratio was lower in LXA4-treated mice (Fig. 2G). These data indicate that LV macrophage accumulation is associated with diabetes and LXA4 may limit monocyte-macrophages polarization to M1-like (pro-inflammatory) macrophages.
Fig. 2.
Effects of lipoxin A4 on macrophage polarization in LV. A Representative images of macrophages in the left ventricle, co-stained with nuclei (blue, DAPI) and macrophage (red, CD68). Scale bar = 200 μm. B Quantitative data of CD68-positive macrophages. C Representative images of M1-like macrophages in the left ventricle, co-stained with nuclei (cyan, DAPI) and M1-like macrophage (magenta, iNOS). Scale bar = 200 μm. D Quantitative data of iNOS-positive macrophages. E Representative images of M2-like macrophages in the left ventricle, co-stained with nuclei (cyan, DAPI) and M2-like macrophage (grey, CD206). Scale bar = 200 μm. F Quantitative data of CD206-positive macrophages. G M1/M2 macrophage ratio H mRNA expression of mCd86 in mouse left ventricle. I mRNA expression of mArg1 in mouse left ventricle. Data are presented as mean ± SEM, Two-way ANOVA followed by Fisher’s LSD post hoc test was used to compare the effects of phenotype and treatment. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Arg1, arginase-1
Lipoxin A4 attenuates LV inflammation
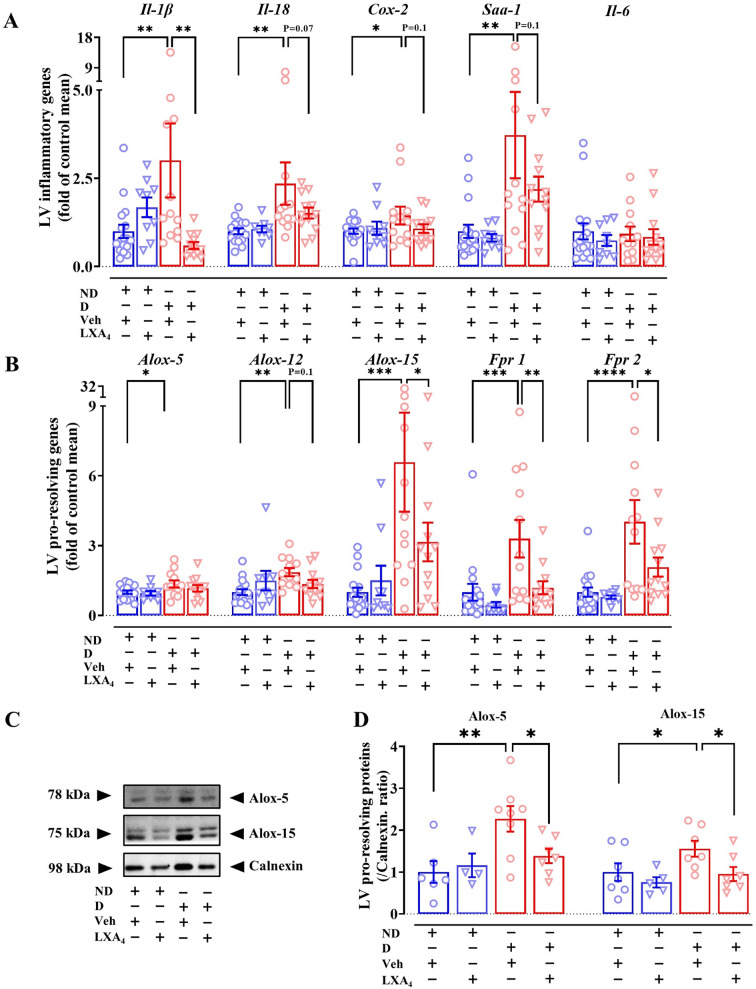
Cytokines play important roles in inflammation, such as in the regulation of cell proliferation, migration and adhesion. Relevant to inflammatory markers, we observed an upregulation of mIl-1β, mIl-18, cyclooxygenase-2 (mCox-2) and serum amyloid A1 (mSaa1) in vehicle-treated diabetic mice compared with ND mice (Fig. 3A). LXA4 treatment of diabetic mice reduced the expression of mIl-1β, and tended to downregulate mIl-18, mCox-2 and mSaa1. There was, however, no difference in mIl-6 expression between the groups (Fig. 3A). Lipoxin-synthesizing arachidonate 5/12/15-lipoxygenase (Alox-5/12/15) is the enzyme responsible for the biosynthesis of pro- and anti-inflammatory lipid mediators. The expression of mAlox-5, mAlox-12 and mAlox-15 in the vehicle-treated diabetic group was significantly upregulated compared to the ND group (Fig. 3B). Treatment with LXA4 showed a tendency toward decreased mAlox-12 expression and a significant downregulation of mAlox-15 expression compared to the diabetic group (Fig. 3B). We also confirmed Alox-5 and Alox-15 protein levels were elevated in vehicle-treated diabetic group compared to the ND group (Figs. 3C and D). Furthermore, treatment with LXA4 reduced Alox-5 and Alox-15 protein levels (Fig. 3C and D). The expression of formylpeptide receptors (FPR1 and FPR2), which are involved in both pro and anti-inflammatory responses, involved in both pro- and anti-inflammatory responses) expression were also assessed using qRT-PCR analysis. Vehicle-treated diabetic mice exhibited an upregulated expression of mFpr1 and mFpr2 (Fig. 3B), and when the mice were treated with LXA4, the expression of both receptor transcripts was significantly downregulated (Fig. 3B).
Fig. 3.
Effects of lipoxin A4 on cardiac inflammation in diabetic mice. A mRNA levels of inflammatory markers. B mRNA levels of pro-resolving markers. C Representative LV immunoblot of Alox-5, Alox-15 and calnexin. D LV protein expression of Alox-5 and Alox-15. Data are presented as mean ± SEM, Two-way ANOVA followed by Fisher’s LSD post hoc test was used to compare the effects of phenotype and treatment. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Il, interleukin; Cox, cyclooxygenase; Saa1, serum amyloid A1; Alox, arachidonate lipoxygenase; Fpr, formylpeptide receptor
Lipoxin A4 alleviates LV fibrosis in diabetic mice
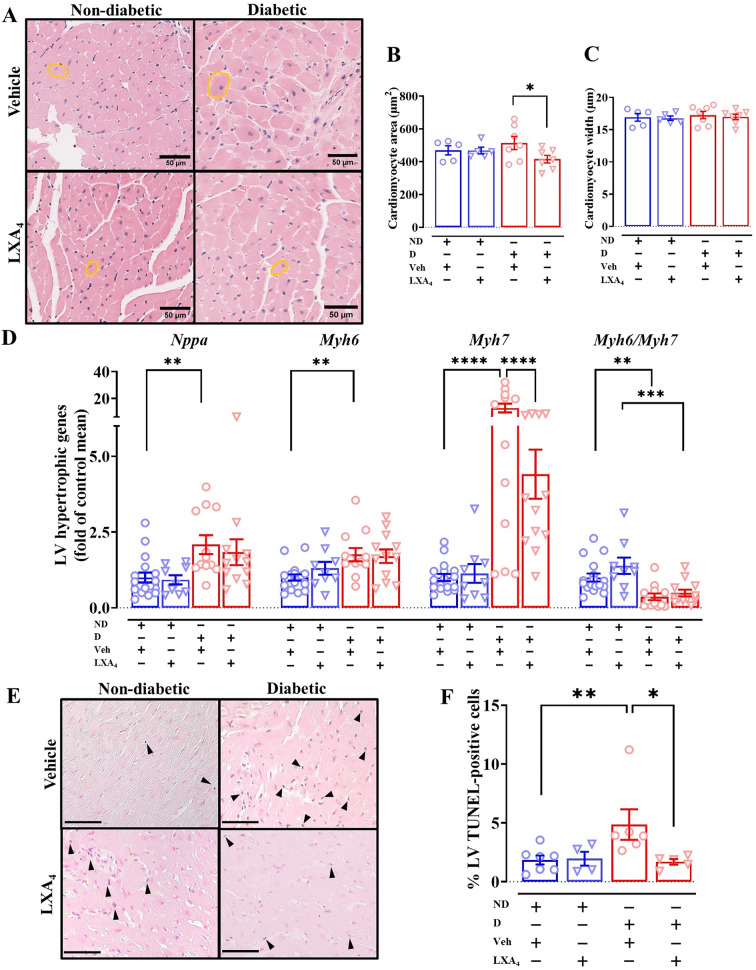
Cardiac fibrosis is a key structural feature of the diabetic heart that is evident both in individuals with diabetes (with and without HF) and in pre-clinical animal models of diabetes [2, 26, 27]. There was a significant increase in total LV collagen deposition in vehicle-treated diabetic mice, compared with ND mice (Fig. 4A and B). Importantly, LV interstitial and perivascular collagen levels were significantly increased in vehicle-treated diabetic mice (Fig. 4C and D). Treatment of diabetic mice with LXA4 significantly reduced the collagen content in all assessed regions of the LV (Fig. 4B–D). Consistent with this histological data, we found that the mRNA level of the pro-fibrotic genes, such as connective tissue growth factor (mCcn2) and matrix metallopeptidase 9 (mMmp9) in the LV, was upregulated in diabetic mice compared with ND mice (Fig. 4E). Other fibrotic genes such as mMmp2 and fibronectin (mFn) showed a tendency towards upregulation in diabetic mice (Fig. 4E), while the expression of vascular endothelial growth factor (mVegf) was not affected. mCcn2 and mFn were significantly downregulated in LXA4-treated diabetic mice (Fig. 4E), and there was a tendency towards downregulating of mMmp9 in the LXA4-treated diabetic group. Furthermore, LV protein expression of fibronectin was significantly higher in diabetic mice, while collagen III exhibited a trend toward increased expression in diabetic mice compared to ND mice (Fig. 4F and G). These findings suggest enhanced extracellular matrix remodeling in the diabetic group, consistent with the pathological alterations observed in diabetic cardiomyopathy, with LXA4 attenuating collagen deposition in the LV of diabetic mice.
Lipoxin A4 attenuates LV cardiomyocyte hypertrophy and apoptosis
Cardiomyocyte hypertrophy is one of the pathological changes commonly observed in diabetic cardiomyopathy [2]. Cardiac-specific biomarkers for cardiac hypertrophy (e.g. β-myosin heavy chain and atrial natriuretic peptide) are valuable for assessing cardiac remodeling and heart failure. Moreover, there are reports that, in heart failure, the α-myosin heavy chain and β-myosin heavy chain ratio (mMyh6/mMyh7) is decreased in animal models [28]. In the present study we show that diabetic mice had a significantly elevated expression of atrial natriuretic peptide A (mNppa), mMyh6 and mMyh7, as well as a decreased mMyh6/mMyh7 ratio (Fig. 5A). No change in mRNA expression of mNppa was observed in mice treated with LXA4, but the expression of mMyh7 was significantly downregulated in LXA4-treated diabetic mice compared to vehicle-treated diabetic mice (Fig. 5A). We performed H&E staining to measure the cross-sectional area and width of cardiomyocytes as a measure of hypertrophy (Fig. 5B). The cross-sectional area and width were not different in diabetic mice compared to ND mice (Fig. 5C and D), but treatment with LXA4 appeared to reduce the area (but not the 2D width) of cardiomyocytes, compared to vehicle-treated mice (Fig. 5C). These findings suggest that our model showed a mild cardiac hypertrophy phenotype at the gene expression level, and LXA4 may attenuate the development of cardiac hypertrophy. Moreover, a higher number of TUNEL-positive cells was evident in diabetic mice, compared to ND mice (Fig. 5E and F). Treatment with LXA4 showed fewer TUNEL-positive cells in the LV (Fig. 5F). Taken together, the higher number of apoptotic cells in the LV in our model is consistent with the previous study using STZ-induced T1DM mice [29]. Additionally, LXA4 may protect against cardiomyocyte apoptosis.
Fig. 5.
Effects of lipoxin A4 on cardiac hypertrophy and cardiomyocyte apoptosis in diabetic mice. A mRNA levels of hypertrophic markers. B Representative images of the left ventricle with H&E staining. Scale bar = 50 µm. C and D Quantification of cardiomyocyte area and width in the indicated groups. E Representative images of the left ventricle with TUNEL staining, scale bar = 100 µm. F Quantification of the percentage of apoptotic cardiomyocytes (blue and pink co-stained nuclei) to total cells (pink-stained nuclei). Data are presented as mean ± SEM. Two-way ANOVA followed by Fisher’s LSD post hoc test was used to compare the effects of phenotype and treatment. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001. Nppa, natriuretic peptide A; Myh7, β-myosin heavy chain; Myh6, α-myosin heavy chain
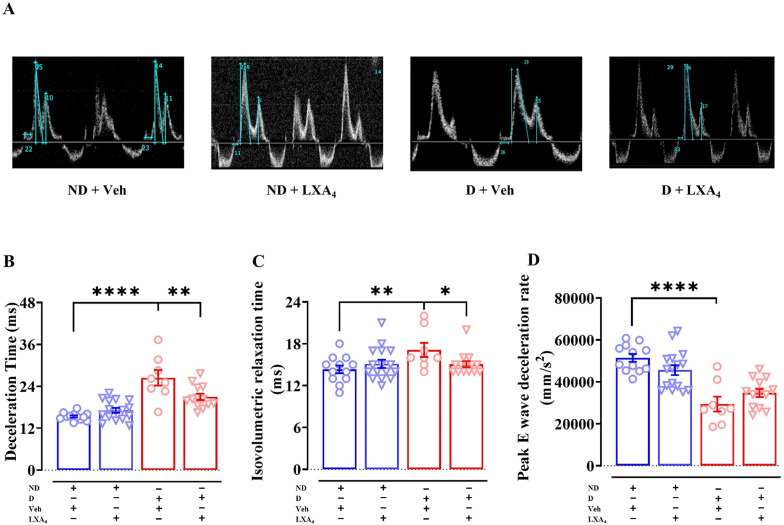
Lipoxin A4 improves LV diastolic function
PW Doppler echocardiography was performed to assess LV diastolic function (Fig. 6A). Vehicle-treated diabetic mice exhibited a prolonged deceleration time (DT, approximately 11 ms) compared to vehicle-treated ND mice (Fig. 6B, Table 2). Treatment with LXA4 shortened the DT compared to vehicle-treated diabetic mice, but did not restore it to the level observed in ND (Fig. 6B). Furthermore, isovolumetric relaxation time (IVRT), another measure of LV diastolic function, was significantly prolonged in vehicle-treated diabetic mice compared to ND mice (Fig. 6C). The IVRT was restored to ND levels by LXA4 in diabetic mice (Fig. 6C). The rate of E-wave deceleration of vehicle-treated diabetic mice was slower than vehicle-treated ND mice, but this was not impacted by LXA4 treatment in either ND or diabetic mice (Fig. 6D). Diabetic mice showed a lower LV volume, area and cardiac output, compared to ND mice (Supplemental Tables S2). There were no significant differences between vehicle treated ND mice and vehicle treated diabetic mice in LV posterior wall diastolic thickness (PWd), LV anterior wall diastolic thickness (AWd), LV end-systolic dimension (LVESD), LV end-diastolic dimension (LVEDD) and fractional shortening assessed by M-mode echocardiography. However, compared to LXA4 treated ND mice, there is a thinner PWd, LVESD, LVEDD and a higher fractional shortening in LXA4 treated diabetic mice (Supplemental Tables S3). Some parameters show greater variability perhaps due to the lower sample size in vehicle-treated diabetic mice. Collectively, these data suggest that STZ-induced diabetic mice on an ApoE−/− background exhibit mild diastolic dysfunction. Notably, treatment with LXA4 appears to partially ameliorate this dysfunction.
Fig. 6.
Effects of Lipoxin A4 on diastolic function in diabetic mice. A Representative Doppler image from each group B Deceleration time. C Isovolumetric relaxation time. D Rate of E-wave deceleration. Data are presented as mean ± SEM, n = 8–16. Two-way ANOVA followed by Fisher’s LSD post hoc test was used to compare the effects of phenotype and treatment; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Table 2.
Assessment of LV diastolic function via PW Doppler and tissue Doppler echocardiography
| Non-diabetic mice | Diabetic mice | |||
|---|---|---|---|---|
| Vehicle | LXA4 | Vehicle | LXA4 | |
| PW Doppler and tissue Doppler echocardiography | ||||
| N | 12 | 16 | 8 | 13 |
| Heart rate (bpm) | 434 ± 9 | 421 ± 8 | 384 ± 12# | 427 ± 8** |
| Peak E wave velocity (mm/s) | 788 ± 24 | 760 ± 18 | 731 ± 38 | 709 ± 18 |
| Peak A wave velocity (mm/s) | 505 ± 32 | 467 ± 15 | 405 ± 21^ | 427 ± 10 |
| Peak e’ velocity (mm/s) | 23.9 ± 1.1 | 25.0 ± 0.7 | 23.4 ± 2.3 | 20.7 ± 1.3$ |
| Peak a’ velocity (mm/s) | 22.6 ± 1.1 | 21.4 ± 0.7 | 20.5 ± 0.9 | 21.5 ± 0.6 |
| E/A ratio | 1.62 ± 0.08 | 1.64 ± 0.05 | 1.83 ± 0.14 | 1.67 ± 0.06 |
| e’/a’ ratio | 1.09 ± 0.09 | 1.19 ± 0.05 | 1.15 ± 0.14 | 0.95 ± 0.06$ |
| E/e’ ratio | 33.7 ± 2.0 | 30.8 ± 1.1 | 32.4 ± 1.8 | 35.7 ± 2.1$ |
| Deceleration time (ms) | 15.4 ± 0.3 | 17.1 ± 0.7 | 26.4 ± 2.2## | 21.0 ± 0.9**, $$ |
| Isovolumetric relaxation time (ms) | 14.3 ± 0.6 | 15.1 ± 0.6 | 17.1 ± 1.0# | 15.1 ± 0.4* |
#P < 0.01, ##P < 0.0001 vs non-diabetic + vehicle; *P < 0.05, **P < 0.01 vs diabetic + vehicle; $P < 0.05, $$P < 0.001 vs non-diabetic + lipoxin A4 (2-way ANOVA, Fisher’s post hoc for multiple comparisons)
Discussion
This study demonstrated that ApoE−/− diabetic mice exhibited cardiac inflammation, myocardial fibrosis, cardiomyocyte apoptosis, increased gene expression associated with cardiomyocyte hypertrophy, and early signs of LV diastolic dysfunction. Notably, the inflammatory response was associated with an increased number of macrophages in the heart, particularly with an increase in the M1-like macrophage population, which was also consistent with the elevated LV expression of pro-inflammatory mediators. Moreover, the expression of mFpr1 and mFpr2 was also increased in the diabetic heart. Our study demonstrated for the first time that chronic treatment with LXA4 protected against cardiac fibrosis, cardiomyocyte apoptosis and cardiomyocyte hypertrophy. Furthermore, a significantly reduced expression of some pro-inflammatory cytokines and mFprs was observed, and these changes were associated with improved in LV function.
Our current data indicate that LXA4 does not significantly impact glucose control, suggesting that it may not influence the synthesis of ketone bodies. However, Börgeson et al. have demonstrated that lipoxin A4 and its analogue, Benzo-LXA4, can limit liver expansion, reduce serum alanine aminotransferase levels, and decrease hepatic triglyceride levels in models of obesity-induced liver disease [30]. However, the effects of LXA4 on ketone body metabolism remain largely unknown, particularly in the context of diabetic ketoacidosis. Further investigation is needed to elucidate the potential interactions between LXA4 and ketone body dynamics in this pathological state.
Our study employed robust T1DM models, pharmacologically induced by STZ [2]. This model not only replicates diabetic-induced fibrosis, apoptosis, hypertrophy and cardiac inflammation, but also exhibits the functional phenotype, especially diastolic dysfunction, mirroring the pathological features observed in the diabetic human heart [31]. Given that chronic low-grade inflammation plays an important role in the development of diabetic cardiomyopathy [32, 33], we utilized ApoE−/− mice on a C57BL/6 background in this study. Due to their deficiency in ApoE, even on a regular diet, these mice demonstrate elevated cholesterol levels, macrophage accumulation, and increased release of cytokines and chemokines, resulting in heightened systemic inflammation [34]. These characteristics provide a valuable platform for studying chronic low-grade inflammatory diseases, including cardiovascular and metabolic disorders [35].
A previous study in our laboratory has demonstrated that diabetes increases cardiac macrophage content, which was evident in diabetic mice 8 weeks post STZ-induction, and this persisted beyond 16 weeks of diabetes, at least on an FVB background [31]. In the present study, an increased cardiac macrophage number, particularly M1-like macrophages, was confirmed by histological analysis in the diabetic heart. The total number of pan macrophages in the heart was not affected by LXA4. However, our observations suggest that LXA4 may limit the polarization of recruited monocyte macrophages towards an M1-like phenotype, which may have important implications for the pathophysiology of the diabetic heart. Following heart injury or other inflammatory cardiac insults, circulating monocytes can infiltrate the heart and polarize towards the M1-like (pro-inflammatory) macrophage phenotype, as previously reported in both animal models and people with diabetes [36, 37]. Our results demonstrated that M1-like macrophages were upregulated in diabetic mice, while M2-like macrophages were not. Treatment with LXA4 reduced the number of M1-like macrophages, and increased the number of M2-like macrophages, consistent with previous reports [30, 38].
FPR1 and FPR2 play critical roles in both the induction and resolution of inflammation[39–41]. FPR2 is the primary target for LXA4 and has multiple roles in cardiometabolic diseases including in aging and obesity [42]. The current study showed a similar upregulation of expression of mFpr1 and mFpr2 in the vehicle-treated diabetic mice, as reported in other chronic inflammatory diseases [43–45]. The induction and resolution of inflammatory responses are not only receptor-dependent, but also highly ligand-dependent, as highlighted in recent reviews [41, 46]. In chronic inflammatory conditions, such as T1DM and T2DM, several animal models have demonstrated reduced plasma levels of LXA4 and annexin A1 [46]. This is consistent with human data, where individuals with diabetes exhibit lower circulating levels of LXA4 and annexin A1 when compared to healthy controls [47]. A cohort study further revealed that diminished LXA4 levels are positively associated with the onset of T2DM [48]. These findings suggest that the resolution phase of inflammation may be overwhelmed by persistent inflammatory stimuli in diabetic conditions. The current study showed an increased gene expression of mSaa1 (pro-inflammatory endogenous mFpr2 ligand) in the vehicle-treated diabetic mice, and a similar upregulation of mFpr1 and mFpr2 was observed. Taken together, the diabetes-induced increase in the expression of mFpr1 and mFpr2 suggests an enhanced opportunity for pro-inflammatory ligands to engage these receptors, potentially exacerbating the inflammatory response. Interestingly, treatment with LXA4 significantly attenuated the diabetes-induced increase in mFpr1 and mFpr2 expression, as well as downregulating mSaa1, consistent with the observations of Aubeux and colleagues who reported that the gene expression of FPR2 was downregulated in M2-like macrophages [38]. Our data suggest that reduced mFpr2 expression may be related to the phenotype switching of macrophages in the LV. However, we could not obtain unequivocal results with a commercially available antibody.
ALOX-5, 12 and 15 are critical inflammatory enzymes and it has been demonstrated that the location of ALOX-5 within leukocytes is vital for its function. For the biosynthesis of leukotriene B4, a pro-inflammatory lipid mediator, ALOX-5 is located in the nuclear envelope. However, for the biosynthesis of LXA4, a pro-resolving lipid mediator, ALOX-5 is located in the cytoplasm [49, 50]. Moreover, it has been shown that ALOX-5 was more highly expressed in M1-like macrophages than in M2-like macrophages [51]. Our study demonstrated a higher expression of Alox-5 in diabetic mice, while treatment with LXA4 showed a lower protein level of Alox-5. Taken together, the lower expression of Alox-5 may be related to a lower number of M2-like macrophage in diabetic mice treated with LXA4. LXA4 may also affect the intercellular localization of ALOX-5 [49, 50], which could impact the biosynthesis of lipid mediators, but this requires further investigation. In the current study, mAlox12 and 15, exhibited upregulation in the vehicle-treated diabetic cohort, consistent with previous studies in other diabetes-associated comorbidities [52, 53]. Overexpression of 12/15-LOX in a transgenic mice model revealed an increased cardiac macrophage content, a high level of cardiac inflammation, cardiac fibrosis and LV systolic dysfunction [54]. A novel observation in this study was that treatment with LXA4 blunted the LV gene expression of mAlox12 and 15 in diabetic mice and Alox-15 protein levels.
Chronic inflammation is a primary basis for cardiac pathobiology, as a result of poorly-balanced diets in addition to deficiencies in both exercise and sleep habits [55, 56]. Cardiac fibrosis, including increased collagen deposition can result in LV diastolic dysfunction by stiffening the myocardium [2]. In this study, we observed a apparent increase in myocardial interstitial and perivascular collagen deposition, consistent with previous observations in both T1DM and T2DM in mice [53, 57, 58] and biopsies of human hearts [59]. In the current study, treatment with LXA4 limited the cardiac fibrosis in both myocardial interstitial and perivascular areas. Cardiomyocyte apoptosis is well recognized in both animals and individuals with diabetes [2, 4, 60]. In this study, we found a significantly higher number of apoptotic cardiomyocytes in diabetic mice, and a lower number of apoptotic cardiomyocytes following the administration of LXA4.
LV diastolic dysfunction is commonly noted as one of the earliest functional abnormalities, observed in the progression of diabetic heart disease [2]. In the present study, there were some early signs of impaired LV diastolic function (prolonged times for deceleration and isovolumetric relaxation) in our vehicle-treated diabetic mice, although not all of the parameters of diastolic function were impaired. The increased time for deceleration in the diabetic animals was normalized in the LXA4 treated diabetic animals. The differences observed between our study and previous reports may be related to different mouse strains. Over 6 weeks in diabetic ApoE−/− mice, the attenuation of inflammatory responses by LXA4 may not sufficiently translate to a measurable improvement in cardiac function. A longer treatment regimen should be explored to fully assess the therapeutic potential of LXA4, provided that the stability and viability of this animal model can be optimized. Extending the duration of LXA4 administration may reveal more pronounced effects on cardiac function and better simulate the resolution of inflammatory responses to limit chronic inflammation in diabetic cardiomyopathy.
Limitations and future direction
The model used in this study provides valuable insight into how macrophages play a crucial role in the progression of diabetic cardiomyopathy. It further highlights how chronic inflammation may lead to cardiac remodeling and early signs of cardiac dysfunction, which could represent a mild or early stage of diabetic heart disease. However, this model does not fully represent the pathophysiology of patients suffering from diabetic cardiomyopathy, so other models may be required to confirm the protective effects of LXA4. We exclusively used male mice as a proof-of-concept study, and future research should incorporate female mice, especially given that females are more susceptible to diabetic cardiovascular complications [61], and that there are observed sex-specific differences in the biosynthesis of SPMs [62]. Including both sexes will provide a more comprehensive understanding of the pathophysiological mechanisms and therapeutic responses. Previous studies have shown that with every percentage increase in HbA1c levels, the risk of heart failure increases by 30% in T1DM and 8% in T2DM [63, 64]. Compared to healthy subjects, individuals with T1DM develop heart disease 10 to 15 years earlier [65]. As mentioned earlier, both T1DM and T2DM share certain pathological features of diabetic cardiomyopathy. In this study, we used a T1DM model to test the therapeutic potential of LXA4. However, using STZ-induced models may introduce toxic effects that influence cardiac phenotype, and may not mimic some specific pathological features of T2DM patients. Studies have shown that there are differences in cardiac remodeling and function between T1DM and T2DM-induced heart disease. For example, in T1DM (STZ-induced diabetes) animal models, mitochondrial dysfunction, inflammation, fibrosis and apoptotic pathways were more affected than control mice. In contrast, in T2DM (induced by STZ and a high-fat diet) animals, mice exhibited impaired glucose metabolism, nitric oxide signaling, and CXR4 signaling pathways compared to the control (normal chow diet) [21]. These differences may contribute to the different phenotypes of heart failure in the two models (heart failure with reduced ejection fraction for T1DM and heart failure with preserved ejection fraction for T2DM) [29]. Therefore, it is important to validate the therapeutic effects of LXA4 in alternative T1DM models (e.g., BioBreeding diabetes-prone mice), as well as in T2DM (e.g., db/db mice) [66, 67]. Moreover, the dynamic nature of the inflammatory response poses a challenge in evaluating the therapeutic efficacy of anti-inflammatory agents at a single time point in chronic inflammatory disease models. Future studies may include assessment of the inflammatory response at earlier stages (e.g., 4 weeks after the onset of diabetes) to capture the temporal dynamics of inflammation and better understand the therapeutic window for LXA4 [31]. Use of genetically modified animals (silenced receptor, etc.) that can demonstrate the role of FPR2 in the progression of diabetic cardiomyopathy, and further confirm targeting resolution of inflammation is a viable approach for treating diabetic cardiomyopathy. We attempted to quantify the cardiac concentration of LXA4; however, our measurements fell below the detectable limit. This limitation is likely attributable to the sensitivity of the analytical equipment used and the stability of LXA4 under the experimental conditions. Given the short half-life of lipoxin, an analysis of the plasma and cardiac concentration of LXA4 is needed [68]. Also, future translational studies will benefit from developing more stable lipoxin analogues. These analogues will enable a more accurate assessment of therapeutic efficacy, facilitating the translation of preclinical findings to clinical applications [69, 70].
Conclusions
In conclusion, our study provides compelling evidence that diabetic ApoE−/− mice exhibit hallmark features of diabetic cardiomyopathy (such as adverse remodeling and diastolic dysfunction). Notably, treatment with LXA4 significantly mitigated diabetes-induced cardiac structural remodeling, as evidenced by reduced cardiac fibrosis, decreased the number of apoptotic cardiomyocytes and downregulation of hypertrophy-related genes. LXA4 also ameliorated early-stage left ventricular diastolic dysfunction. Mechanistically, LXA4 selectively reduced the prevalence of M1-like macrophages without altering the overall macrophage population in the diabetic heart. Furthermore, LXA4 downregulated the expression of both FPR subtypes, correlating with decreased levels of pro-inflammatory mediators. These findings suggest that LXA4 could serve as a valuable adjunct to current standard-of-care therapies, potentially enhancing clinical outcomes for patients with diabetic heart disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the technical assistance of Monash Histology Platform, Monash University.
Abbreviations
- Alox 5/12/15
Arachidonate 5/12/15-lipoxygenase
- ApoE−/−
Apolipoprotein E-deficient
- Arg1
Arginase-1
- AWd
Anterior wall diastolic thickness
- Ccn2
Connective tissue growth factor
- Cox
Cyclooxygenase
- DT
Deceleration time
- EF
Ejection fraction
- Fn
Fibronectin
- FPR
Formylpeptide receptor
- FS
Fractional shortening
- HbA1c
Glycated hemoglobin
- H&E
Hematoxylin and eosin
- IL
Interleukin
- i.p.
Intraperitoneal injection
- IVRT
Isovolumetric relaxation time
- LV
Left ventricle
- LVEDD
LV end-diastolic dimension
- LVESD
LV end-systolic dimension
- LXs
Lipoxins
- LXA4
Lipoxin A4
- LXB4
Lipoxin B4
- MI
Myocardial infarction
- Mmps
Matrix metalloproteinases
- Myh
Myosin heavy chain
- ND
Non-diabetic
- NF-κB
Nuclear factor κ–light-chain-enhancer of activated B cells
- Nppa
Natriuretic peptide A
- KXA
Ketamine/xylazine/atropine
- PW Doppler
Pulsed wave Doppler
- PWd
Posterior wall diastolic thickness
- qRT-PCR
Quantitative real-time polymerase chain reaction
- RV
Right ventricle
- Saa
Serum amyloid A
- SPMs
Specialized pro-resolving lipid mediators
- STZ
Streptozotocin
- T1DM
Type 1 diabetes mellitus
- TNF-α
Tumor necrosis factor-α
- Vegf
Vascular endothelial growth factor
Author contributions
E.P.B., C.G., R.H.R., P.K., and C.X.Q. conceived and designed the study; T.F., M.M., M.B., M.D., and P.K. performed the experiments; T.F., P.S.Z., B.K.K.-H., O.L.W., R.H.R., and C.X.Q. interpreted the results; H.K. acquired echocardiography images and performed echo data validation. C.J.N. wrote the script for analyzing the picrosirius red staining; E.P.B., C.G., P.K., R.H.R., and C.X.Q. acquired funding; T.F. analyzed the data, prepared the figures, drafted, and reviewed the manuscript. All authors contributed to and edited the article and approved the submitted version.
Funding
This work was partly funded by Juvenile Diabetes Research Foundation Strategic Research Award (to E.P.B, C.G. and P.K), and the Victorian Government of Australia’s Operational Infrastructure Support Program. P.K is supported by the National Health and Medical Research Council (NHMRC), APP1183737. P.K and M.M are supported by Diabetes Australia Research Program Y22G-MOHM. C.G. and E.P.B are supported by the UCD foundation. P.S.Z. is supported by an Australian Research Council Future Fellowship, FT200100218. R.H.R. was supported by an NHMRC Senior Research Fellowship ID1059960. C.X.Q. is supported by a National Heart Foundation of Australia Future Leader Fellowship ID102787. T.F. is supported by the Monash Graduate Scholarship and Monash International Tuition Scholarship.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rebecca H. Ritchie, Phillip Kantharidis and Cheng Xue Qin are joint senior authors of this work.
Change history
11/29/2024
The degree tagging was removed for the first author
References
- 1.Marx N, et al. 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44(39):4043–140. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126(11):1501–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes, A., 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S125–S150. [DOI] [PubMed]
- 4.Marwick TH, et al. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–51. [DOI] [PubMed] [Google Scholar]
- 5.Packer M. Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart Fail. 2021;9(8):535–49. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen M, et al. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev. 2003;124(4):495–502. [DOI] [PubMed] [Google Scholar]
- 7.Zaharieva E, et al. Interleukin-18 serum level is elevated in type 2 diabetes and latent autoimmune diabetes. Endocr Connect. 2018;7(1):179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. [DOI] [PubMed]
- 9.Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol Chem. 2012;287(13):10070–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27(3):200–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halade GV et al. Splenic leukocytes define the resolution of inflammation in heart failure. Sci Signal 2018;11(520). [DOI] [PMC free article] [PubMed]
- 13.Fu T, et al. Therapeutic potential of lipoxin A(4) in chronic inflammation: focus on cardiometabolic disease. ACS Pharmacol Transl Sci. 2020;3(1):43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reina-Couto M, et al. Impaired resolution of inflammation in human chronic heart failure. Eur J Clin Invest. 2014;44(6):527–38. [DOI] [PubMed] [Google Scholar]
- 15.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92(21):9475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jozsef L, et al. Lipoxin A4 and Aspirin-triggered 15-epi-lipoxin A4 Inhibit Peroxynitrite Formation, NF-kappa B and AP-1 Activation, and IL-8 gene Expression in Human Leukocytes. Proc Natl Acad Sci U S A. 2002;99(20):13266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan EP, et al. Lipoxins regulate the early growth response-1 network and reverse diabetic kidney disease. J Am Soc Nephrol. 2018;29(5):1437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan EP, et al. Lipoxins protect against inflammation in diabetes-associated atherosclerosis. Diabetes. 2018;67(12):2657–67. [DOI] [PubMed] [Google Scholar]
- 19.Kain V, et al. Resolution agonist 15-epi-Lipoxin A(4) programs early activation of resolving phase in post-myocardial infarction healing. Sci Rep. 2017;7(1):9999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drucker DJ. Never waste a good crisis: confronting reproducibility in translational research. Cell Metab. 2016;24(3):348–60. [DOI] [PubMed] [Google Scholar]
- 21.Khan HA, et al. Evaluation of Hba1c criteria for diagnosis of diabetes mellitus: a retrospective study of 12785 type 2 Saudi male patients. Endocr Res. 2014;39(2):61–5. [DOI] [PubMed] [Google Scholar]
- 22.Donner DG, et al. Improving the quality of preclinical research echocardiography: observations, training, and guidelines for measurement. Am J Physiol Heart Circ Physiol. 2018;315(1):H58–70. [DOI] [PubMed] [Google Scholar]
- 23.Tate M, et al. Bone morphogenetic protein 7 gene delivery improves cardiac structure and function in a murine model of diabetic cardiomyopathy. Front Pharmacol. 2021;12:719290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin CX, et al. Small-molecule-biased formyl peptide receptor agonist compound 17b protects against myocardial ischaemia-reperfusion injury in mice. Nat Commun. 2017;8:14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velagic A, et al. A high-sucrose diet exacerbates the left ventricular phenotype in a high fat-fed streptozotocin rat model of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2023;324(2):H241–57. [DOI] [PubMed] [Google Scholar]
- 26.Wong TC, et al. myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35(10):657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuleta I, Frangogiannis NG. Fibrosis of the diabetic heart: clinical significance, molecular mechanisms, and therapeutic opportunities. Adv Drug Deliv Rev. 2021;176:113904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morkin E. Control of cardiac myosin heavy chain gene expression. Microsc Res Tech. 2000;50(6):522–31. [DOI] [PubMed] [Google Scholar]
- 29.Marino F, et al. Streptozotocin-induced type 1 and 2 diabetes mellitus mouse models show different functional, cellular and molecular patterns of diabetic cardiomyopathy. Int J Mol Sci. 2023;24(2):1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borgeson E, et al. Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metab. 2015;22(1):125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Blasio MJ, et al. Defining the progression of diabetic cardiomyopathy in a mouse model of type 1 diabetes. Front Physiol. 2020;11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. [DOI] [PubMed] [Google Scholar]
- 33.Ururahy MA, et al. Increased TLR2 Expression in patients with type 1 diabetes: evidenced risk of microalbuminuria. Pediatr Diabetes. 2012;13(2):147–54. [DOI] [PubMed] [Google Scholar]
- 34.Lo Sasso G, et al. The Apoe(-/-) mouse model: a suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction. J Transl Med. 2016;14(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petri MH, et al. Resolution of inflammation through the lipoxin and ALX/FPR2 receptor pathway protects against abdominal aortic aneurysms. JACC Basic Transl Sci. 2018;3(6):719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tate M, et al. Exendin-4 attenuates adverse cardiac remodelling in streptozocin-induced diabetes via specific actions on infiltrating macrophages. Basic Res Cardiol. 2016;111(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devaraj S, et al. Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes. 2006;55(3):774–9. [DOI] [PubMed] [Google Scholar]
- 38.Yuan J, et al. Lipoxin A4 regulates M1/M2 macrophage polarization via FPR2-IRF pathway. Inflammopharmacology. 2022;30(2):487–98. [DOI] [PubMed] [Google Scholar]
- 39.Caso VM, et al. Regulation of inflammation and oxidative stress by formyl peptide receptors in cardiovascular disease progression. Life. 2021;11(3):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oldekamp S, et al. Lack of formylpeptide receptor 1 and 2 leads to more severe inflammation and higher mortality in mice with of pneumococcal meningitis. Immunology. 2014;143(3):447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yi X, et al. The formyl peptide receptors FPR1 and FPR2 as targets for inflammatory disorders: recent advances in the development of small-molecule agonists. Eur J Med Chem. 2024;265:115989. [DOI] [PubMed] [Google Scholar]
- 42.Tourki B, et al. Lack of resolution sensor drives age-related cardiometabolic and cardiorenal defects and impedes inflammation-resolution in heart failure. Mol Metab. 2020;31:138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bozinovski S, et al. Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2012;109(3):935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kain V, et al. Inhibition of FPR2 impaired leukocytes recruitment and elicited non-resolving inflammation in acute heart failure. Pharmacol Res. 2019;146:104295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petri MH, et al. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc Res. 2015;105(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bathina S, Das UN. PUFAs, BDNF and Lipoxin A4 inhibit chemical-induced cytotoxicity of RIN5F cells in vitro and streptozotocin-induced type 2 diabetes mellitus in vivo. Lipids Health Dis. 2019;18(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu L, et al. Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 2021;100(1):107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S, et al. The protective effects of lipoxin A4 on type 2 diabetes mellitus: a Chinese prospective cohort study. Front Endocrinol (Lausanne). 2023;14:1109747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woods JW, et al. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J Exp Med. 1993;178(6):1935–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fredman G, et al. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc Natl Acad Sci U S A. 2014;111(40):14530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sorgi CA, et al. Dormant 5-lipoxygenase in inflammatory macrophages is triggered by exogenous arachidonic acid. Sci Rep. 2017;7(1):10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M, et al. Elevated ALOX12 in renal tissue predicts progression in diabetic kidney disease. Ren Fail. 2024;46(1):2313182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzuki H, et al. Arachidonate 12/15-lipoxygenase-induced inflammation and oxidative stress are involved in the development of diabetic cardiomyopathy. Diabetes. 2015;64(2):618–30. [DOI] [PubMed] [Google Scholar]
- 54.Kayama Y, et al. Cardiac 12/15 lipoxygenase-induced inflammation is involved in heart failure. J Exp Med. 2009;206(7):1565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westermann D, et al. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. 2007;56(3):641–6. [DOI] [PubMed] [Google Scholar]
- 56.Halade GV, Lee DH. Inflammation and resolution signaling in cardiac repair and heart failure. EBioMedicine. 2022;79:103992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zile MR, et al. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Heart Fail.2011;4(3):246–56. [DOI] [PMC free article] [PubMed]
- 58.Li Q, et al. Inhibition of Mir-21 alleviated cardiac perivascular fibrosis via repressing EndMT in T1DM. J Cell Mol Med. 2020;24(1):910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimizu M, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46(1):32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prakoso D, et al. Current landscape of preclinical models of diabetic cardiomyopathy. Trends Pharmacol Sci. 2022;43(11):940–56. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, et al. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019;17(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pullen AB, et al. Molecular and cellular differences in cardiac repair of male and female mice. J Am Heart Assoc. 2020;9(8):e015672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stratton IM, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kristofi R, et al. Cardiovascular and renal disease burden in type 1 compared with type 2 diabetes: a two-country nationwide observational study. Diabetes Care. 2021;44(5):1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soedamah-Muthu SS, et al. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care. 2006;29(4):798–804. [DOI] [PubMed] [Google Scholar]
- 66.Holscher ME, Bode C, Bugger H. Diabetic cardiomyopathy: does the type of diabetes matter? Int J Mol Sci. 2016;17(12):2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heather LC, et al. Guidelines on models of diabetic heart disease. Am J Physiol Heart Circ Physiol. 2022;323(1):H176–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pamplona FA, et al. Age-linked suppression of lipoxin A4 associates with cognitive deficits in mice and humans. Transl Psychiatry. 2022;12(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Godson C, Guiry P, Brennan E. Lipoxin mimetics and the resolution of inflammation. Annu Rev Pharmacol Toxicol. 2023;63:429–48. [DOI] [PubMed] [Google Scholar]
- 70.Jaen RI, et al. BML-111 treatment prevents cardiac apoptosis and oxidative stress in a mouse model of autoimmune myocarditis. FASEB J. 2020;34(8):10531–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.