Abstract
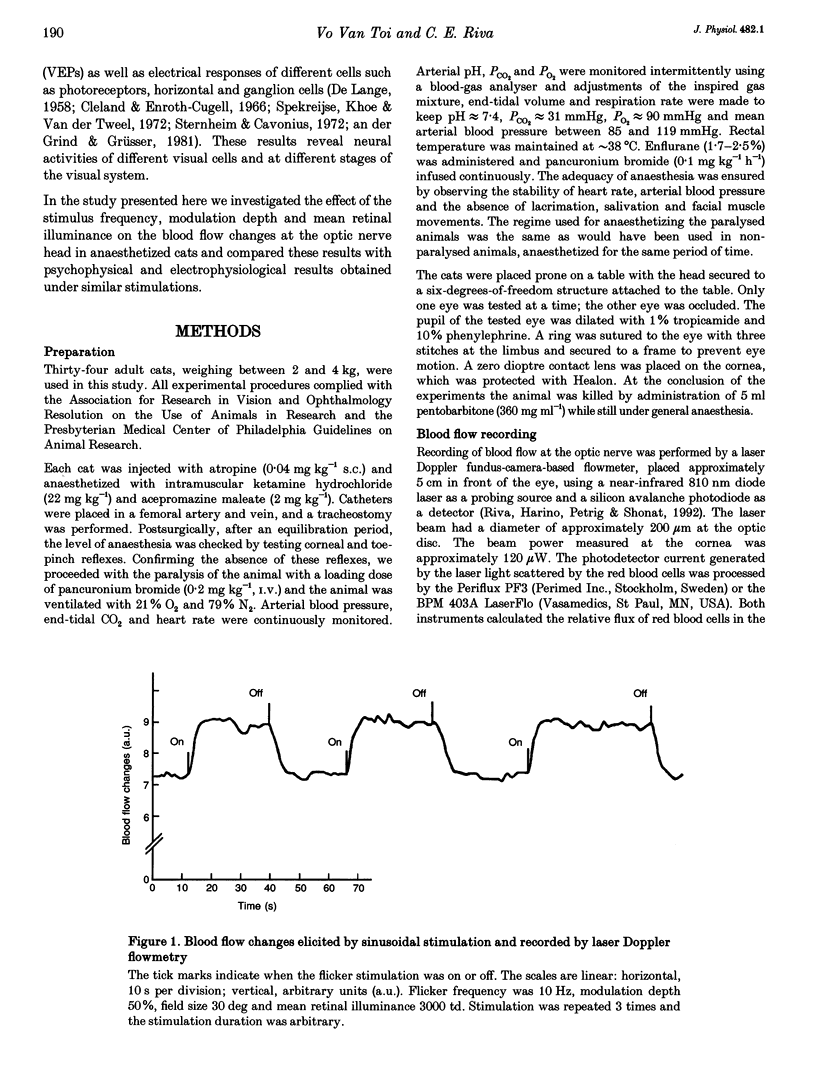
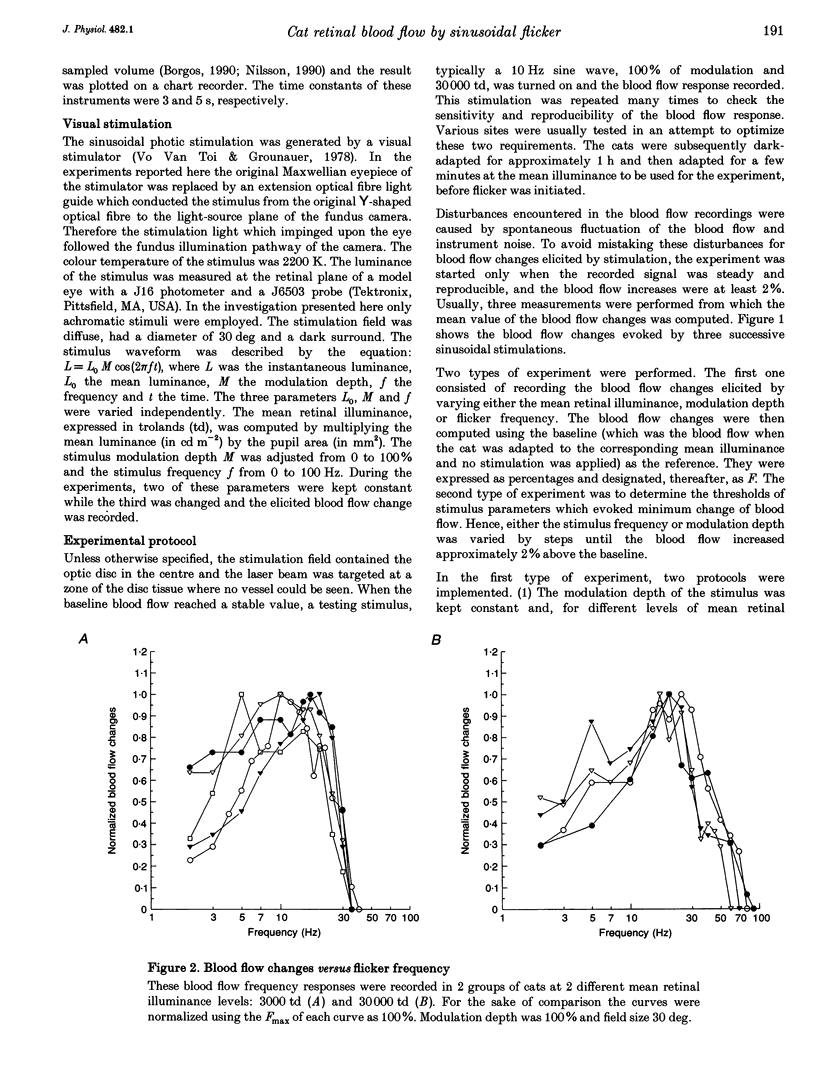
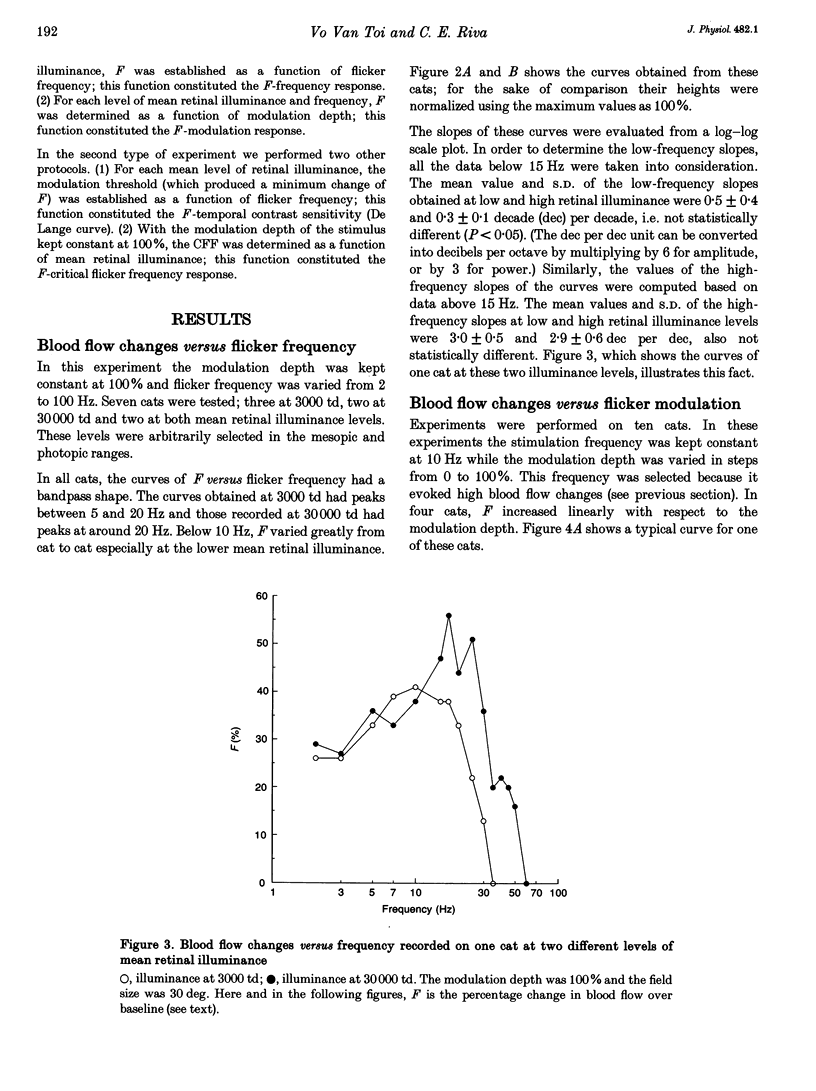
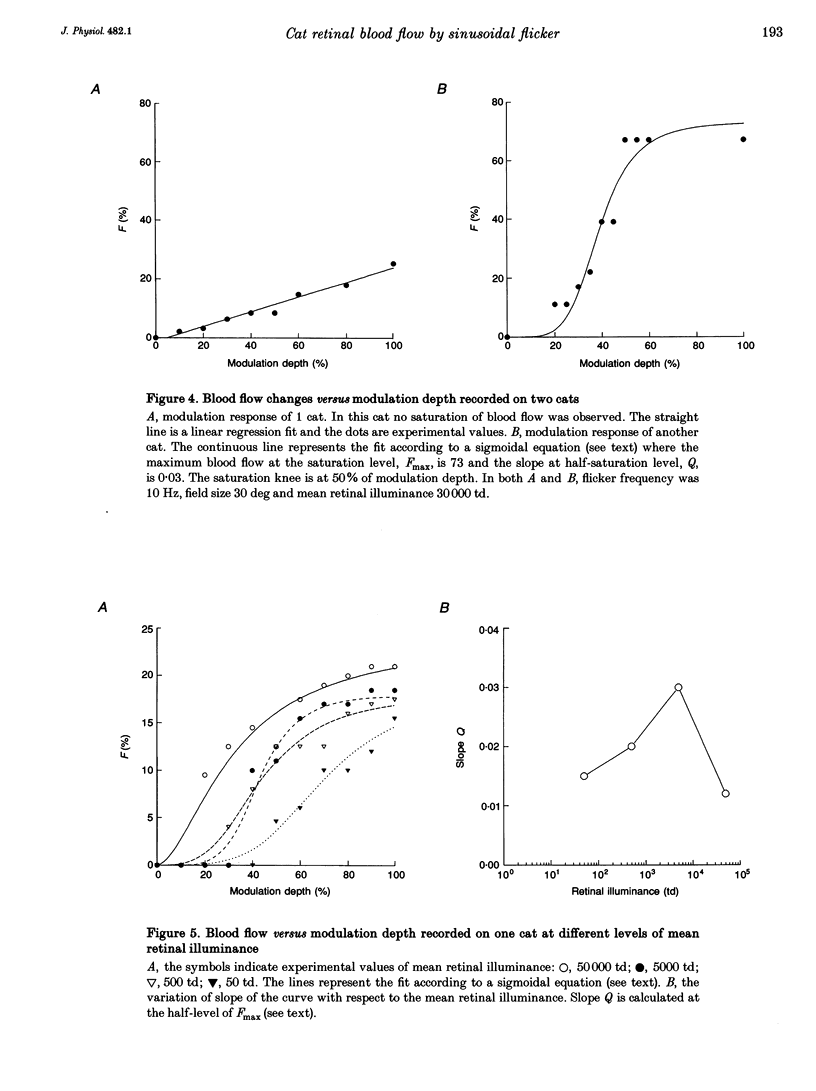
1. The present investigation explored, in thirty-four anaesthetized cats, the blood flow changes at the optic nerve head elicited by sinusoidally modulated photic stimuli. 2. The stimuli were achromatic, diffuse and had 30 deg diameter field size; the stimulus frequency was varied from 0 to 100 Hz, modulation depth from 0 to 100% and mean retinal illuminance up to 50,000 trolands (td); the blood flow was measured with a near-infrared (810 nm) laser Doppler flowmeter. 3. At various frequencies, modulation depths and mean retinal illuminance, sinusoidal flicker stimulation always caused an increase in blood flow at the optic nerve head relative to steady stimulation. 4. The frequency response and temporal contrast sensitivity function of the blood flow changes had a bandpass shape; the high-frequency slope of the frequency response was 3 decades (dec) per decade and that of the temporal contrast sensitivity function was 1.7 dec per dec, close to the slope for cat 'on' ganglion cells (2.6 dec per dec). 5. In most cats, the magnitude of the increase in blood flow was a sigmoidal function of modulation depth; in the remainder, the relationship was close to linear. 6. The threshold of blood flow changes varied with respect to mean retinal illuminance similar to Ferry-Porter's law and the photopic linear slope was 50 Hz dec-1. 7. In comparison with reported psychophysical and electrophysiological responses elicited by similar stimulations, the results of the present study resemble more those obtained from ganglion cells than those from electroretinograms, visual-evoked potentials and psychophysics. It is suggested that the blood flow changes at the optic nerve head are induced by the activity of ganglion cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. L., Jr, Hess R. F. Linear and nonlinear components of human electroretinogram. J Neurophysiol. 1984 May;51(5):952–967. doi: 10.1152/jn.1984.51.5.952. [DOI] [PubMed] [Google Scholar]
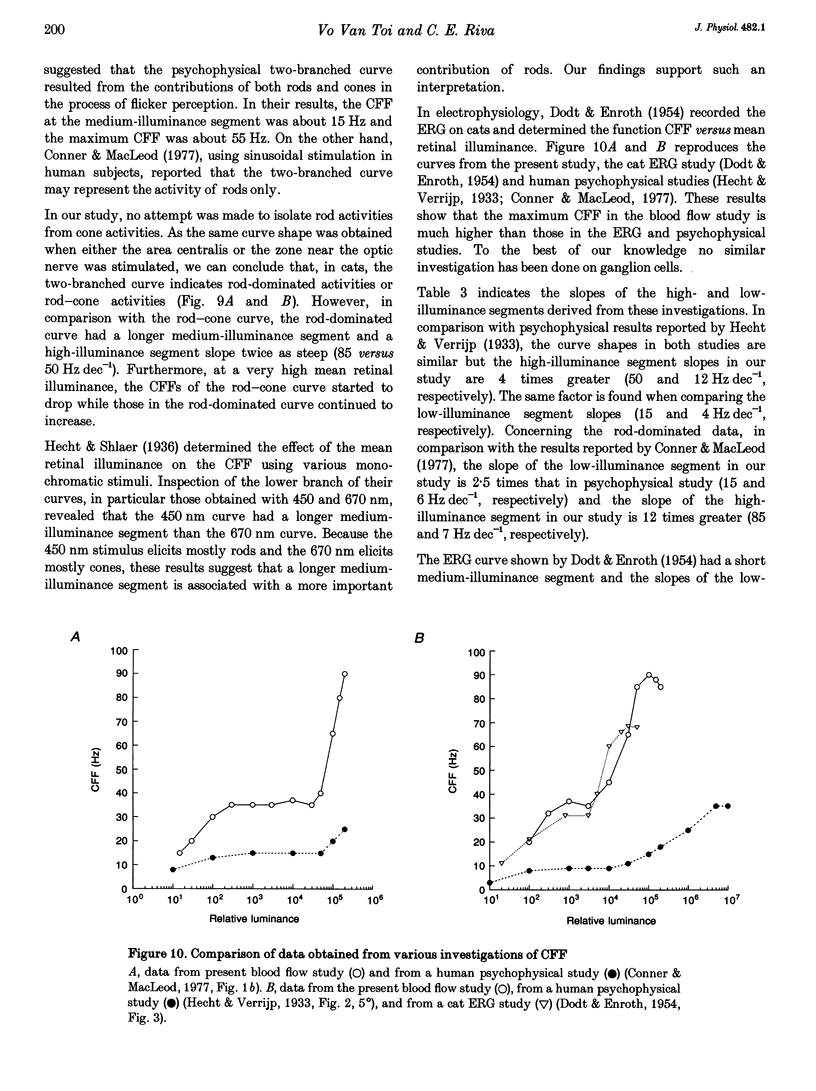
- Conner J. D., MacLeod D. I. Rod photoreceptors detect rapid flicker. Science. 1977 Feb 18;195(4279):698–699. doi: 10.1126/science.841308. [DOI] [PubMed] [Google Scholar]
- DE LANGE DZN H. Research into the dynamic nature of the human fovea-cortex systems with intermittent and modulated light. I. Attenuation characteristics with white and colored light. J Opt Soc Am. 1958 Nov;48(11):777–784. doi: 10.1364/josa.48.000777. [DOI] [PubMed] [Google Scholar]
- DODT E., ENROTH C. Retinal flicker response in cat. Acta Physiol Scand. 1954 May 15;30(4):375–390. doi: 10.1111/j.1748-1716.1954.tb01076.x. [DOI] [PubMed] [Google Scholar]
- Frishman L. J., Freeman A. W., Troy J. B., Schweitzer-Tong D. E., Enroth-Cugell C. Spatiotemporal frequency responses of cat retinal ganglion cells. J Gen Physiol. 1987 Apr;89(4):599–628. doi: 10.1085/jgp.89.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Saito H. The relationship between response characteristics to flicker stimulation and receptive field organization in the cat's optic nerve fibers. Vision Res. 1971 Mar;11(3):227–240. doi: 10.1016/0042-6989(71)90187-8. [DOI] [PubMed] [Google Scholar]
- Lou H. C., Edvinsson L., MacKenzie E. T. The concept of coupling blood flow to brain function: revision required? Ann Neurol. 1987 Sep;22(3):289–297. doi: 10.1002/ana.410220302. [DOI] [PubMed] [Google Scholar]
- Regan D. A high frequency mechanism which underlies visual evoked potentials. Electroencephalogr Clin Neurophysiol. 1968 Sep;25(3):231–237. doi: 10.1016/0013-4694(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Regan D., Beverley K. I. Relation between the magnitude of flicker sensation and evoked potential amplitude in man. Perception. 1973;2(1):61–65. doi: 10.1068/p020061. [DOI] [PubMed] [Google Scholar]
- Riva C. E., Harino S., Petrig B. L., Shonat R. D. Laser Doppler flowmetry in the optic nerve. Exp Eye Res. 1992 Sep;55(3):499–506. doi: 10.1016/0014-4835(92)90123-a. [DOI] [PubMed] [Google Scholar]
- Riva C. E., Harino S., Shonat R. D., Petrig B. L. Flicker evoked increase in optic nerve head blood flow in anesthetized cats. Neurosci Lett. 1991 Jul 22;128(2):291–296. doi: 10.1016/0304-3940(91)90282-x. [DOI] [PubMed] [Google Scholar]
- Roy C. S., Sherrington C. S. On the Regulation of the Blood-supply of the Brain. J Physiol. 1890 Jan;11(1-2):85–158.17. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spekreijse H., Khoe L. H., van der Tweel L. H. A case of amblyopia; electrophysiology and psychophysics of luminance and contrast. Adv Exp Med Biol. 1972;24(0):141–156. doi: 10.1007/978-1-4684-8231-7_15. [DOI] [PubMed] [Google Scholar]
- Steinberg R. H., Reid M., Lacy P. L. The distribution of rods and cones in the retina of the cat (Felis domesticus). J Comp Neurol. 1973 Mar 15;148(2):229–248. doi: 10.1002/cne.901480209. [DOI] [PubMed] [Google Scholar]
- Sternheim C. E., Cavonius C. R. Sensitivity of the human ERG and VECP to sinusoidally modulated light. Vision Res. 1972 Oct;12(10):1685–1695. doi: 10.1016/0042-6989(72)90039-9. [DOI] [PubMed] [Google Scholar]
- van de Grind W., Grüsser O. J. Frequency transfer properties of cat retina horizontal cells. Vision Res. 1981;21(11):1565–1572. doi: 10.1016/0042-6989(81)90033-x. [DOI] [PubMed] [Google Scholar]


