Abstract
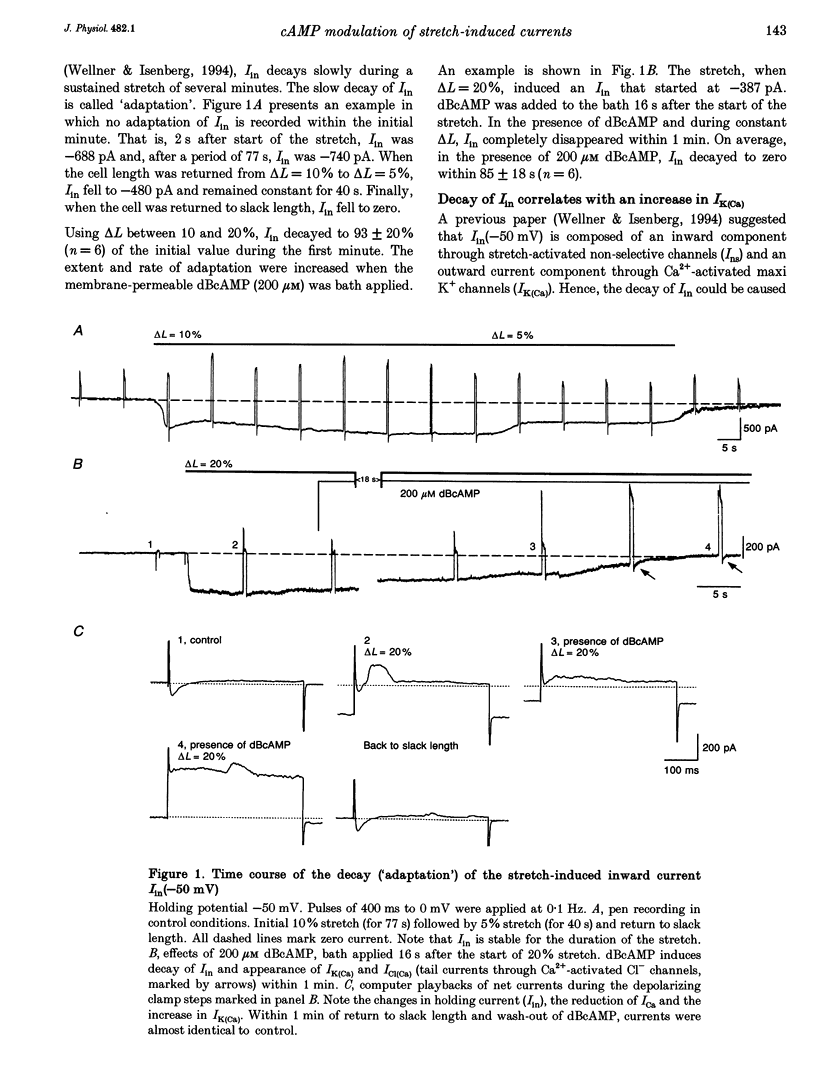
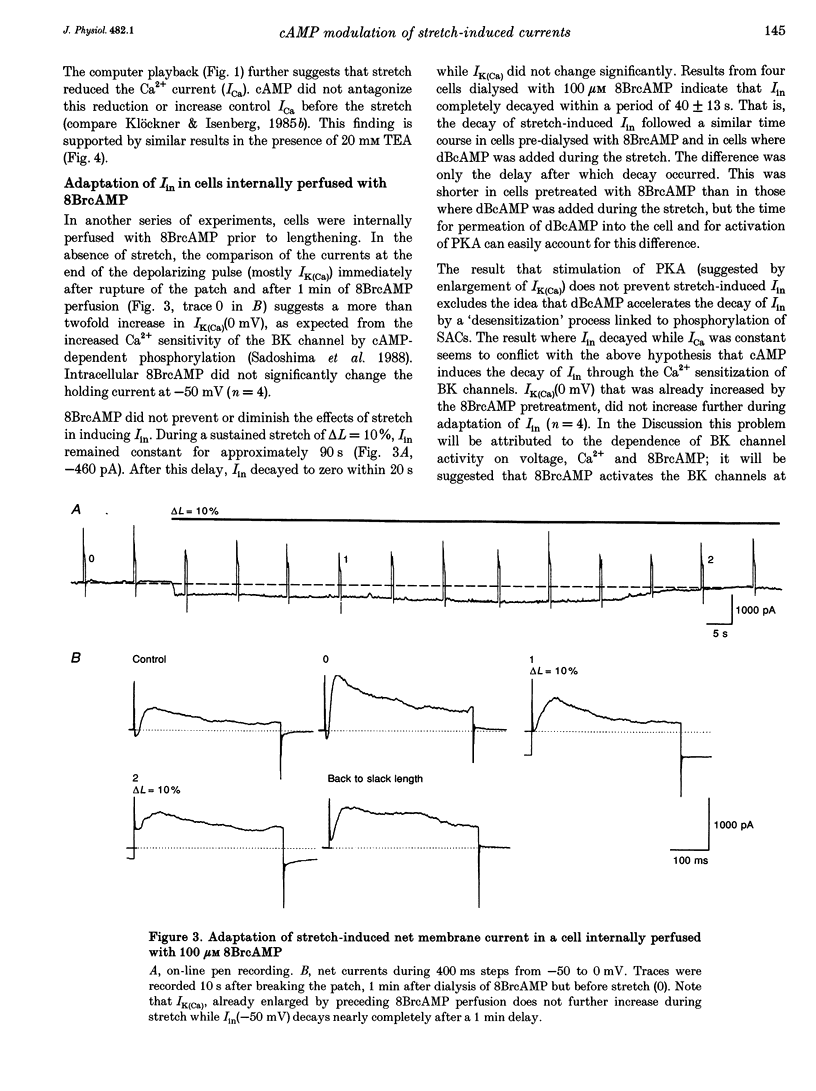
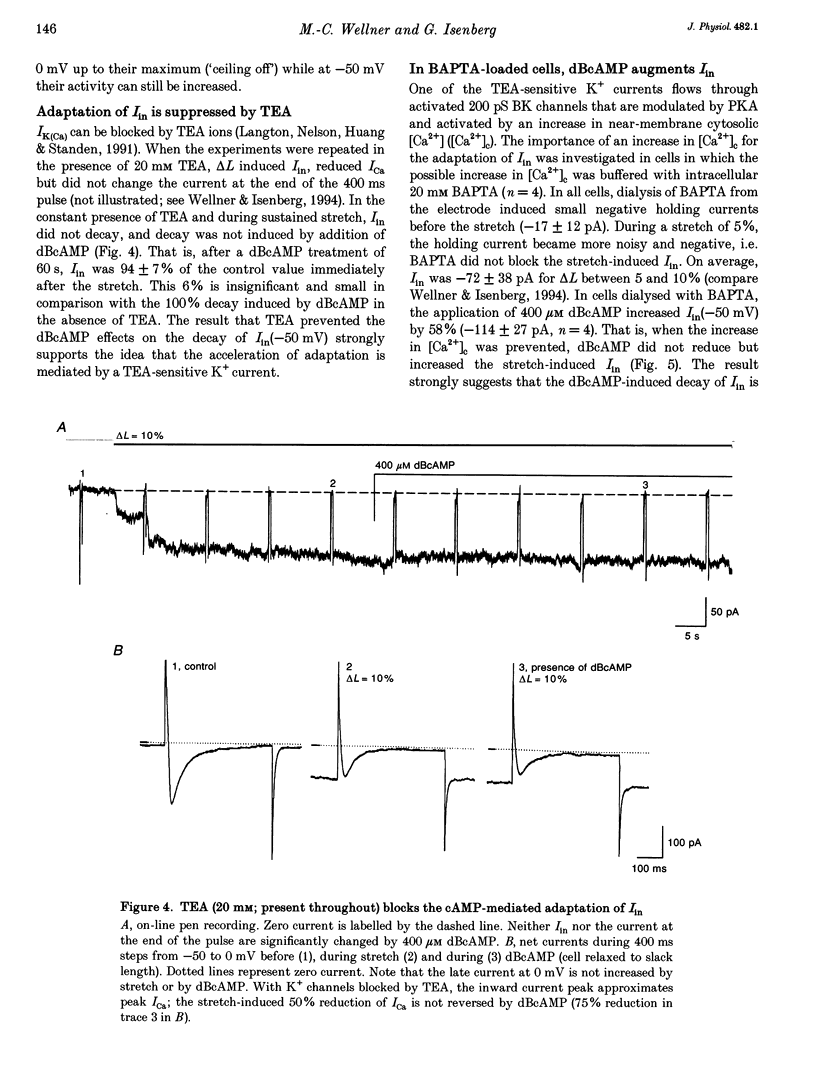
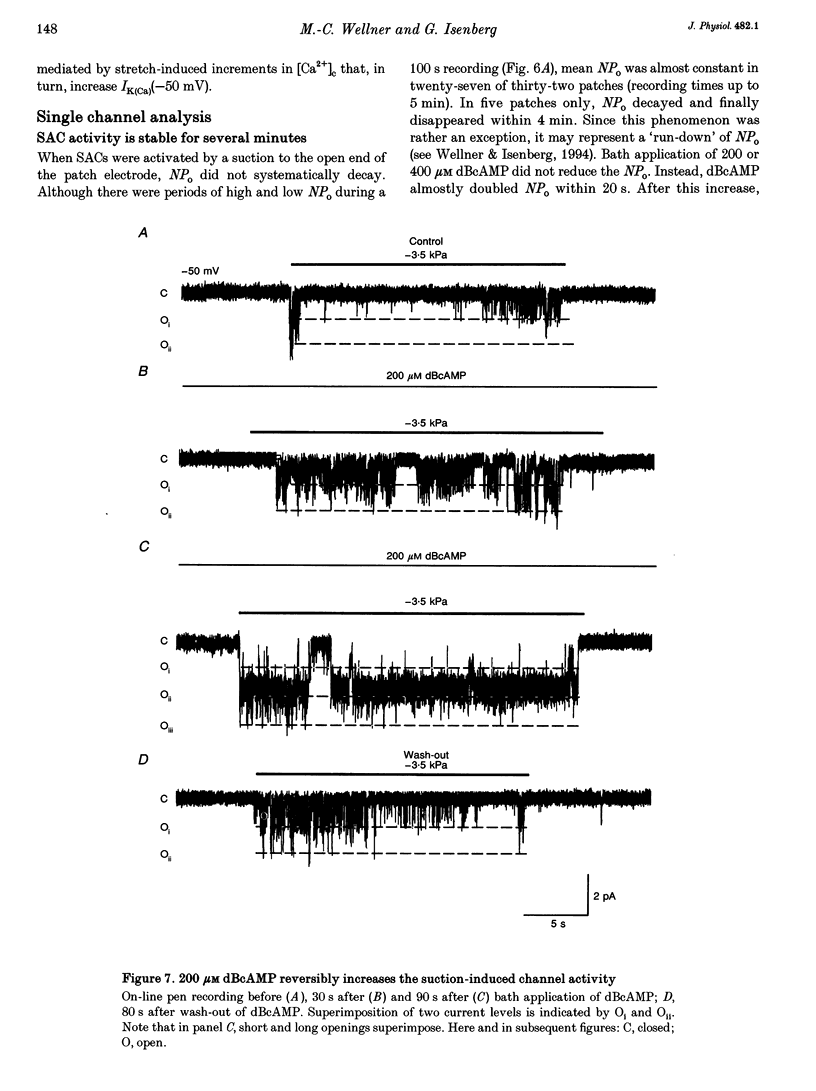
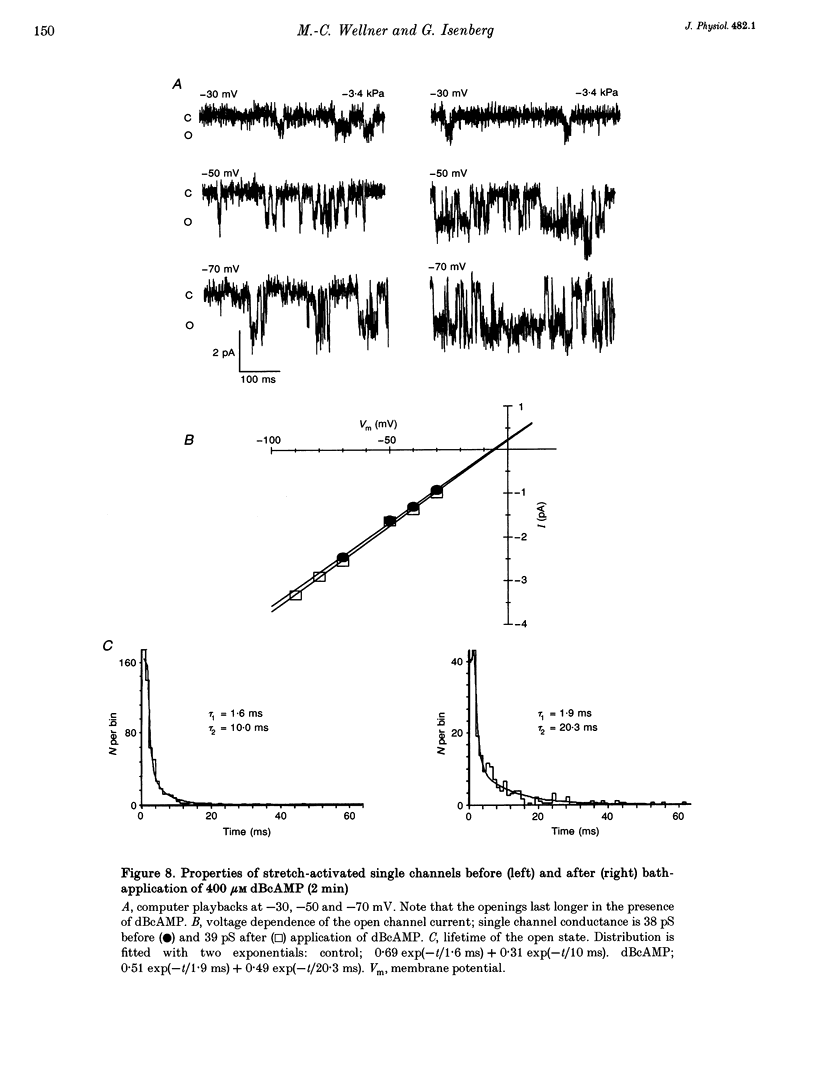
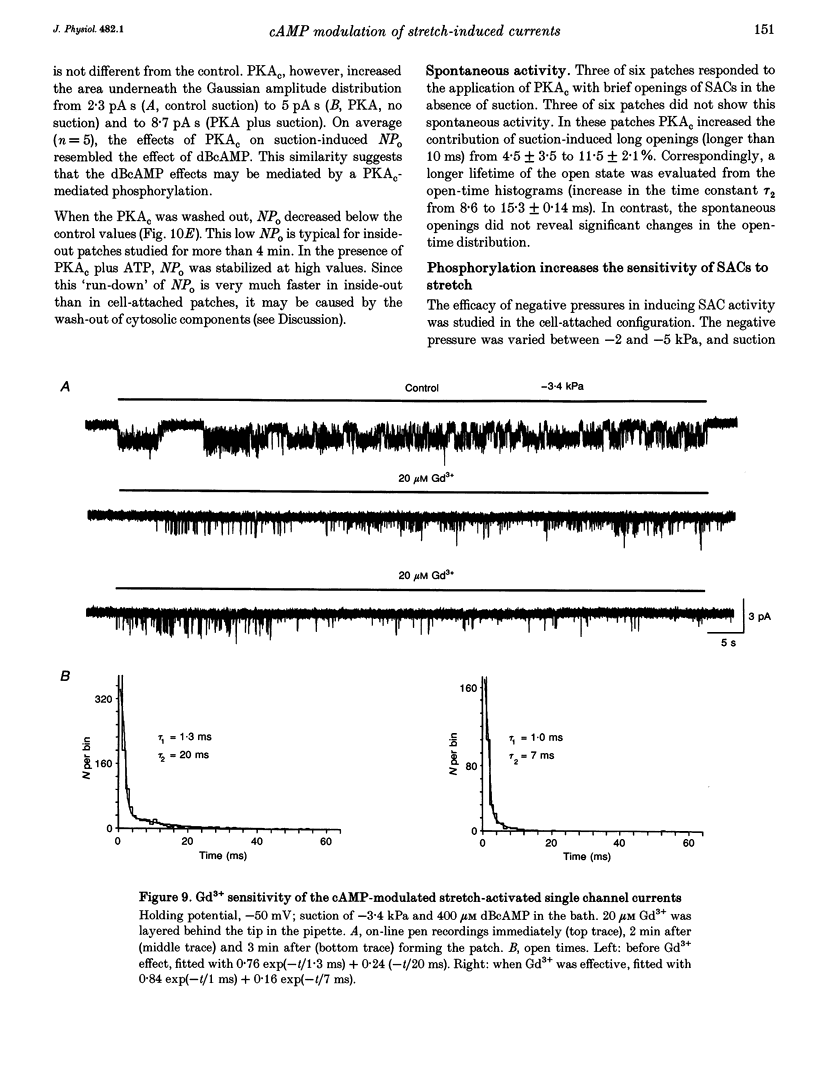
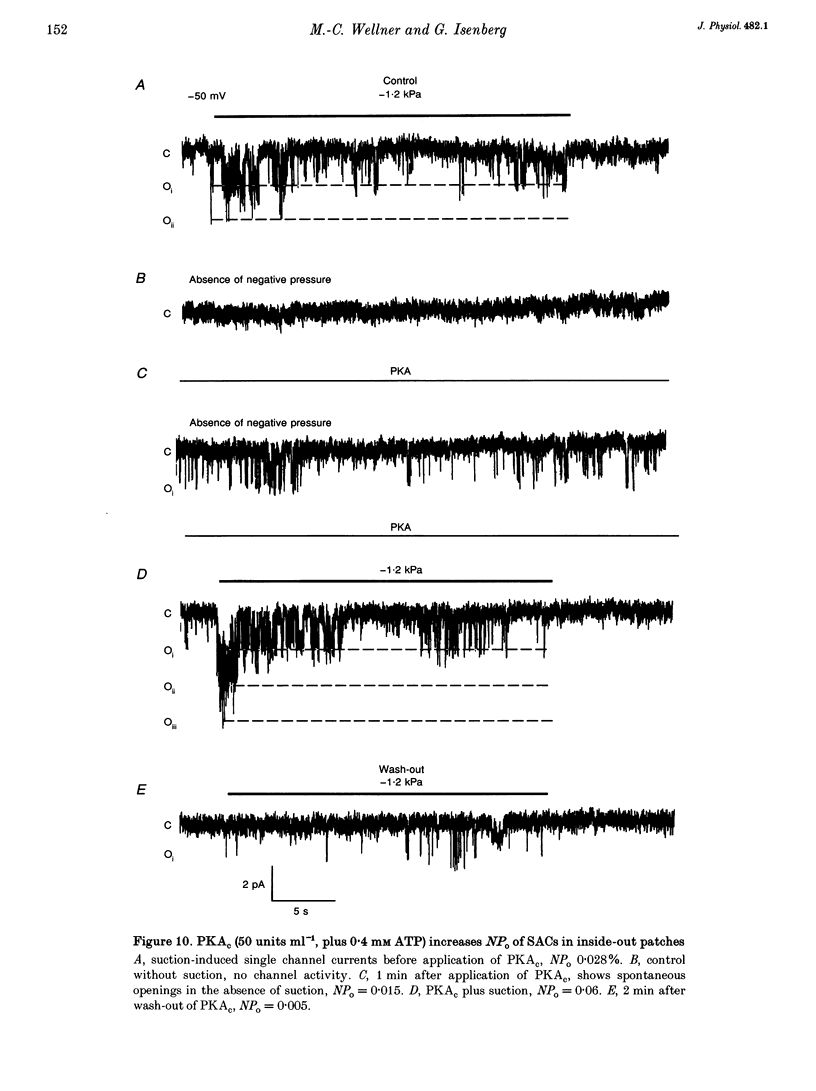
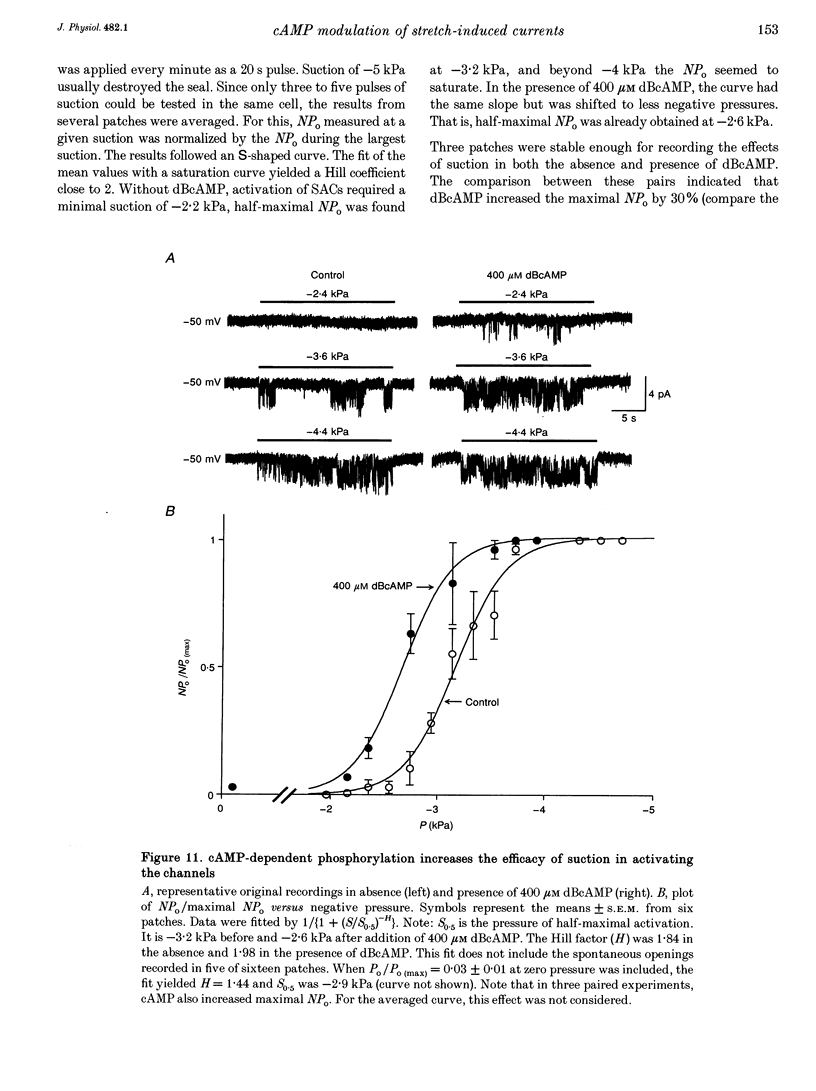
1. Myocytes from the urinary bladder were stretched longitudinally by 5-20%. At -50 mV, stretch induced whole-cell inward currents (Iin) between -100 and -600 pA. Iin decayed slowly with time to 93 +/- 20% (mean +/- S.E.M., n = 6) of the initial value in 1 min. The mechanisms of this 'adaptation' and its modulation by dibutyryl cAMP (dBcAMP) were analysed with whole-cell and single channel currents. 2. When the cells were internally perfused with 100 microM 8-bromo-cAMP (8BrcAMP), stretch induced an Iin of the usual amplitude that decayed completely within 40 +/- 13 s. When 200 microM dBcAMP was bath applied 10 s after the start of the stretch, Iin decayed to zero within 85 +/- 18 s. 3. dBcAMP increased the K+ current through Ca(2+)-activated BK channels (IK(Ca)) at 0 mV with a time course that correlated well with the decay of Iin, and block of IK(Ca) by TEA suppressed the dBcAMP-induced decay of Iin. In the presence of intracellular BAPTA, dBcAMP increased the stretch-induced Iin. The results suggest that adaptation is caused by superimposition of IK(Ca) which is increased through elevation of near-membrane [Ca2+] and by cAMP-dependent phosphorylation. 4. Single channel analysis was carried out with 140 mM KCl electrode solution and at -50 mV. Stretch-activated channels (SACs) were recorded during pulses of negative pressures between -2 and -5 kPa. Activity (NPo) of SACs was constant for at least 4 min, e.g. evidence for adaptation was missing. dBcAMP (200 microM) increased NPo of SACs by 142 +/- 35% (n = 16). 5. dBcAMP increased NPo via frequency of openings and channel open time. In five of sixteen patches, dBcAMP induced openings without suction. Similar effects were induced by the catalytic subunit of cAMP-dependent protein kinase (PKAc), applied to inside-out patches. 6. NPo, normalized by its maximum, increased with more negative pressure along an S-shaped curve. dBcAMP increased the sensitivity of SACs to stretch by shifting the point of half-maximal activity from -3.2 to -2.6 kPa. 7. The augmentation of NPo by dBcAMP is attributed to the phosphorylation of SACs promoting their opening. Adaptation of Iin is discussed as a 'secondary' effect of stretch-activated channels: Ca2+ influx through SACs increases the Ca2+ concentration that activates BK channels whose Ca2+ sensitivity is increased by cAMP.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dopico A. M., Kirber M. T., Singer J. J., Walsh J. V., Jr Membrane stretch directly activates large conductance Ca(2+)-activated K+ channels in mesenteric artery smooth muscle cells. Am J Hypertens. 1994 Jan;7(1):82–89. doi: 10.1093/ajh/7.1.82. [DOI] [PubMed] [Google Scholar]
- Erxleben C. F. Calcium influx through stretch-activated cation channels mediates adaptation by potassium current activation. Neuroreport. 1993 Jun;4(6):616–618. doi: 10.1097/00001756-199306000-00003. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Hogg R. C., Wang Q., Helliwell R. M., Large W. A. Properties of spontaneous inward currents in rabbit pulmonary artery smooth muscle cells. Pflugers Arch. 1993 Nov;425(3-4):233–240. doi: 10.1007/BF00374172. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kirber M. T., Ordway R. W., Clapp L. H., Walsh J. V., Jr, Singer J. J. Both membrane stretch and fatty acids directly activate large conductance Ca(2+)-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 1992 Feb 3;297(1-2):24–28. doi: 10.1016/0014-5793(92)80319-c. [DOI] [PubMed] [Google Scholar]
- Klöckner U. Intracellular calcium ions activate a low-conductance chloride channel in smooth-muscle cells isolated from human mesenteric artery. Pflugers Arch. 1993 Aug;424(3-4):231–237. doi: 10.1007/BF00384347. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium currents of cesium loaded isolated smooth muscle cells (urinary bladder of the guinea pig). Pflugers Arch. 1985 Dec;405(4):340–348. doi: 10.1007/BF00595686. [DOI] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Nelson M. T., Huang Y., Standen N. B. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol. 1991 Mar;260(3 Pt 2):H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- Low A. M., Darby P. J., Kwan C. Y., Daniel E. E. Effects of thapsigargin and ryanodine on vascular contractility: cross-talk between sarcoplasmic reticulum and plasmalemma. Eur J Pharmacol. 1993 Jan 5;230(1):53–62. doi: 10.1016/0014-2999(93)90409-b. [DOI] [PubMed] [Google Scholar]
- Markwardt F., Isenberg G. Gating of maxi K+ channels studied by Ca2+ concentration jumps in excised inside-out multi-channel patches (myocytes from guinea pig urinary bladder). J Gen Physiol. 1992 Jun;99(6):841–862. doi: 10.1085/jgp.99.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina I., Bregestovski P. Sensitivity of stretch-activated K+ channels changes during cell-cleavage cycle and may be regulated by cAMP-dependent protein kinase. Proc Biol Sci. 1991 Sep 23;245(1314):159–164. doi: 10.1098/rspb.1991.0103. [DOI] [PubMed] [Google Scholar]
- Minami K., Fukuzawa K., Nakaya Y., Zeng X. R., Inoue I. Mechanism of activation of the Ca(2+)-activated K+ channel by cyclic AMP in cultured porcine coronary artery smooth muscle cells. Life Sci. 1993;53(14):1129–1135. doi: 10.1016/0024-3205(93)90549-i. [DOI] [PubMed] [Google Scholar]
- Ono K., Fozzard H. A. Phosphorylation restores activity of L-type calcium channels after rundown in inside-out patches from rabbit cardiac cells. J Physiol. 1992 Aug;454:673–688. doi: 10.1113/jphysiol.1992.sp019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoson D., Swerup C. Effects of intracellular TEA injection on early adaptation of crustacean stretch receptor. Brain Res. 1985 Jun 10;336(1):9–17. doi: 10.1016/0006-8993(85)90410-x. [DOI] [PubMed] [Google Scholar]
- Sachs F. Baroreceptor mechanisms at the cellular level. Fed Proc. 1987 Jan;46(1):12–16. [PubMed] [Google Scholar]
- Sadoshima J., Akaike N., Kanaide H., Nakamura M. Cyclic AMP modulates Ca-activated K channel in cultured smooth muscle cells of rat aortas. Am J Physiol. 1988 Oct;255(4 Pt 2):H754–H759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Both G., Lechene C. Effect of cell spreading on cytoplasmic pH in normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4525–4529. doi: 10.1073/pnas.86.12.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerup C., Rydqvist B., Ottoson D. Time characteristics and potential dependence of early and late adaptation in the crustacean stretch receptor. Acta Physiol Scand. 1983 Sep;119(1):91–99. doi: 10.1111/j.1748-1716.1983.tb07310.x. [DOI] [PubMed] [Google Scholar]
- Trischmann U., Klöckner U., Isenberg G., Utz J., Ullrich V. Carbon monoxide inhibits depolarization-induced Ca rise and increases cyclic GMP in visceral smooth muscle cells. Biochem Pharmacol. 1991 Jan 15;41(2):237–241. doi: 10.1016/0006-2952(91)90482-k. [DOI] [PubMed] [Google Scholar]
- Watson P. A. Direct stimulation of adenylate cyclase by mechanical forces in S49 mouse lymphoma cells during hyposmotic swelling. J Biol Chem. 1990 Apr 25;265(12):6569–6575. [PubMed] [Google Scholar]
- Watson P. A. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 1991 Apr;5(7):2013–2019. doi: 10.1096/fasebj.5.7.1707019. [DOI] [PubMed] [Google Scholar]
- Wellner M. C., Isenberg G. Properties of stretch-activated channels in myocytes from the guinea-pig urinary bladder. J Physiol. 1993 Jul;466:213–227. [PMC free article] [PubMed] [Google Scholar]
- Wellner M. C., Isenberg G. Stretch effects on whole-cell currents of guinea-pig urinary bladder myocytes. J Physiol. 1994 Nov 1;480(Pt 3):439–448. doi: 10.1113/jphysiol.1994.sp020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- von Harsdorf R., Lang R., Fullerton M., Smith A. I., Woodcock E. A. Right atrial dilatation increases inositol-(1,4,5)trisphosphate accumulation. Implications for the control of atrial natriuretic peptide release. FEBS Lett. 1988 Jun 6;233(1):201–205. doi: 10.1016/0014-5793(88)81384-x. [DOI] [PubMed] [Google Scholar]


