Abstract
Background
This study evaluates the effectiveness of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria, typically applied to singleton pregnancies, in managing gestational diabetes mellitus (GDM) in twin pregnancies. Focusing on a Chinese cohort, it contrasts the clinical outcomes and complications in twin pregnancies with and without GDM.
Methods
We conducted a retrospective cohort study at our hospital from January 2019 to December 2021, including all twin deliveries except those before 28 weeks of gestation, with prior diabetes, or unknown GDM status. GDM was diagnosed using a 75 g oral glucose tolerance test based on the IADPSG criteria, and management involved dietary or insulin interventions. We assessed outcomes such as hypertensive disorders (gestational hypertension, preeclampsia, and eclampsia), membrane rupture, preterm birth, small for gestational age (SGA), large for gestational age (LGA), and neonatal intensive care unit (NICU) admissions.
Results
Among 1003 twin pregnancies, 21.7% had GDM, with 11.5% receiving insulin. GDM was associated with older maternal age, higher BMI, and a family history of diabetes. Pregnant women with GDM had lower weekly weight gain (0.44 kg/week vs. 0.58 kg/week, p < 0.001) and experienced a higher risk of SGA neonates (aOR = 1.68, 95% CI: 1.06–2.67) and increased NICU admissions (aOR = 1.30, 95% CI: 1.00-1.69) compared to those without GDM. Additionally, dichorionic twins with GDM showed higher risks of SGA and NICU admissions, while monochorionic twins had no significant differences. A U-shaped relationship was identified between weekly weight gain and the rates of SGA and NICU admissions, with the lowest risk observed at a weekly weight gain of 0.75 kg for SGA and 0.57 kg for NICU admissions.
Conclusions
Applying singleton-derived IADPSG criteria to twin pregnancies may mitigate some maternal risks but elevates the risk for SGA neonates, suggesting a need for tailored diagnostic and management strategies for twin pregnancies.
Trial registration
Retrospectively registered.
Keywords: Gestational diabetes mellitus, Twin pregnancy, Chorionicity, Small-for-gestational-age
Introduction
Gestational diabetes mellitus (GDM) elevates perinatal risks in singleton pregnancies, including preeclampsia (PE), premature rupture of membranes, macrosomia, small for gestational age (SGA), neonatal hypoglycemia, and respiratory distress syndrome (RDS) [1]. Effective management of GDM in singletons is known to mitigate these risks [2]. Conversely, twin pregnancies inherently carry higher risks of GDM and related complications than singletons [3]. However, the extent to which GDM exacerbates perinatal outcomes in twins remains unclear, due to mixed findings in current research. Evidence on the risks associated with GDM in twin pregnancies is contradictory; some studies report increased adverse outcomes [4–8], such as gestational hypertension and PE, while others note minimal associations [9, 10]. These discrepancies are often attributed to variations in study methodologies, including inconsistent diagnostic criteria and missing data on pre-pregnancy body mass index (BMI) and chorionicity.
Diagnostic guidelines for GDM differ widely [11, 12], still predominantly based on studies involving singletons [3]. The appropriateness of applying these singleton-based guidelines to twins has not been substantiated, leading to uncertainties around the benefits of strict glycemic control in such cases [13–15].
This study aimed to evaluate adverse perinatal outcomes between twin pregnancies with GDM diagnosed with the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria and those without. It particularly focuses on the differential outcomes in dichorionic versus monochorionic twin pregnancies. By doing so, it contributes to a more nuanced understanding of how existing GDM management protocols may perform when extended to twin scenarios, which may inform future research and adjustments in clinical practice guidelines.
Materials and methods
Study population
This retrospective study encompassed all twin pregnancies delivered at our institution in Eastern China from January 1, 2019, to December 31, 2021. We excluded cases involving singleton pregnancies, delivered at less than 28 weeks of gestation, absent early ultrasound scans for chorionicity or gestational age determination, prior diabetes mellitus, or missing GDM screening results.
Data collection
Data were extracted from the hospital’s medical records system, covering maternal demographics, clinical histories, and obstetric-pediatric outcomes. The study included dichorionic (DC) and monochorionic (MC) twins. The MC group was further classified into monochorionic diamniotic (MCDA) and monoamniotic (MCMA) twins. In the case of MCDA twins, they were either naturally MCDA or became MCDA after mid-trimester fetal reduction surgery performed on MCTA pregnancies. Chorionicity was assessed via prenatal ultrasound and confirmed by surgical and pathological reports post-delivery. Gestational age for naturally conceived twins was calculated from the last menstrual period or early ultrasound if discrepancies exceeded five days. For assisted reproduction cases, gestational age was based on the date of embryo implantation.
GDM diagnosis and management
GDM screening was performed using a 75 g oral glucose tolerance test (OGTT), with diagnoses confirmed upon exceeding thresholds of fasting glucose ≥ 5.1 mmol/L, 1-hour postprandial glucose ≥ 10.0 mmol/L, or 2-hour postprandial glucose ≥ 8.5 mmol/L. Management included self-monitored blood glucose, tailored dietary and exercise plans, and, if necessary, insulin therapy based on guidelines from the IADPSG and other standards [1, 16]. All management protocols were uniform across the patient population, overseen by our maternal-fetal medicine team.
Outcomes measured
Outcomes assessed included hypertensive disorders (gestational hypertension, PE, and eclampsia), premature rupture of membranes, preterm delivery, fetal distress, fetal death, and delivery modes. Gestational hypertension was defined as a new development of a blood pressure of ≥ 140/90 mmHg after 20 weeks’ gestation without proteinuria, and PE was defined as the onset of a blood pressure of ≥ 140/90 mmHg and proteinuria of ≥ 300 mg/24 h [17]. Neonatal outcomes measured were NICU admission, SGA, LGA [18], birth weight discordance, and conditions like RDS, neonatal hypoglycemia, necrotizing enterocolitis, infections and jaundice. The intertwin birth weight discordance was calculated using the following formula: (birth weight of the heavier twin − birth weight of the lighter twin) / birth weight of the heavier twin × 100% [19]. A discordant twin pair was defined as having a BWD greater than 25%, based on the NICE guidelines [20], which indicate that BWD exceeding 25%, when combined with other ultrasound findings, is more effective for diagnosing selective intrauterine growth restriction (sIUGR). Outcomes were stratified by chorionicity into monochorionic and dichorionic groups.
Statistical analysis
Descriptive statistics quantified maternal and perinatal data. Group comparisons employed t-tests or Analysis of variance (ANOVAs) for parametric data and Mann-Whitney U tests for non-parametric data. Categorical outcomes were analyzed using Pearson’s chi-squared or Fisher’s exact tests. Generalized estimating equations (GEE) assessed differences in neonatal outcomes between GDM and non-GDM groups, with marginal standardization to estimate risk differences. All statistical analyses were conducted using SPSS 24.0 and the R programming language, considering p-values < 0.05 as statistically significant.
Results
Study population characteristics
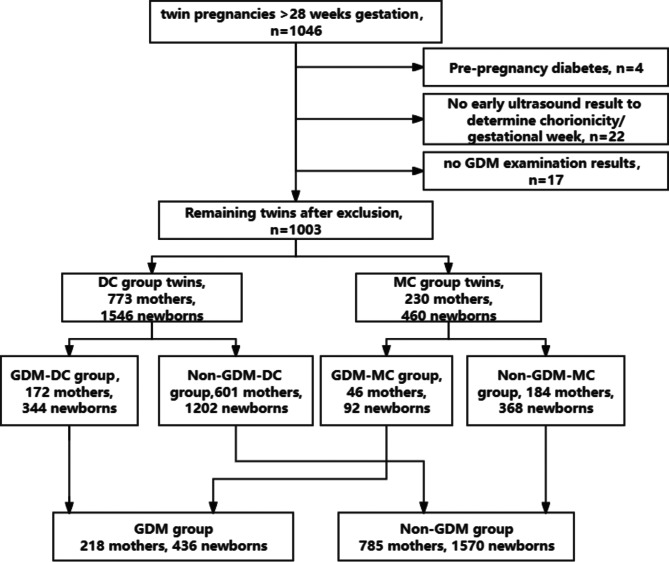
We included 1046 women with twin pregnancies, excluding 43 due to our criteria, resulting in 1003 cases included in the analysis. Of these, 773 (77.1%) were dichorionic, and 230 (22.9%) were monochorionic. Among the monochorionic cases, 225 were MCDA, with 223 initially classified as MCDA and 2 converted from MCTA following fetal reduction at 15–16 weeks, while the remaining 5 were MCMA. GDM was diagnosed in 218 women (21.7%) following the IADPSG criteria. These women underwent standardized dietary and exercise interventions, with 25 (11.5%) requiring insulin therapy for inadequate glycemic control (Fig. 1).
Fig. 1.
Flow chart of selection of the study population
Maternal characteristics
Table 1 contrasts maternal characteristics between the GDM and non-GDM groups. Women with GDM were older (average age 33.20 vs. 32.02 years; p < 0.001) and had higher pre-pregnancy BMI (22.22 vs. 21.40; p < 0.001). Significant associations were found for previous GDM history (2.3% in GDM vs. 0.3% in non-GDM; p = 0.007), family diabetes history (13.3% vs. 5.9%; p < 0.001), and higher local GDM prevalence (51.8% vs. 42.2%; p = 0.011). Post-diagnosis, the GDM group exhibited significantly lower weekly weight gains (0.44 vs. 0.58 kg/week; p < 0.001) and total gestational weight gain (GWG) (14.08 vs. 17.13 kg; p < 0.001).
Table 1.
Characteristics of women with twin pregnancies 2019–2021 (n = 1003)
| Characteristics | Non-GDM group (n = 785) | GDM group (n = 218) | p-value |
|---|---|---|---|
| Maternal age (year) | 32.02 ± 3.92 | 33.20 ± 3.69 | 0.000 |
| Maternal age ≥ 35 years | 201(25.6) | 83(38.1) | 0.000 |
| Pre-pregnancy BMI (kg/m2) | 21.40 ± 3.04 | 22.22 ± 3.02 | 0.000 |
| Underweight: BMI < 18.5 | 78(9.9) | 16(7.3) | 0.006 |
| Normal weight:18.5 ≤ BMI < 24 | 593(75.5) | 149(68.3) | |
| Overweight:24 ≤ BMI < 28 | 88(11.2) | 42(19.3) | |
| Obese: BMI ≥ 28 | 26(3.3) | 11(5.0) | |
| Multiparity | 123(15.7) | 28(12.8) | 0.294 |
| Assisted Reproductive Technology (ART) pregnancy | 489(62.3) | 140(64.2) | 0.603 |
| Chorionicity (dichorionic) | 601(77.8) | 172(78.9) | 0.524 |
| chronic hypertension | 9(1.1) | 5(2.3) | 0.200 |
| History of GDM | 2(0.3) | 5(2.3) | 0.007 |
| History of polycystic ovary syndrome | 20(2.5) | 9(4.1) | 0.218 |
| Family history of type II diabetes | 46(5.9) | 29(13.3) | 0.000 |
| Geography | 0.011 | ||
| Shanghai | 331(42.2) | 113(51.8) | |
| Foreign/expatriate | 454(57.8) | 105(48.2) | |
| Educational level | 0.405 | ||
| Master and above | 130(16.6) | 44(20.2) | |
| Undergraduate/Specialist | 532(67.8) | 136(62.4) | |
| High school and below | 123(15.7) | 38(17.4) | |
| Smoking | 6(0.8) | 1(0.5) | 1.000 |
| Early pregnancy | |||
| Ferritin | 90.15 ± 68.73 | 90.3 ± 71.99 | 0.854 |
| Fasting blood glucose | 4.54 ± 0.42 | 4.64 ± 0.43 | 0.000 |
| Glycated hemoglobin | 5.23 ± 0.29 | 5.35 ± 0.38 | 0.000 |
| Middle pregnancy (OGTT) | |||
| Fasting blood glucose | 4.16 ± 0.38 | 4.52 ± 0.53 | 0.000 |
| 1 h after | 7.64 ± 1.21 | 10.18 ± 1.17 | 0.000 |
| 2 h after | 6.42 ± 1.04 | 8.87 ± 1.45 | 0.000 |
| Glycated hemoglobin | 4.96 ± 0.32 | 5.14 ± 0.42 | 0.000 |
| Early and middle pregnancy (conception to OGTT) * | |||
| Weight gain (kg) | 10.20 ± 4.16 | 9.20 ± 4.12 | 0.027 |
| BMI gain (kg/m2) | 3.85 ± 1.57 | 3.53 ± 1.59 | 0.082 |
| Middle and late pregnancy (OGTT to labor) * | |||
| Weight gain (kg) | 6.87 ± 3.38 | 4.87 ± 3.76 | 0.000 |
| Weight gain per week (kg/W) | 0.58 ± 0.29 | 0.44 ± 0.47 | 0.000 |
| BMI gain per week (kg/m2/W) | 0.22 ± 0.11 | 0.17 ± 0.18 | 0.000 |
| Gestational weight gain (GWG) (kg) | 17.13 ± 6.01 | 14.08 ± 4.94 | 0.000 |
The impact of GDM on maternal outcomes
Following adjustments for maternal age, pre-pregnancy BMI, early and middle pregnancy BMI gain, history of GDM, family history of type II diabetes, and geographic differences, no significant differences in maternal outcomes were observed in the twin pregnancy GDM group compared to the non-GDM group (Table 2).
Table 2.
Impact of GDM on maternal outcomes in twin pregnancies (n = 1003)
| maternal outcomes | Non-GDM group (n = 785) | GDM group (n = 218) | OR (95%CI) | aOR (95% CI) | p-Value Adjusted | |
|---|---|---|---|---|---|---|
| hypertensive disorders | 170(21.7) | 44(20.2) | 0.92(0.63,1.33) | 0.88(0.60,1.29) | 0.515 | |
| pre-eclampsia | total | 114(14.5) | 27(12.4) | 0.83(0.53,1.30) | 0.84(0.53,1.34) | 0.458 |
| < 37 weeks | 100(12.7) | 23(10.6) | 0.81(0.50,1.31) | 0.82(0.50,1.35) | 0.442 | |
| < 34 weeks | 20(2.5) | 4(1.8) | 0.72(0.24,2.11) | 0.80(0.26,2.45) | 0.701 | |
| preterm rupture of membranes | 114(14.5) | 37(17) | 1.20(0.80,1.81) | 1.26(0.82,1.93) | 0.288 | |
| preterm delivery | < 37 weeks | 496(63.2) | 134(61.5) | 0.93(0.68,1.27) | 0.97(0.70,1.33) | 0.836 |
| < 34 weeks | 83(10.6) | 29(13.3) | 1.30(0.83,2.04) | 1.38(0.85,2.24) | 0.194 | |
| excessive amniotic fluid | 15(1.9) | 4(1.8) | 0.96(0.32,2.92) | 1.08(0.33,3.47) | 0.902 | |
| fetal distress | 58(7.4) | 20(9.2) | 1.27(0.74,2.16) | 1.25(0.71,2.19) | 0.443 | |
| fetal death | 7(0.9) | 1(0.5) | 0.51(0.06,4.19) | 0.20(0.01,3.77) | 0.280 | |
| mode of delivery (Cesarean section) | 766(97.6) | 212(97.2) | 0.88(0.35,2.22) | 1.17(0.41,3.36) | 0.766 | |
| postpartum hemorrhage | 32(4.1) | 11(5.0) | 1.25(0.62,2.52) | 1.22(0.59,2.53) | 0.591 | |
aOR: Adjusted odds ratio.CI: Confidence interval. AOR was corrected for age, pre-pregnancy BMI, early and middle pregnancy BMI gain, history of GDM, family history of type II diabetes, and geographic differences as covariates with P-values < 0.05 for all models
Neonatal outcomes
GDM-associated pregnancies showed a higher risk of SGA neonates (7.1% in GDM vs. 4.5% in non-GDM; aOR = 1.68; p = 0.027) and a trend towards more frequent NICU admissions (49.1% vs. 43.6%, aOR = 1.30; p = 0.051), but a reduced risk of LGA neonates (3.7% vs. 7.2%, aOR = 0.49; p = 0.014). Dichorionic (DC) twins with GDM had increased risks of SGA (7.0% vs. 3.8%, aOR = 1.91; p = 0.017) and NICU admission (44.5% vs. 38.5%, aOR = 1.35; p = 0.046). The absolute risks for monochorionic twins were similar to those for dichorionic twins, although the statistical significance was not reached likely due to the smaller sample size (Table 3).
Table 3.
Impact of GDM on neonatal outcomes in twin pregnancies (n = 2006)
| neonatal outcomes | Non-GDM group (n = 1570) |
GDM group (n = 436) |
OR (95% CI) | aOR (95% CI) | P-value Adjusted |
|---|---|---|---|---|---|
| Total (n = 2006) | |||||
| NICU admission | 684(43.6) | 214(49.1) | 1.25(0.96,1.62) | 1.30(1.00,1.69) | 0.051 |
| SGA | 70(4.5) | 31(7.1) | 1.64(1.05,2.57)* | 1.68(1.06,2.67)* | 0.027 |
| LGA | 113(7.2) | 16(3.7) | 0.49(0.28,0.86)* | 0.49(0.28,0.87)* | 0.014 |
| DC (n = 1546) | |||||
| NICU admission | 463(38.5) | 153(44.5) | 1.31(0.72,2.39) | 1.35(1.01,1.81)* | 0.046 |
| SGA | 45(3.8) | 24(7.0) | 1.92(1.15,3.23)* | 1.91(1.12,3.26)* | 0.017 |
| LGA | 91(7.6) | 16(4.7) | 0.60(0.34,1.05) | 0.59(0.33,1.04) | 0.070 |
| MC (n = 460) | |||||
| NICU admission | 221(60.1) | 61(66.3) | 1.31(0.72,2.39) | 1.29(0.70,2.38) | 0.408 |
| SGA | 25(6.8) | 7(7.6) | 1.13(0.44,2.91) | 1.27(0.48,3.30) | 0.631 |
| LGA | 22(6.0) | 0(0.0) | NA | NA | NA |
Generalized estimating equations (GEE) models adjusted for maternal age, pre-pregnancy BMI, early and middle pregnancy BMI gain, history of GDM, family history of type II diabetes, and geographic differences as covariates. *: P < 0.05
We observed no significant differences in neonatal sex ratio or other adverse neonatal outcomes (including prematurity, RDS, neonatal asphyxia, delivery weight discordance, neonatal hypoglycemia, necrotizing enterocolitis, neonatal infection, neonatal jaundice, neonatal malformation, respiratory system diseases, and hyperbilirubinemia), as shown in Appendix Table 1. The number of cases of neonatal deaths is quite limited, with only 1 in the GDM group and 7 in the non-GDM group. Given the small sample size, statistical analysis would not yield meaningful comparisons, and there was no significant difference in neonatal death rates between the two groups. Therefore, we did not include these data in the primary outcome analysis.
Effect of gestational weight gain on outcomes
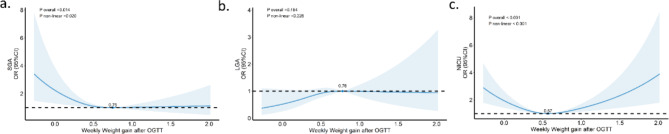
Analysis revealed a U-shaped relationship between weekly weight gain and rates of SGA and NICU admissions, with the lowest risk at a weekly weight gain of 0.75 kg (SGA) and 0.57 kg (NICU admissions). No such relationship was observed for LGA (Fig. 2).
Fig. 2.
RCS-curves correlating weekly weight gain after OGTT with neonatal SGA, LGA and NICU admission rates. (a) weekly weight gain after OGTT with neonatal SGA, P < 0.05, n = 2006. (b) weekly weight gain after OGTT with neonatal LGA, P > 0.05, n = 2006. (c) weekly weight gain after OGTT with neonatal NICU admission rates, P < 0.05, n = 2006. The statistics were analyzed with other factors taken into account. Covariates: age, pre-pregnancy BMI, fasting blood glucose and glycated hemoglobin
Discussion
The study’s primary objective was to assess how the IADPSG criteria for GDM diagnosis, initially developed for singleton pregnancies, affected key perinatal outcomes in twin pregnancies. We identified a GDM prevalence of 21.7% within our twin pregnancy cohort, which aligns with the higher end of the prevalence spectrum previously reported, ranging from 3.2 to 25.5%. This rate substantiates the findings from a previous retrospective cohort study conducted at our institution. That study documented a threefold increase in gestational diabetes mellitus (GDM) incidence following the shift from the conventional two-step diagnostic procedure, which includes an initial 50 g glucose screening test followed by a 100 g diagnostic oral glucose tolerance test according to the Carpenter-Coustan criteria, to the simplified one-step International Association of Diabetes and Pregnancy Study Groups (IADPSG) protocol [21]. Similar trends have been documented in studies of singleton pregnancies [22, 23].
Risk factors for GDM in women with twin pregnancies include advanced maternal age, primiparity, higher pre-pregnancy BMI and assisted reproductive technology(ART) conception [5, 8, 24]. Our study revealed that women with GDM in twin pregnancies were more likely to be aged 35 or older, overweight or obese, and have a personal or family history of diabetes, as well as being registered residents of Shanghai. These findings are consistent with meta analyses focusing on Asian populations [25], emphasizing consistent risk patterns across different studies. Although our data indicated an increased prevalence of GDM among ART-conceived and primigravida, these differences did not reach statistical significance. Furthermore, our analysis showed no significant variation in GDM incidence across different chorionicity of twins, confirming recent findings [26].
Maternal outcomes
The relationship between GDM in twin pregnancies and hypertensive disorders remains a subject of debate. In our study, after controlling for variables such as maternal age and pre-pregnancy BMI, no statistically significant differences were observed in the incidence of hypertension and PE between twin pregnancies affected by GDM and those that were not. This observation aligns with some segments of the literature [8, 26], which report no direct correlation between GDM and hypertensive outcomes, while other studies suggest a strong association [10, 27]. Notably, previous research indicates that typical weight gain during pregnancy for Chinese twins ranges from 15 to 21 kg [28], and excessive weight gain is often cited as a contributing factor to hypertensive complications [29, 30].
In contrast to these broader trends, our GDM cohort, managed actively through diet and exercise protocols, gained significantly less weight, an average of only 14.08 kg, compared to the non-GDM group. This moderated weight gain likely played a role in mitigating hypertensive complications traditionally associated with GDM. We postulate that this effective management of weight gain could underlie the absence of increased hypertension and PE rates in our GDM-affected twin pregnancies. This hypothesis is supported by results previously published by our institution [31], indicating that a comprehensive management approach, including specific dietary recommendations, can substantially reduce the risk of gestational hypertension, particularly in twins diagnosed with obesity. Furthermore, the reduction in the incidence of early-onset PE in our cohort may be partly attributed to the proactive use of aspirin in twin pregnancies managed at our hospital, aligning with recent trends towards preventive pharmacological interventions in high-risk populations. This proactive strategy has demonstrated effectiveness in decreasing the prevalence of this severe pregnancy complication, underscoring the value of integrative care strategies in managing complex cases like twin pregnancies with GDM.
Neonatal outcomes
Building upon our findings from maternal outcomes, where strict dietary and exercise regimens were seen to mitigate hypertensive disorders in GDM-affected twin pregnancies, our study further explored the neonatal consequences of these management strategies. Contrary to certain previous studies [26, 32], we observed an increase in neonatal SGA and a decrease in LGA within the GDM group. This trend could be attributed to the notably lower weight gain during pregnancy, particularly after GDM diagnosis, as stringent glycemic and weight control were prioritized. Indeed, meta-analyses suggest that insufficient weight gain in twin pregnancies is linked to increased risks of SGA and low birth weight [29, 33], potentially due to inadequate nutritional intake. The physiological dynamics of twin pregnancies, which include heightened glucose consumption and a higher basal metabolic rate, suggest that some degree of hyperglycemia might be naturally compensatory [8, 26], the greater transient increase in insulin resistance observed in twin pregnancies is merely a physiologic exaggeration rather than a pathology that requires treatment [15]. The recent studies from the American Journal of Obstetrics and Gynecology (AJOG) indicated that while strict glycemic control is associated with reduced LGA incidence, it does not necessarily improve overall neonatal outcomes and may increase SGA prevalence [15, 34]. These findings raise critical questions about the applicability of standard GDM glycemic targets, originally designed for singleton pregnancies, to the unique metabolic requirements of twins. This discrepancy underscores the need for tailored guidelines that specifically address the dual challenges of managing GDM and optimizing fetal growth in twin pregnancies.
Diagnostic criteria for GDM in twins
In singleton pregnancies, established interventions such as dietary control and insulin therapy are known to benefit both severe and mild cases of GDM, adhering to the National Diabetes Data Group (NDDG) criteria [35]. However, the evidence base concerning the impact of changing diagnostic criteria on maternal and neonatal outcomes in twin pregnancies remains limited. The introduction of the IADPSG criteria has led to increased GDM diagnosis rates, which necessitates an evaluation of cost-effectiveness due to potential increases in healthcare expenditures.
The 2018 guidelines from the American College of Obstetricians and Gynecologists (ACOG) [12] continue to advocate for the two-step diagnostic approach, citing a lack of sufficient evidence to support that a higher diagnosis rate significantly enhances maternal or neonatal outcomes in clinical settings. These guidelines caution against hasty adoption of new criteria without robust national-level research, although they acknowledge that hospitals and organizations may adopt the IADPSG criteria at their discretion.
Our study indicates that applying single-fetus GDM diagnostic criteria to twin pregnancies has resulted in increased GDM diagnosis rates. This heightened detection could lead to potential overdiagnosis and overtreatment, contributing to adverse perinatal outcomes, such as increased incidences of SGA and neonatal intensive care unit (NICU) admissions. These findings underscore the necessity for a tailored approach to diagnosing and managing GDM in twin pregnancies. It is imperative to further investigate and possibly revise the diagnostic criteria specifically for twin pregnancies to avoid the pitfalls of overdiagnosis and ensure optimal perinatal care.
Twin pregnancy weight gain targets
Effective glycemic and weight management in twin pregnancies is essential for reducing risks associated with gestational hypertension and PE [1], though it may increase the likelihood of SGA. This presents a clinical dilemma: balancing weight control with the prevention of adverse perinatal outcomes.
The Institute of Medicine (IOM) guidelines from 2009 recommend a GWG of 16.8–24.5 kg for twins with a normal pre-pregnancy BMI, suggesting variations in these outcomes across different racial groups [36]. For instance, adequate GWG as per IOM standards correlated with increased gestational hypertension risks in Japanese populations [37], while Soichiro Obata proposed lower GWG ranges of 10.3–16.0 kg for twins in Japan, excluding GDM cases [38]. In China, current GWG guidelines are tailored only for singleton pregnancies [39], highlighting the need for specific standards for twins. Studies exploring the application of IOM recommendations to Chinese twins have shown inconsistent results, often neglecting the impact of varying rates of GWG across different pregnancy stages [40–42].
Our research focused on the correlation between weekly weight gain post-OGTT and neonatal outcomes such as SGA, LGA, and NICU admissions. We identified optimal outcomes at a GWG rate of 0.75 kg/week (SGA) and 0.57 kg/week (NICU admissions) during mid-to-late pregnancy (Fig. 2), compared to just 0.44 kg/w in the GDM group, indicating a direct connection between lower weight gain rates and increased SGA/NICU admission risk. These findings underline the importance of establishing specific GWG targets for twin pregnancies in China. Given the limited sample size (436 newborns in the GDM group), expanding this research is essential to confirm these preliminary results and refine GWG recommendations.
Outcomes in dichorionic versus monochorionic twins
Understanding the influence of placental factors is crucial in managing gestational diabetes mellitus (GDM), hypertensive disorders, and fetal growth issues in twin pregnancies. This study examines the perinatal outcomes of GDM in twins categorized by their chorionicity. Our findings indicate that the incidence of GDM is similar across both DC and MC twins, which is consistent with the results reported by Dave et al. [10]. Notably, the neonatal outcomes in the DC twin group mirrored the general twin outcomes, suggesting that GDM increases the likelihood of having a neonate SGA and the risk of NICU admission. A relevant Taiwanese study highlighted that GDM triples the risk of NICU admissions [32], although it did not show a difference in the occurrence of SGA.
Interestingly, in our study, GDM did not lead to a higher incidence of SGA or NICU admissions in the MC twin group, possibly due to the shared placenta, which may dilute the individual impact of GDM typically observed in singletons. This phenomenon suggests that the twin MC group’s outcomes might be less affected by the maternal GDM status compared to DC twins, where each fetus has its own placenta.
Furthermore, contrasting findings by Y. Mei indicated that GDM is associated with fewer instances of SGA in monochorionic diamniotic twins [43], which diverges from our observations where no significant differences in weight gain were noted between the GDM and non-GDM groups during pregnancy. Our study, however, had a limited sample size of 460 newborns in the MC group, which may not sufficiently represent broader population dynamics. Expanding the sample size in future research will be crucial to confirm these preliminary findings and to refine management strategies for this high-risk group.
Advantages and limitations
Our study benefits from several notable strengths, including a robust sample size from a single institution which ensures uniformity in the diagnosis and management of GDM. We specifically focused on twin pregnancies characterized by a high rate of ART usage (up to 62.7%), which provided a unique cohort for examining GDM impacts. Participants showed excellent adherence to prescribed dietary and exercise regimens, enhancing the reliability of treatment outcomes. These factors contributed to the standardized delivery of treatments and allowed us to propose specific weight gain targets tailored for Chinese twin pregnancies, anticipated to improve both obstetric and neonatal outcomes. Additionally, we adjusted for variables such as pre-pregnancy BMI, and weight gain during early and mid-pregnancy, alongside family history, to control for potential confounders. Furthermore, our study’s approach of stratifying GDM-affected pregnancies by chorionicity is an aspect that is less commonly addressed in existing literature, providing deeper insights into how GDM impacts different twin types.
Despite these strengths, our study faces certain limitations due to its retrospective design. We analyzed data on weight gain and glucose levels at various stages of pregnancy, but did not comprehensively examine other vital factors like physical activity and detailed glycemic control, which are crucial for understanding the full spectrum of GDM development and management. This omission means we cannot definitively conclude how these factors might influence pregnancy outcomes. Additionally, the study’s design did not allow for the tracking of long-term maternal and neonatal health outcomes, which are critical for assessing the enduring impacts of GDM management strategies. Given these gaps, further prospective studies are necessary to validate our findings and refine the guidelines for managing GDM in twin pregnancies. As a next step, we aim to expand our sample size and employ a prospective study design to explore more deeply the diagnostic criteria for GDM in twins and to optimize strategies for glycemic control and weight management.
Conclusions
Our study aimed to evaluate the effectiveness of the IADPSG criteria, originally developed for singleton pregnancies, in managing GDM in twin pregnancies. We found that while these criteria effectively reduce the prevalence of LGA neonates through targeted dietary and pharmacological interventions, they concurrently increase the risk of SGA neonates and potentially elevate NICU admissions. These findings suggest that a tailored approach to managing GDM in twin pregnancies is necessary, incorporating specific diagnostic, glycemic control, and weight management strategies to optimize outcomes for both the mother and neonates. Future research should focus on longitudinal studies to refine these strategies and validate their effectiveness in improving overall perinatal outcomes in twins.
Abbreviations
- ANOVA
Analysis of variance
- aOR
adjusted odds ratio
- ART
Assisted reproductive technology
- BMI
Body mass index
- CI
Confidence interval
- DC
Dichorionic
- GDM
Gestational diabetes mellitus
- GEE
General estimated equation
- GWG
Gestational weight gain
- IADPSG
International Association of Diabetes and Pregnancy Study Groups
- IOM
Institute of Medicine
- LGA
Large for gestational age
- MC
Monochorionic
- NICU
Neonatal intensive care unit
- OGTT
Oral glucose tolerance test
- RDS
Respiratory distress syndrome
- SGA
Small for gestational age
Appendix table 1. Impact of GDM on other neonatal outcomes in twin pregnancies (n = 2006)
| neonatal outcomes | Non-GDM group (n = 1570) |
GDM group (n = 436) |
OR (95% CI) | aOR (95% CI) | P-value Adjusted | |
|---|---|---|---|---|---|---|
| prematurity | < 37 weeks | 992(63.2) | 268(61.5) | 0.93(0.68,1.27) | 0.94(0.69,1.29) | 0.712 |
| < 34 weeks | 166(10.6) | 58(13.3) | 1.30(0.83,2.04) | 1.39(0.87,2.21) | 0.164 | |
| sex(male) | 781(49.7) | 206(47.2) | 0.90(0.71,1.14) | 0.87(0.68,1.11) | 0.260 | |
| RDS | 113(7.2) | 38(8.7) | 1.23(0.76,1.99) | 1.31(0.80,2.14) | 0.289 | |
| neonatal asphyxia | 47(3.0) | 20(4.6) | 1.56(0.80,3.05) | 1.93(0.97,3.87) | 0.063 | |
| delivery weight discordance > 25% | 100(6.4) | 30(6.9) | 1.09(0.60,1.97) | 1.07(0.58,1.98) | 0.836 | |
| hypoglycemia | 57(3.6) | 17(3.9) | 1.08(0.60,1.94) | 1.04(0.58,1.88) | 0.887 | |
| necrotizing enterocolitis | 60(3.8) | 16(3.7) | 0.96(0.51,1.79) | 1.00(0.53,1.87) | 0.995 | |
| neonatal infection | 55(3.5) | 16(3.7) | 1.05(0.57,1.92) | 1.25(0.70,2.31) | 0.472 | |
| neonatal jaundice | 321(20.4) | 90(20.6) | 1.01(0.73,1.41) | 098(0.70,1.38) | 0.912 | |
| neonatal malformation | 20(1.3) | 10(2.3) | 1.82(0.75,4.44) | 1.86(0.75,4.58) | 0.178 | |
| respiratory system diseases | 269(17.1) | 84(19.3) | 1.15(0.83,1.61) | 1.20(0.86,1.69) | 0.281 | |
| hyperbilirubinemia | 55(3.5) | 22(5.0) | 1.46(0.85,2.54) | 1.63(0.94,2.85) | 0.084 | |
Generalized estimating equations (GEE)models adjusted for maternal age, pre-pregnancy BMI, early and middle pregnancy BMI gain, history of GDM, family history of type II diabetes, and geographic differences as covariates. *:P < 0.05
Author contributions
Jue Ma: The conception, design of the work, writing-original draft; Dongjian Yang: Analysis of the data; Juanxiu Lv: Methodology; Shujing Liu: Data curation; Li Gao: Supervision; Yan Bi: Substantively revised the work; Yanlin Wang: The conception & design of the work, funding acquisition.
Funding
This project is supported by the Key Research and Development Program of the Ministry of Science and Technology (2023YFC2705901), Shanghai Municipal Science and Technology Commission (22Y11902300, 23DZ2300300), Shanghai Jiao Tong University STAR Grant (YG2023ZD26, YG2024QNA59).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The institutional review board approved the study (approval reference number: GKLW-A-2024-023-01). Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jue Ma and Dongjian Yang contributed equally to this work.
Contributor Information
Yan Bi, Email: byan98@sjtu.edu.cn.
Yanlin Wang, Email: wyanlin@163.com.
References
- 1.International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy[J]. Diabetes Care. 2010;33(3):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartling L, Dryden DM, Guthrie A, et al. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research[J]. Ann Intern Med. 2013;159(2):123–9. [DOI] [PubMed] [Google Scholar]
- 3.on behalf of the WHO Multi-Country Survey on Maternal and Newborn Health Research Network, Santana DS, Silveira C, et al. Perinatal outcomes in twin pregnancies complicated by maternal morbidity: evidence from the WHO Multicountry Survey on maternal and Newborn Health[J]. BMC Pregnancy Childbirth. 2018;18(1):449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes Screening[J]. N Engl J Med. 2021;384(10):895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gortazar L, Flores-Le Roux JA, Benaiges D, et al. Trends in Prevalence of Diabetes among Twin pregnancies and Perinatal outcomes in Catalonia between 2006 and 2015: the DIAGESTCAT Study[J]. J Clin Med. 2021;10(9):1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu X, Huang C, Wu L et al. Perinatal Outcomes and Related Risk Factors of Single vs Twin Pregnancy Complicated by Gestational Diabetes Mellitus: Meta-Analysis[J]. Computational and Mathematical Methods in Medicine, 2022, 2022: 3557890. [DOI] [PMC free article] [PubMed]
- 7.Tu F, Fei A. Maternal and neonatal outcomes of singleton versus twin pregnancies complicated by gestational diabetes mellitus: a systematic review and meta-analysis[J]. PLoS ONE. 2023;18(1):e0280754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiersch L, Berger H, Okby R, et al. Gestational diabetes mellitus is associated with adverse outcomes in twin pregnancies[J]. Am J Obstet Gynecol. 2019;220(1):102. .e1-102.e8. [DOI] [PubMed] [Google Scholar]
- 9.Lin D, Fan D, Li P, et al. Perinatal outcomes among twin pregnancies with gestational diabetes mellitus: a nine-year retrospective cohort study[J]. Front Public Health. 2022;10:946186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dave ED, Bodnar LM, Vani K, et al. Perinatal outcomes in twin pregnancies complicated by gestational diabetes[J]. Am J Obstet Gynecol MFM. 2021;3(5):100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R BB. U, P S, Screening and diagnosis of gestational diabetes mellitus - relevance to low and middle income countries[J]. Clin Diabetes Endocrinol, 2016, 2. [DOI] [PMC free article] [PubMed]
- 12.ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus[J]. Obstetrics and Gynecology. 2018, 131(2): e49-e64. [DOI] [PubMed]
- 13.Ooi S, Wong VW. Twin pregnancy with gestational diabetes Mellitus: a double whammy?[J]. Diabetes Care. 2018;41(2):e15–6. [DOI] [PubMed] [Google Scholar]
- 14.Fox NS, Gerber RS, Saltzman DH, et al. Glycemic control in twin pregnancies with gestational diabetes: are we improving or worsening outcomes?[J]. The Journal of maternal-fetal & neonatal medicine: the Official Journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies. Int Soc Perinat Obstetricians. 2016;29(7):1041–5. [DOI] [PubMed] [Google Scholar]
- 15.Melamed N, Avnon T, Barrett J, et al. Gestational diabetes in twin pregnancies—a pathology requiring treatment or a benign physiological adaptation?[J]. Am J Obstet Gynecol. 2024;231(1):92–e1044. [DOI] [PubMed] [Google Scholar]
- 16.Diabetes in pregnancy. management from preconception to the postnatal period[EB/OL]//PubMed. (2020-12-16)[2024-07-18]. https://pubmed.ncbi.nlm.nih.gov/32212588/ [PubMed]
- 17.Fishel Bartal M, Sibai BM. Eclampsia in the 21st century[J]. Am J Obstet Gynecol. 2022;226(2):S1237–53. [DOI] [PubMed] [Google Scholar]
- 18.Dai L, Deng C, Li Y, et al. Population-based birth weight reference percentiles for Chinese twins[J]. Ann Med. 2017;49(6):470–8. [DOI] [PubMed] [Google Scholar]
- 19.Mascio DD, Acharya G, Khalil A et al. Birthweight discordance and neonatal morbidity in twin pregnancies: A systematic review and meta-analysis[J]. [DOI] [PubMed]
- 20.National Institute for Health and Care Excellence (Great Britain). Twin and triplet pregnancy[M]. London: National Institute for Health and Care Excellence, 4.
- 21.Liu X, Chen Y, Zhou Q, et al. Utilization of International Association of Diabetes and pregnancy study groups criteria vs. a two-step approach to screening for gestational diabetes mellitus in Chinese women with twin pregnancies[J]. Diabet Med. 2015;32(3):367–73. [DOI] [PubMed] [Google Scholar]
- 22.Hyperglycemia and Adverse Pregnancy Outcomes: The HAPO Study Cooperative Research Group[J]. Obstet Gynecol Surv. 2008;63(10):615–6. [Google Scholar]
- 23.Waters TP, Dyer AR, Scholtens DM, et al. Maternal and neonatal morbidity for women who would be added to the diagnosis of GDM using IADPSG criteria: a secondary analysis of the hyperglycemia and adverse pregnancy outcome Study[J]. Diabetes Care. 2016;39(12):2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGrath RT, Hocking SL, Scott ES, et al. Outcomes of twin pregnancies complicated by gestational diabetes: a meta-analysis of observational studies[J]. J Perinatol. 2017;37(4):360–8. [DOI] [PubMed] [Google Scholar]
- 25.Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis[J]. BMC Pregnancy Childbirth. 2018;18(1):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Mei L, Li L, et al. Maternal and neonatal outcomes of twin pregnancies complicated by gestational diabetes mellitus[J]. Endocrine. 2023;84(2):388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mourad M, Too G, Gyamfi-Bannerman C, et al. Hypertensive disorders of pregnancy in twin gestations complicated by gestational diabetes[J]. J Maternal-Fetal Neonatal Med. 2021;34(5):720–4. [DOI] [PubMed] [Google Scholar]
- 28.Gao L, Lei C, Zhou S, et al. Investigation of optimal gestational weight gain for twin pregnancy in Southwest China: a retrospective study[J]. Sci Rep. 2023;13(1):5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Yan M, Xu Z et al. Maternal and neonatal outcomes in women with twin pregnancies based on gestational weight gain: an updated systematic review and meta-analysis[J]. Pakistan J Med Sci, 2023, 39(4). [DOI] [PMC free article] [PubMed]
- 30.H L, N M, H B, et al. Maternal weight gain and pregnancy outcomes in twin gestations[J]. Am J Obstet Gynecol, 2021, 225(5). [DOI] [PubMed]
- 31.Gao L, Lyu SP, Zhao XR, et al. Systematic management of twin pregnancies to reduce pregnancy complications[J]. Chin Med J. 2020;133(11):1355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung T, Hsieh T, Shaw SW, et al. Risk factors and adverse maternal and perinatal outcomes for women with dichorionic twin pregnancies complicated by gestational diabetes mellitus: a retrospective cross-sectional study[J]. J Diabetes Invest. 2021;12(6):1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong W, Fan X, Hu F, et al. Gestational weight gain and its effects on maternal and neonatal outcome in women with twin pregnancies: a systematic review and Meta-Analysis[J]. Front Pead. 2021;9:674414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berezowsky A, Ardestani S, Hiersch L, et al. Glycemic control and neonatal outcomes in twin pregnancies with gestational diabetes mellitus[J]. Am J Obstet Gynecol. 2023;229(6):682. .e1-682.e13. [DOI] [PubMed] [Google Scholar]
- 35.Harper LM, Mele L, Landon MB et al. Carpenter-Coustan Compared With National Diabetes Data Group Criteria for Diagnosing Gestational Diabetes[J]. Obstetrics & Gynecology, 2016, 127(5): 893–8. [DOI] [PMC free article] [PubMed]
- 36.Rasmussen KM, Yaktine AL. Institute of Medicine (U.S.). Weight gain during pregnancy: reexamining the guidelines[M]. Washington, DC: National Academies; 2009. [PubMed] [Google Scholar]
- 37.S MS. O, T M, Are the Institute of Medicine guidelines for optimal gestational weight gain in twin pregnancies applicable to Japanese women?[J]. J Obstet Gynaecol Res, 2021, 47(1). [DOI] [PubMed]
- 38.Obata S, Shimura M, Misumi T, et al. Weight gain during twin pregnancy with favorable pregnancy outcomes in Japan: a retrospective investigation for new criteria based on perinatal registry data[J]. PLoS ONE. 2021;16(7):e0253596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Standard of Recommendation for Weight Gain during Pregnancy Period. Biomed Environ Sci. 2022;35(10):875–877. 10.3967/bes2022.114. Available at https://pubmed.ncbi.nlm.nih.gov/36443264/. [DOI] [PubMed]
- 40.Lin Lhua, Weng Y lin, Lin Yying et al. Examining the effects of second-and third-trimester gestational weight gain rates on the perinatal outcomes among Chinese twin pregnancies: a retrospective cohort study[J]. BMC Pregnancy and Childbirth, 2022, 22(1): 137. [DOI] [PMC free article] [PubMed]
- 41.Lin D, Fan D, Wu S, et al. The effect of gestational weight gain on perinatal outcomes among Chinese twin gestations based on Institute of Medicine guidelines[J]. BMC Pregnancy Childbirth. 2019;19(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Wen L, Zheng Y, et al. Association between Gestational Weight Gain and pregnancy complications or adverse delivery outcomes in Chinese Han Dichorionic Twin pregnancies: validation of the Institute of Medicine (IOM) 2009 Guidelines[J]. Med Sci Monit. 2018;24:8342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mei Y, Yu J, Wen L, et al. Perinatal outcomes and offspring growth profiles in twin pregnancies complicated by gestational diabetes mellitus: a longitudinal cohort study[J]. Diabetes Res Clin Pract. 2021;171:108623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.