Abstract
Objective
This study investigated whether short-term incremental prednisone therapy decreases the risk of relapse without increasing adverse events (AEs) in patients with serologically active, clinically quiescent lupus nephritis (LN).
Methods
After standardized treatment, 153 patients with serologically active, clinically quiescent LN were included. Clinical data were retrospectively reviewed. The patients were divided into two groups: the control group (n = 58) received prednisone or prednisone and immunosuppressant maintenance therapy, the prednisone increment group (n = 95) received additional prednisone doses of up to 10 mg/day as maintenance therapy, which were then gradually reduced back to the original dose at 3 months. Lupus activity, renal involvement, and AEs during follow-up in the two groups were analyzed within 18 months.
Results
No significant differences in sex, age, disease course, maintenance treatment composition, or laboratory tests between the two groups were observed, except for serum complement C3 levels, which were significantly lower in patients in the prednisone increment group than in controls (P = 0.025). The prednisone increment group had significantly lower recurrence rates than the control group (P = 0.002), with only 3 patients (5.2%) in the prednisone increment group and 24 patients (25.3%) in the control group experiencing relapse. Renal recurrence was significantly lower in the prednisone increase group (P = 0.013). Nine AEs occurred in the prednisone-modulated group and 11 AEs occurred in controls, with infection being the main cause for both groups.
Conclusion
Short-term incremental prednisone therapy is safe in reducing recurrence rates in serologically active and clinically quiescent patients with LN.
Key points
Incremental prednisone is safe and effective for patients with serologically active clinically quiescent LN.
Keywords: Systemic lupus erythematosus, Lupus nephritis, Serologically active and clinically quiescent, Incremental prednisone therapy, Recurrence rate
Introduction
Lupus nephritis (LN) is a serious and common complication of systemic lupus erythematosus (SLE), affecting more than 50–60% of patients [1]. Circulating immune complex deposits in the glomeruli activate complement systems to damage resident cells in the glomeruli, eventually leading to glomerulonephritis and proteinuria. The disease follows a natural course of alternating disease activity and remission, and approximately 70% of patients experience a relapse-remission course [2]. Despite the effectiveness of standard induction therapy and new biologics, the high heterogeneity and long course of the disease result in a relapse rate of 30–60%. Recurrent renal seizures are an independent risk factor for renal deterioration in patients with LN, highlighting the importance of early identification of risk factors and appropriate interventions to reduce relapse [3].
Currently, the identification of renal involvement in patients with LN is based mainly on the monitoring of changes in serum creatinine, urinary protein, and urinary sediment. In addition, some studies have shown that changes in serum anti-dsDNA antibodies and complement are closely related to the pathogenesis of LN. Anti-dsDNA antibody is considered not only a specific marker of SLE, but also a marker of disease activity, especially kidney injury [4–8]. In addition, complement components C3 and C4 have been identified as sensitive markers of SLE activity [9]. Some early prospective studies have also shown that SLE recurrence is predictable and associated with serological abnormalities and that renal and extrarenal activities of SLE are significantly correlated with high levels of anti-dsDNA antibody and low complement levels [10]. A >50% increase in anti-dsDNA antibody titer and a 10% decrease in complement C3 may indicate the initiation of immune dysregulation in vivo, suggesting an increased risk of disease activity [11, 12].
Patients with LN and serological activity in the absence of clinical symptoms (SACQ) are not rare and have a higher probability of attacks during the follow-up period [13, 14]. Early identification of risk factors for recurrence and appropriate interventions in patients with SACQ are critical [15, 16]. Although early serological remission is associated with a low risk of renal recurrence, opinions still differ on whether to intervene in clinically quiescent patients with SLE with serological activity and whether preemptive immunosuppression is necessary in all patients with asymptomatic serum reactivation. Many patients who exhibit serological activity may remain clinically quiescent for several years [17, 18]. In addition, pre-emptive immunosuppression may increase the risk of AEs, such as infection and metabolic complications [19]. The treat-to-target (T2T) strategy recommends monitoring clinically asymptomatic patients with quiescent or persistent serological activity rather than upgrading treatment. In contrast, some studies have shown that early and appropriate interventions can reduce the proportion of patients who relapse [15, 20–22]. Therefore, in the present study, we investigated whether short-term incremental prednisone therapy could decrease the risk of relapse without increasing the incidence of adverse drug reactions in patients with serologically active and clinically quiescent LN.
Materials and methods
Patients and clinical data
This retrospective case–control study included patients diagnosed with LN attending the Affiliated Hospital of the Guangdong Medical University between January 2014 and September 2022. Patients who were clinically quiescent and only serologically active after maintenance treatment were enrolled in the study. Data sources included the medical record homepage system, HIS system, Chronic kidney disease management system, and outpatient telephone follow-ups. The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University, and the ethics approval number was PJ2013115. Informed consent was obtained from all patients.
Inclusion criteria were as follows: (1) patients with LN diagnosed with confirmed SLE with renal impairment who achieved complete remission after induction therapy; (2) age ≥18 years, ≤80 years, regular outpatient follow-up, and complete clinical data; (3) clinical stability; and (4) serological activity.
Exclusion criteria were as follows: (1) co-infection, such as with hepatitis B virus, hepatitis C virus, and HIV infection; (2) serious complications requiring surgical treatment; (3) pregnancy; or (4) history of mental illness, poor compliance, and inability to cooperate with treatment.
Definitions
Serologically active and clinically quiescent was defined as at least 6 months of serological activity (serum anti-dsDNA antibody titers increased by ≥50% from the previous time with a value of ≥60 IU/mL and/or complement C3 or C4 decreased by ≥10%). Each patient in both groups was observed to be serologically active for a duration of at least 6 months at the time of enrollment. In contrast to the prednisone increment group, the control group did not receive any intervention for 18 months following enrollment. The intervention involved an initial increase in the prednisone dose to 30 mg/day for a period of 2 weeks, as part of a hormonal or prednisone-plus immunosuppressant maintenance regimen. This was followed by a gradual 10% reduction to reach the lowest maintenance dose (≤7.5 mg/day) over the course of 1–2 weeks, and this reduction process continued for the subsequent 3 months. Intervention therapy was initiated after the patient’s serological activity persisted for 6 months. Relapse was defined as: (1) extrarenal lupus activity and the presence of new or worsening clinical symptoms (fever, hair loss, specific rash, mouth ulcers, and organ damage other than the kidney) indicating increased disease activity in one or more organ systems other than the kidney, which was considered clinically significant and required change or increased treatment; (2) renal recurrence: urinary sediment indicating aggravation; urinary protein level increased from a urinary protein/creatinine ratio of <500 mg/g (complete response) to 1000 mg/g and increased from >500 mg/g (partial remission) to ≥2000 mg/g. Complete response was defined as normal urinary protein (urinary protein < 0.5 g/24 h or UPCR < 500 mg/g), inactive urinary sediment, serum albumin ≥ 35 g/L, and normal or elevated serum creatinine, with no more than a 10% increase from the baseline value. Partial response was defined as a more than 50% reduction in urinary protein compared with the baseline, urinary protein < 3.0 g/24 h, serum albumin > 30 g/L, and an increase in blood creatinine of less than 10% compared with the baseline.
Statistical analyses
Data with a normal distribution are presented as means and standard deviations. The t-test was used for comparisons between the two groups. Non-normally distributed data are presented as means and 25 or 75 quartiles, and comparisons between the two groups were performed using the Mann–Whitney U test. Count data are presented as the number of cases and percentages. The chi-square test or Fisher’s exact probability test was used for comparisons between the two groups. Statistical significance was established at P < 0.05. SPSS (version 20.0) was used for statistical analysis of the data.
Results
Demographic data and symptoms at the time of initial diagnosis
This study included 153 patients with clinically quiescent and serologically active LN: 58 patients (37.9%) in the prednisone increment group and 95 patients (62.1%) in the control group. In the prednisone increment group of 7 men and 51 women, the age of onset of LN was 32.91 ± 10.11 years. In the control group, the age of onset of LN was 32.60 ± 11.56 years, and included 9 men and 86 women. There were no significant differences in sex, age, disease course, or maintenance treatment composition between the two groups (P > 0.05; Table 1). In addition, during this trial, every patient in both groups was administered hydroxychloroquine and did not receive any biologic therapies.
Table 1.
Demographic data of the patients and symptoms at initial diagnosis
| Clinical parameters | Control group (n = 95) | Prednisone increment group (n = 58) | P values |
|---|---|---|---|
| Age (age) | 32.60 ± 11.56 | 32.91 ± 10.11 | 0.510 |
| Sex (%) | 0.611 | ||
| Male | 9 (9.5) | 7 (12.1) | |
| Female | 86 (90.5) | 51 (87.9) | |
| Course of disease (%) | 0.666 | ||
| 1–5 years | 46 (48.4) | 26 (44.8) | |
| >5 years | 49 (51.6) | 32 (55.2) | |
| WBC (109/L) | 6.10 (4.76, 8.27) | 6.76 (5.19, 8.58) | 0.303 |
| PLT (109/L) | 249.55 (219.78, 291.00) | 239.20 (204.40, 290.65) | 0.387 |
| Hb (g/L) | 123.35 (114.45, 131.50) | 130.20 (117.05, 137.40) | 0.117 |
| BUN (mmol/L) | 4.40 (3.50, 5.28) | 4.30 (3.66, 5.00) | 0.889 |
| ALB (g/L) | 44.59 ± 4.18 | 44.73 ± 3.18 | 0.827 |
| Scr (μmol/L) | 57.50 (50.00, 73.00) | 62.00 (54.50, 69.50) | 0.237 |
| SUA (μmol/L) | 318.00 (258.48, 366.50) | 290.00 (248.00, 356.65) | 0.270 |
| C3 (g/L) | 0.63 ± 0.13 | 0.59 ± 0.12 | 0.025 |
| C4 (g/L) | 0.13 ± 0.05 | 0.12 ± 0.05 | 0.148 |
| Anti-ds-DNA (IU/mL) | 90.0 ± 71.9 | 96.3 ± 83.6 | 0.605 |
| Renal biopsy (n, %) | 66 (69.5) | 40 (69.0) | 0.947 |
| Class of LN | 0.354 | ||
| Class I, II, and V (n, %) | 12 (18.2) | 7 (17.5) | |
| Class III (n, %) | 2 (3.0) | 4 (10) | |
| Class IV (n, %) | 30 (45.5) | 20 (50) | |
| Class III/IV+V (n, %) | 22 (33.3) | 9 (22.5) | |
| Maintenance treatment (%) | 0.281 | ||
| Prednisone | 37 (38.9) | 15 (25.9) | |
| Prednisone + MMF | 38 (40) | 24 (41.4) | |
| Prednisone + AZA | 7 (7.4) | 6 (10.3) | |
| Prednisone + other | 13 (13.7) | 13 (22.4) | |
| Hydroxychloroquine | 95 (100) | 58(100) |
WBC white blood cell, PLT platelet count, Hb hemoglobin, BUN blood urea nitrogen, ALB albumin, Scr serum creatinine, SUA serum uric acid, C3 complement C3, C4 complement C4, LN lupus nephritis, MMF mycophenolate mofetil, AZA azathioprine
Each patient received treatment with hydroxychloroquine, and no biologics were administered.
Compared with laboratory results at baseline, there were no significant differences in white blood cell count, platelet count, hemoglobin, urea nitrogen, albumin, creatinine, uric acid, C4 or anti-dsDNA titers between the two groups. Furthermore, the level of serum complement C3 was significantly lower in the prednisone increment group than in the control group (0.59 ± 0.12 vs. 0.63 ± 0.13 g/L; P = 0.025, Table 1).
There was no difference in the number of cases (P = 0.947) or the renal pathological classification (P = 0.354) between the prednisone increment and control groups on renal biopsy. A total of 40 patients (69.0%) in the prednisone increment group and 66 patients (69.5%) in the control group completed the renal puncture biopsy, with both groups having a higher incidence of type IV LN.
Analysis of end-point events
After 3 months of prednisone increment therapy, patients in the prednisone increment group and those in the control group were monitored for a duration of 18 months. During this period, we analyzed lupus activity, kidney involvement, and adverse reactions among patients in both groups. In the prednisone increment group, there were three cases (5.2%) of total recurrence, one case (1.7%) of renal recurrence, and two cases (3.4%) of extrarenal recurrence. In the control group, there were 24 (25.3%) cases with total recurrence, 13 (13.7%) with renal recurrence, and 11 (11.6%) with extrarenal recurrence. Furthermore, the probability of renal recurrence was significantly lower in the prednisone increment group than in the control group (P = 0.013). According to the analysis of survival data, the prognosis of patients in the prednisone increment group was significantly better than that of patients in the control group (P = 0.002, Table 2).
Table 2.
Relapsed patients in the control group and the prednisone increment group
| Endpoint | Control group (n = 95) | Prednisone increment group (n = 58) | P value |
|---|---|---|---|
| Recurrence (n, %) | 24 (25.3) | 3 (5.2) | 0.002 |
| Renal recurrence (n, %) | 13 (13.7) | 1 (1.7) | 0.013 |
| Extrarenal recurrence (n, %) | 11 (11.6) | 2 (3.4) | 0.080 |
The assessment of recurrence-free and renal recurrence-free rates in the prednisone increment group commenced 3 months after the initiation of prednisone increment therapy, with an observation period extending for 18 months. In contrast, the control group was monitored for 18 months following the achievement of SACQ, during which time the recurrence-free and renal recurrence-free rates were evaluated. Kaplan–Meier survival curves showed that the prognosis of patients in the prednisone increment group was significantly higher than that of patients in the control group (P = 0.002), and the cumulative relapse-free survival at 6, 12, and 18 months in the prednisone increment group was higher than that of the control group (100 vs. 96.6%, 94.8 vs. 94.7%, 89.5 vs. 74.7%) (Fig. 1); Compared to the prednisone increment group, the probability of renal recurrence was significantly higher in the control group (P = 0.009), and the cumulative renal relapse-free survival at 6, 12, and 18 months was higher in the prednisone increment group than in the control group (100 vs. 100%, 98.3 vs. 93.7%, 90.5 vs. 85.3%) (Fig. 2).
Fig. 1.
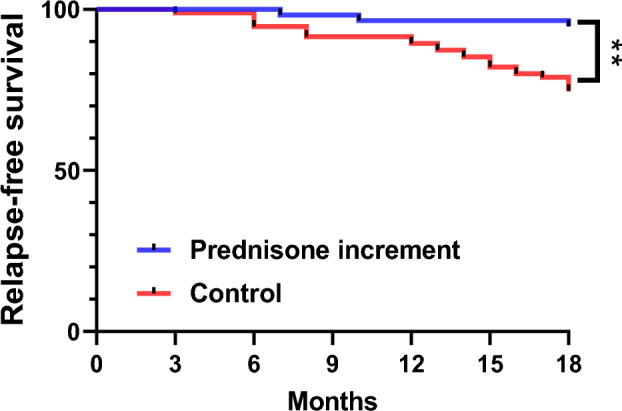
Relapse-free survival in the control group and the prednisone increment group (vs. control **P < 0.01)
Fig. 2.
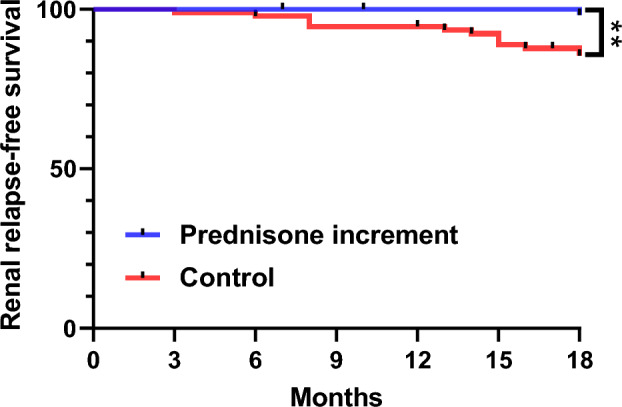
Renal relapse-free survival in the control group and the prednisone increment group (vs. control **P < 0.01)
Adverse events
During follow-up, nine cases of adverse reactions occurred in the prednisone increment group, including six cases of infection and one case each of liver function impairment, gastrointestinal reaction, and cytomegalovirus infection. In the control group, 11 adverse reactions occurred, including five cases of infection, two cases of impairment of liver function, one case of gastrointestinal reaction, two cases of herpes zoster, and one case of necrosis of the femoral head. The adverse reactions of the two groups were mainly infection (including upper respiratory tract and urinary system infection, no hospitalization), and no death occurred; however, there was no significant difference in the incidence of ADR between the two groups (P = 0.483, Table 3).
Table 3.
Adverse events in the control group and the prednisone increment group
| Adverse events | Control group (n = 95) | Prednisone increment group (n = 58) | P values |
|---|---|---|---|
| Adverse reaction (%) | 11 (18.97) | 9 (15.52) | 0.483 |
| Infection episodes, n (%) | 5 (5.26) | 6 (10.34) | 0.238 |
| Liver dysfunction, n (%) | 2 (2.11) | 1 (1.72) | 0.869 |
| Gastrointestinal reaction, n (%) | 1 (1.05) | 1 (1.72) | 0.723 |
| Cytomegalovirus infection, n (%) | 0 | 1 (1.72) | 0.199 |
| Herpes zoster, n (%) | 2 (2.11) | 0 | 0.266 |
| Femoral head necrosis, n (%) | 1 (1.05) | 0 | 0.433 |
Discussion
In the present study, we found that short-term incremental prednisone therapy decreases the risk of total and renal recurrence without increasing adverse events in patients with serologically active, clinically quiescent LN.
Patients with LN undergo a prolonged period of maintenance therapy after induction therapy. Patients with SACQ are not rare among patients with SLE who experience an attack during the follow-up period, and the attack probability is significantly higher than that of clinically quiescent and serologically quiescent SLE patients [15, 16].
As in other retrospective studies, the results can be confounded by selection bias. In our cohort, we found that patients in the prednisone increment group had lower baseline serum complement levels than those in the control group. According to previous studies, patients with lower serum complement levels had a higher probability of recurrence, causing attending physicians to initiate treatment. However, compared to the control group, patients in the prednisone increment group were more serologically active and had a higher probability of recurrence. However, patients in the prednisone increment group had a significantly better prognosis than those in the control group, with recurrence-free survival rates of 94.8 and 74.7% in the prednisone increment and control groups, respectively, at 18 months. Recurrent renal attacks are an independent risk factor for renal deterioration in patients with LN [23], and recurrent renal recurrence leads to cumulative depletion of renal nephrons and gradual reduction in renal reserves, resulting in adverse long-term renal survival.
In this study, two groups of patients with LN were observed among patients with renal recrudescence. Comparing the survival curves of the prednisone increment and control groups, the probability of renal recurrence was significantly lower in the prednisone increment group than in the control group. At the 18-month follow-up, patients in the prednisone increment group without renal recurrence had a survival rate of 98.3%, whereas for the control group, survival was 85.3%. This suggests that interventional therapy may have had a protective effect against renal recurrence in LN.
For all asymptomatic serologically active patients, increased prednisone dosage can increase the risk of AEs, such as infection and metabolic complications [19]. The risk of AEs after dosing should be evaluated when adjusting drug regimens in patients with LN. In this study, infection was the main AE in both groups, but no serious AEs were observed in either group, and the risk of AEs in the prednisone increment group was not significantly higher than that in the control group. Thus, an appropriate dose of the intervention drug may be considered relatively safe in the short-term, which is of great significance for future prospective trials.
This study is based on a single-center retrospective study of LN patients in the Affiliated Hospital of Guangdong Medical University, but there are still some limitations, such as a small sample size of cases, which can be further confirmed by a multi-center, large-sample randomized controlled study (RCT) in the future. BILAG score and SDI score are useful tools to assess disease activity in patients with SLE. However, this study is a retrospective study, and some examinations, such as fundus examination and lung X-ray, are missing to provide an accurate BILAG score and SDI score. We will add BILAG score and SDI scores in future studies to assess organ damage in patients. In addition, because cumulative doses of glucocorticoids are associated with increased damage, the effects of interventions on long-term toxicity, such as osteoporosis and cardiovascular risk, can only be determined by long-term follow-up.
In summary, short-term incremental prednisone therapy is safe for reducing the rate of recurrence in serologically active and clinically quiescent patients with LN.
Author contributions
Ning An: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Funding acquisition, Resources, Writing – original draft. Hao-tao Chen: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft. Wen-bo Deng: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft. Le Zhang: Data curation, Methodology. Jin-xia Chen: Data curation, Methodology. Cui-wei Yao: Data curation, Methodology. Yong-zhi Xu: Funding acquisition, Supervision, Investigation, Writing – review & editing. Hua-feng Liu: Supervision, Funding acquisition, Writing – review & editing.
Funding
This work was supported by the National Clinical Key Specialty Construction Project (Institute of Nephrology, Affiliated Hospital of the Guangdong Medical University), the Natural Science Foundation of the Guangdong Province (2021A1515011581, 2023A1515030024), and Guangdong Provincial Key Laboratory of Autophagy and Major Chronic Non-Communicable Diseases (2022B1212030003).
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University, and the ethics approval number was PJ2013115. Informed consent was obtained from all patients.
Competing interests
The authors declare no competing interests.
Footnotes
This work was supported by National Clinical Key Specialty Construction Project (Institute of Nephrology, Affiliated Hospital of Guangdong Medical University).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ning An, Hao-tao Chen, and Wen-bo Deng are the authors contributed equally to this work.
Contributor Information
Hua-feng Liu, Email: liuhf@gdmu.edu.cn.
Yong-zhi Xu, Email: lxyzhi@126.com.
References
- 1.Morales E, Galindo M, Trujillo H, Praga M. Update on lupus nephritis: looking for a new vision. Nephron. 2021;145:1–13. 10.1159/000511268. [DOI] [PubMed] [Google Scholar]
- 2.Tselios K, Gladman DD, Touma Z, Su J, Anderson N, Urowitz MB. Disease course patterns in systemic lupus erythematosus. Lupus. 2019;28:114–22. 10.1177/0961203318817132. [DOI] [PubMed] [Google Scholar]
- 3.Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020;6:7. 10.1038/s41572-019-0141-9. [DOI] [PubMed] [Google Scholar]
- 4.Förger F, Matthias T, Oppermann M, Becker H, Helmke K. Clinical significance of anti-dsDNA antibody isotypes: IgG/IgM ratio of anti-dsDNA antibodies as a prognostic marker for lupus nephritis. Lupus. 2004;13:36–44. 10.1191/0961203304lu485oa. [DOI] [PubMed] [Google Scholar]
- 5.Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? Rheumatology. 2007;46:1052–6. 10.1093/rheumatology/kem112. [DOI] [PubMed] [Google Scholar]
- 6.Koffler D, Schur PH, Kunkel HG. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967;126:607–24. 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witte T, Hartung K, Matthias T, Sachse C, Fricke M, Deicher H, Kalden JR, Lakomek HJ, Peter HH, Schmidt RE. Association of IgA anti-dsDNA antibodies with vasculitis and disease activity in systemic lupus erythematosus. SLE Study Group. Rheumatol Int. 1998;18(1998):63–9. 10.1007/s002960050059. [DOI] [PubMed] [Google Scholar]
- 8.Riboldi P, Gerosa M, Moroni G, Radice A, Allegri F, Sinico A, Tincani A, Meroni PL. Anti-DNA antibodies: a diagnostic and prognostic tool for systemic lupus erythematosus? Autoimmunity. 2005;38:39–45. 10.1080/08916930400022616. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein A, Alexander RV, Zack DJ. A review of complement activation in SLE. Curr Rheumatol Rep. 2021;23:16. 10.1007/s11926-021-00984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zonana-Nacach A, Salas M, Sánchez ML, Camargo-Coronel A, Bravo-Gatica C, Mintz G. Measurement of clinical activity of systemic lupus erythematosus and laboratory abnormalities: a 12-month prospective study. J Rheumatol. 1995;22:45–9. [PubMed] [Google Scholar]
- 11.Chi S, Yu Y, Shi J, Zhang Y, Yang J, Yang L, Liu X. Antibodies against C1q are a valuable serological marker for identification of systemic lupus erythematosus patients with active lupus nephritis. Dis Markers. 2015;15:450351. 10.1155/2015/450351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attar SM, Koshak EA. Medical conditions associated with a positive anti-double-stranded deoxyribonucleic acid. Saudi Med J. 2010;31:781–7. [PubMed] [Google Scholar]
- 13.Gladman DD, Urowitz MB, Keystone EC. Serologically active clinically quiescent systemic lupus erythematosus: a discordance between clinical and serologic features. Am J Med. 1979;66:210–5. 10.1016/0002-9343(79)90529-1. [DOI] [PubMed] [Google Scholar]
- 14.Conti F, Ceccarelli F, Perricone C, Miranda F, Truglia S, Massaro L, Pacucci VA, Conti V, Bartosiewicz I, Spinelli FR, Alessandri C, Valesini G. Flare, persistently active disease, and serologically active clinically quiescent disease in systemic lupus erythematosus: a 2-year follow-up study. PLoS ONE. 2012;7: e45934. 10.1371/journal.pone.0045934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Mu L, Zhang Z, Gao D, Hao Y, Zhou W. Treatments and outcomes in Chinese patients with serologically active clinically quiescent systemic lupus erythematosus: a retrospective observational study. Arthritis Res Ther. 2021;23:275. 10.1186/s13075-021-02641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiman AJ, Gladman DD, Ibañez D, Urowitz MB. Prolonged serologically active clinically quiescent systemic lupus erythematosus: frequency and outcome. J Rheumatol. 2010;37:1822–7. 10.3899/jrheum.100007. [DOI] [PubMed] [Google Scholar]
- 17.Gripenberg M, Helve T. Outcome of systemic lupus erythematosus. A study of 66 patients over 7 years with special reference to the predictive value of anti-DNA antibody determinations. Scand J Rheumatol. 1991;20:104–9. 10.3109/03009749109165284. [DOI] [PubMed] [Google Scholar]
- 18.Esdaile JM, Abrahamowicz M, Joseph L, Mackenzie T, Li Y, Danoff D. Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus. Why some tests fail. Arthritis Rheum. 1996;39:370–8. 10.1002/art.1780390304. [DOI] [PubMed] [Google Scholar]
- 19.Austin HA 3rd, Klippel JH, Balow JE, Le Riche NG, Steinberg AD, Plotz PH, Decker JL. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614–9. 10.1056/NEJM198603063141004. [DOI] [PubMed] [Google Scholar]
- 20.Capone G, Lojacono C, Al-Bayitee F, Makvandi S, Hennon T, Wrotniak B, Abdul-Aziz R. Follow-up and management of serologically active clinically quiescent cases in pediatric systemic lupus erythematosus. Reumatologia. 2021;59:244–51. 10.5114/reum.2021.108353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bootsma H, Spronk P, Derksen R, De Boer G, Wolters-Dicke H, Hermans J, Limburg P, Gmelig-Meyling F, Kater L, Kallenberg C. Prevention of relapses in systemic lupus erythematosus. Lancet. 1995;345:1595–9. 10.1016/s0140-6736(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 22.Tseng CE, Buyon JP, Kim M, Belmont HM, Mackay M, Diamond B, Marder G, Rosenthal P, Haines K, Ilie V, Abramson SB. The effect of moderate-dose corticosteroids in preventing severe flares in patients with serologically active, but clinically stable, systemic lupus erythematosus: findings of a prospective, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:3623–32. 10.1002/art.22198. [DOI] [PubMed] [Google Scholar]
- 23.Parikh SV, Nagaraja HN, Hebert L, Rovin BH. Renal flare as a predictor of incident and progressive CKD in patients with lupus nephritis. Clin J Am Soc Nephrol. 2014;9:279–84. 10.2215/CJN.05040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.


