Abstract
Background
Sepsis is a frequent reason for ICU admission and a leading cause of death. Its incidence has been increasing over the past decades. While hospital mortality is decreasing, it is recognized that the sequelae of sepsis extend well beyond hospitalization and are associated with a high mortality rate that persists years after hospitalization. The aim of this study was to disentangle the relative contribution of sepsis (infection with multi-organ failure), of infection and of inflammation, as reasons for ICU admission to long-term survival. This was done as infection and inflammation are both cardinal features of sepsis. We assessed the 3-year mortality of ICU patients admitted with sepsis, with individually matched ICU patients with an infection but not sepsis, and with an inflammatory illness not caused by infection, discharged alive from hospital.
Methods
A multicenter cohort study of adult ICU survivors admitted between January 1st 2007 and January 1st 2019, with sepsis, an infection or an inflammatory illness. Patients were classified within the first 24 h of ICU admission according to APACHE IV admission diagnoses. Dutch ICUs (n = 78) prospectively recorded demographic and clinical data of all admissions in the NICE registry. These data were linked to a health care insurance claims database to obtain 3-year mortality data. To better understand and distinct the sepsis cohort from the non-sepsis infection and inflammatory condition cohorts, we performed several sensitivity analyses with varying definitions of the infection and inflammatory illness cohort.
Results
Three-year mortality after discharge was 32.7% in the sepsis (N = 10,000), 33.6% in the infectious (N = 10,000), and 23.8% in the inflammatory illness cohort (N = 9997). Compared with sepsis patients, the adjusted HR for death within 3 years after hospital discharge was 1.00 (95% CI 0.95–1.05) for patients with an infection and 0.88 (95% CI 0.83–0.94) for patients with an inflammatory illness.
Conclusions
Both sepsis and non-sepsis infection patients had a significantly increased hazard rate of death in the 3 years after hospital discharge compared with patients with an inflammatory illness. Among sepsis and infection patients, one third died in the next 3 years, approximately 10% more than patients with an inflammatory illness. The fact that we did not find a difference between patients with sepsis or an infection suggests that the necessity for an ICU admission with an infection increases the risk of long-term mortality. This result emphasizes the need for greater attention to the post-ICU management of sepsis, infection, and severe inflammatory illness survivors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05165-x.
Keywords: Sepsis survivors, Long-term mortality, Infection, Inflammatory illness
Background
Sepsis is a frequent reason for ICU admission and a leading cause of death in the intensive care unit (ICU). Furthermore, its incidence has been increasing over the past decades [1–3]. While hospital mortality is decreasing, long-term mortality remains high, as many sepsis survivors, i.e. ICU patients discharged alive from hospital, die in the subsequent months [4–7]. It is recognized that the sequelae of sepsis extend well beyond hospitalization and are associated with a high mortality rate that persists years after hospitalization. One-year mortality of sepsis survivors is reported to range from 7 to 44%, and > 2-year mortality is reported to be as high as 53% [5, 8, 9].
It is debated whether this persistent high risk is the effect of sepsis itself or is associated with factors such as multimorbidity, age, and acute illness. A few studies have compared long-term mortality of sepsis survivors with non-sepsis survivors and found that hospitalized sepsis survivors had higher long-term mortality risk compared to non-sepsis survivors [5, 9]. On the other hand, Thompson et al. compared ICU sepsis patients with a non-sepsis cohort and found similar 2-year survival [10]. This stresses the fact that long-term outcome after sepsis reflects a complex interplay between patient characteristics, comorbidities, treatments in the ICU and critical illness itself [6, 11, 12]. Several studies asserted that long-term mortality among sepsis survivors was largely due to comorbidities [6, 13, 14], while others showed that a substantial part of long-term mortality could not be explained by comorbidities but rather by sepsis itself [5, 15]. Others found that acute organ dysfunction was associated with long-term mortality in sepsis survivors [16]. Yet, little is known about the interplay of age, sex, magnitude of acute organ dysfunction, and presence of comorbidities on long-term outcome in sepsis [17, 18]. Both facts, the premorbid states that increase the risk of developing sepsis and the sepsis itself that induces multi-organ dysfunction, may contribute to the increased post-sepsis mortality risk.
The aim of this study was to disentangle the relative contribution of sepsis (infection with multi-organ failure), of infection and of inflammation, to long-term survival of sepsis patients after ICU admission. This was done as infection and inflammation are both cardinal features of sepsis.
We assessed the 3-year mortality of ICU survivors admitted to the ICU with sepsis and compared this with the 3-year mortality of two individually matched control groups, i.e. patients admitted to the ICU with a non-sepsis infection and ICU patients with a non-infectious inflammatory illness, all discharged alive from hospital.
Methods
Study design, study population
We performed a multicenter cohort study using prospectively recorded data from the NICE registry, containing demographic, physiologic and clinical data of ICU patients admitted to 78 participating ICUs [19, 20]. The participating ICUs were mixed medical and surgical ICUs in university, teaching and nonteaching hospitals.
All consecutive patients (> = 18 years) admitted with sepsis, a non-sepsis infection or an inflammatory illness to one of the ICUs in the Netherlands between January 1st 2007 and January 1st 2019, were selected (N = 215,371) (Supplement Fig. 1). The health insurance claims database Vektis was used to obtain long-term outcomes. In case of one or more readmissions during the same hospitalization period, only the first ICU admission was included.
Exclusion criteria were a non-valid APACHE IV probability score and missing or non-valid information on age, sex, or ICU length of stay. For the final selection of the three cohorts of hospital survivors, missing linkage or linkage discrepancies between NICE and Vektis, and death during ICU- or hospital stay were additional exclusion criteria (remaining N = 128,356).
Patients in the last-mentioned selection were classified as follows: (1) Sepsis pool (N = 47,897): patients with at least one sepsis diagnosis in the APACHE IV model as reason for ICU admission, i.e. cutaneous/soft tissue-, gastro-intestinal-, gynecologic-, pulmonary-, renal/urinary tract (including bladder)-, other-, or unknown sepsis. (2) Non-sepsis infection pool (N = 35,888): patients with a proven infection in the first 24 h of ICU admission (i.e. “proven infection: yes/no” is a mandatory item registered in the NICE registry) and at least one infection diagnosis in the APACHE IV model as reason for ICU admission, with exception of the sepsis and inflammatory condition APACHE IV diagnoses. (3) Inflammatory illness pool (N = 44,571): patients with an inflammatory illness diagnosis in the APACHE IV model as reason for ICU admission, with exception of the sepsis and infection APACHE IV diagnoses and without a proven infection in the first 24 h of ICU admission, e.g. patients with traumatic injury, certain fractures, burns, pancreatitis, inflammatory bowel disease, or connective tissue disease. These inflammatory diagnoses were based on the diagnoses used in the study by Prescott et al. [5] and reflect the concept of a non-infectious inflammatory response described in the 2001consensus definitions of sepsis [21]. Three board-certified intensivists defined the above-mentioned three pools (SA, DdL, JvP in Contribution) based on APACHE IV reasons for ICU admission diagnoses, and differences in categorization were resolved through consensus (Supplement Table 1). Coding (of e.g. admission diagnoses, comorbidities, acute diagnoses < 24 h of admission) in the Netherlands is done by board-certified intensivists. In the Netherlands there is a nation-wide training for intensivists, organized multiple times per year by the NICE registry, emphasizing correct registration, and contributing to correct categorization of the reason for admission [22]. Also, there are extensive quality checks of the data, organized by the NICE registry, including yearly on-site quality checks for all ICU’s contributing to the NICE registration [23]. Furthermore, our way of coding is hierarchical and mutually exclusive. Per patient a maximum of two admission diagnoses could be recorded in the NICE registry. Both diagnoses were weighted equally. The hierarchy was such that when the first or second admission diagnosis was one of the seven septic diagnoses, a patient was included in the sepsis pool. Awareness of sepsis in the Netherlands among the intensive care community is high, contributing to correct coding of sepsis patients. Secondly, if an infection (but not sepsis) diagnosis was assigned to the patient and the mandatory question, demanding: “proven infection: yes/no ?” was answered in a confirmative way, then the patient was assigned to the infection pool. Thirdly, when a patient had one of the inflammatory condition diagnoses as admission diagnosis, and no sepsis nor confirmed infection, then the patient was assigned to the inflammatory illness pool. This way patients were assigned to one group only, based on their ICU admission diagnosis.
We performed two steps to create from these three pools three comparable cohorts:
First, the treatment of sepsis patients in the hospital has been shown to have a demonstrable impact on their health status at discharge and affects long-term mortality. It is noteworthy that some patients survive their hospital stay despite their high risk of hospital mortality, while other survivors maintained a consistently lower risk throughout their entire hospitalization period. To account for the health status of the included patients at the time of ICU discharge, we calculated a prognostic score for hospital survival. This was done using the data of all surviving and non-surviving patients with valid data (N = 187,570). This prognostic score was used in a matching procedure to create three cohorts, that were eventually selected for the final analyses, comparable with respect to their estimated chance of hospital survival at the time of ICU admission.
The calculation of the prognostic score was based on a logistic regression model with hospital death as outcome and with the following predictors: type of hospital (university, teaching, nonteaching), year of ICU admission (2007–2019), APACHE III Acute Physiological Score (quintiles), Body Mass Index (quintiles), admission type (medical, elective surgery or urgent surgery), acute diagnoses present at admission (cardiopulmonary resuscitation (y/n), gastrointestinal bleeding (y/n), diabetes (y/n)), indicators of vital organ failure in the first 24 h of ICU admission (mechanical ventilation (y/n), acute renal failure (y/n), vasoactive drugs (y/n)), and chronic comorbidities present before hospitalization (cardiovascular insufficiency, chronic respiratory insufficiency, renal insufficiency, malignancy, immuno-deficiency, cirrhosis) (for definitions see Supplement Table 2).
Second, from the group of eligible sepsis patients (N = 47,897), we randomly selected 10,000 patients whom we matched 1:1, on an individual patient level, within strata of the prognostic score (quintiles), age (quintiles), sex, length of ICU stay (tertiles), to patients admitted with an non-sepsis infection and patients with an inflammatory illness. By restriction to 10,000 patients, we were able to find for all patients in the final sepsis cohort a unique match from the pool of infection patients (N = 10,000) and for almost all a unique match from the pool of inflammation patients (N = 9997). This way we were able to construct two comparison cohorts that were very similar to the sepsis cohort (Supplement Fig. 1).
Outcome
The primary outcome was time to death during a follow up of 3 years after hospital discharge. By linking the NICE registry, that includes data until hospital discharge, to the health care insurance claims database of Vektis, we were able to obtain long-term mortality data [24, 25]. Health care insurance is compulsory for all Dutch citizens, hence the Vektis database includes nearly complete coverage of all medical care in the Netherlands. Conform agreement with Vektis, maximum follow-up period is 3 years. The date of death of patients from the Vektis database was deterministically linked to the NICE registry, based on date of birth, gender, hospital of admission, and ICU admission and discharge dates.
Statistical analysis
The observed mortality for the three cohorts, and of the cohorts of the sensitivity analyses, was analyzed using Kaplan–Meier curves. We visually examined whether the survival curves did not cross to assess the proportional hazard assumption. We applied Cox regression analysis to estimate the hazard ratios (HR) of mortality during the 3 years after hospital discharge in the two matched cohorts compared to the sepsis cohort, and in the cohorts of the sensitivity analyses. This was done without and with adjustment for APACHE IV probability, comorbidities, mechanical ventilation, acute renal failure, and use of vasoactive drugs. Namely, matching on identical prognostic score at hospital discharge could have left room for other components to have their effect on long-term outcome, hence our correction for these factors in the analyses of 3-year mortality.
To deepen our insight into the three cohorts we described the most common second APACHE IV reason for ICU admission, if registered.
Sensitivity analyses
With respect to organ failure at admission, and to check whether sepsis patients were sicker and had more organ failure than non-sepsis infection patients, we compared the three cohorts on the available parameters of organ dysfunction (i.e. use of vasoactive drugs within 24 h, need for mechanical ventilation within 24 h, acute renal failure within 24 h, SOFA score per organ system, mean and median SOFA scores, number (%) of patients with > = 2 failing organ systems). We did this for both the initial population (n = 187,570) and the three matched cohorts (n = 29,997). Daily SOFA scores were not recorded by all hospitals as this was facultative and required an addition to the basic registration module (https://www.stichting-nice.nl/dd/#547) [26].
However, in the NICE database, all data on the six organ systems to approach the SOFA score in the first 24 h of ICU admission are available, also for ICUs that do not participate in the daily SOFA module. Based on the definition of Sepsis-3, we calculated the SOFA score in the first 24 h of ICU admission, from here called SOFAfirst24. Organ dysfunction can be identified as an acute change in total SOFA score > = 2 points consequent to the infection. We assumed the baseline SOFA score to be zero in patients not known to have preexisting organ dysfunction [27].
We first performed a sensitivity analysis on patients admitted to hospitals that registered daily SOFA scores, followed by two sensitivity analyses on the calculated SOFAfirst24. Finally, we performed a sensitivity analysis on the cohort of inflammatory illnesses.
We considered that the infection cohort could include septic patients or patients becoming septic during their ICU stay and developing multi-organ failure. Therefore, we performed an analysis comparing infection patients with a valid SOFA score and without emerging sepsis during the ICU stay, as evidenced by a delta SOFA score < 2 during their ICU stay, with their individually matched sepsis patients.
To make a greater distinction between the sepsis group and the non-sepsis infection group we identified a subgroup of sepsis patients with SOFAfirst24 > = 2, with mechanical ventilation and use of vasoactive medication in the first 24 h of admission. We matched these with infection patients with SOFAfirst24 < 2, without mechanical ventilation and without vasoactive medication in the first 24 h of admission, and with patients with an inflammatory illness and compared their 3-year mortality using Kaplan Meier curves.
We separated the sepsis and infection patients by the number of failing organ systems: after having calculated the SOFAfirst24, we matched patients with sepsis and 2 or more failing organ systems (defined, according to SEPSIS-3 by a SOFAfirst24 > = 2 per organ system) with patients with an infection and one or no failing organ system and with patients with an inflammatory illness. We compared their 3-year mortality using Kaplan Meier curves.
We considered that the cohort of inflammatory illnesses included a mix of conditions, that could have induced more or less inflammation and hence could have induced different effects on 3-year mortality. Therefore, we performed a sub analysis on patients with diagnoses limited to universally accepted inflammatory illnesses (i.e. inflammatory bowel disease, pancreatitis, acute liver failure, rheumatoid arthritis, (mixed) connective tissue disease, systemic lupus, (other) musculoskeletal disorders, vasculitis, surgery for pancreatitis). Next, we performed a sub analysis on patients with diagnoses limited to surgery for severe chest, abdomen, pelvis, extremity and multiple trauma, as these diagnoses are known to cause inflammation.
P values < 0.05 were considered statistically significant. The statistical analyses were performed using statistical environment R, version 3.4.3 (R Foundation) for Statistical Computing, Vienna, Austria).
Ethical considerations
The Institutional Research Board of the Academic Medical Center, AMC, Amsterdam, The Netherlands, reviewed the research proposal and concluded that the anonymized data were not subject to the Dutch Research on Human Subjects Act (in Dutch “WMO”) and waived the need for informed consent (IRB protocol W18_318).
Results
With respect to demographics, 58% consisted of male patients and the median age and Inter Quartile Range (IQR) was 68 (57–76). Median APACHE IV probability (IQR) was 0.203 (0.104–0.369), 0.197 (0.098–0.358) and 0.22 (0.081–0.499) for sepsis, infection and inflammatory illness patients, respectively. For characteristics of the three cohorts see Tables 1 and 2.
Table 1.
Variables used to create prognostic index in the three study cohorts (sepsis, infection, inflammatory illness)
| Group 1 sepsis | Group 2 infection | Group 2 versus 1 p value |
Group 3 inflammatory illness | Group 3 versus 1 p value |
|
|---|---|---|---|---|---|
| Number of patients n (%) | 71,477 (59.3) | 49,125 (40.7) | 66,968 (48.4) | ||
| Type of hospital: university n (%) | 8088 (11.3) | 7117 (14.5) | < 0.001 | 17,063 (25.5) | < 0.001 |
| Type of hospital: non-university, teaching n (%) | 30,484 (42.6) | 21,192 (43.1) | 28,376 (42.4) | ||
| Type of hospital: non-teaching n (%) | 32,753 (45.8) | 20,768 (42.3) | 21,312 (31.8) | ||
| Type of hospital: unknown n (%) | 152 (0.2) | 48 (0.1) | 217 (0.3) | ||
| Calendar year of ICU admission: 2007 n (%) | 1787 (2.5) | 1322 (2.7) | < 0.001 | 1988 (3) | < 0.001 |
| Calendar year of ICU admission: 2008 n (%) | 3190 (4.5) | 2099 (4.3) | 2886 (4.3) | ||
| Calendar year of ICU admission: 2009 n (%) | 4699 (6.6) | 2952 (6) | 4368 (6.5) | ||
| Calendar year of ICU admission: 2010 n (%) | 5246 (7.3) | 3422 (7) | 4621 (6.9) | ||
| Calendar year of ICU admission: 2011 n (%) | 5566 (7.8) | 3627 (7.4) | 4906 (7.3) | ||
| Calendar year of ICU admission: 2012 n (%) | 5973 (8.4) | 3788 (7.7) | 5375 (8) | ||
| Calendar year of ICU admission: 2013 n (%) | 6244 (8.7) | 4203 (8.6) | 5779 (8.6) | ||
| Calendar year of ICU admission: 2014 n (%) | 6908 (9.7) | 4208 (8.6) | 6125 (9.1) | ||
| Calendar year of ICU admission: 2015 n (%) | 6858 (9.6) | 4589 (9.3) | 6130 (9.2) | ||
| Calendar year of ICU admission: 2016 n (%) | 6473 (9.1) | 5129 (10.4) | 6469 (9.7) | ||
| Calendar year of ICU admission: 2017 n (%) | 6139 (8.6) | 4788 (9.7) | 6022 (9) | ||
| Calendar year of ICU admission: 2018 n (%) | 6429 (9) | 4771 (9.7) | 6075 (9.1) | ||
| Calendar year of ICU admission: 2019 n (%) | 5965 (8.3) | 4227 (8.6) | 6224 (9.3) | ||
| BMI: < 18.5 n (%) kg/m2 | 2475 (3.5) | 2291 (4.7) | < 0.001 | 1473 (2.2) | < 0.001 |
| BMI: 18.5–25 n (%) kg/m2 | 27,829 (38.9) | 20,378 (41.5) | 28,577 (42.7) | ||
| BMI: 25–30 n (%) kg/m2 | 22,070 (30.9) | 14,635 (29.8) | 22,000 (32.9) | ||
| BMI: 30–35 n (%) kg/m2 | 8948 (12.5) | 5515 (11.2) | 6780 (10.1) | ||
| BMI: 35–40 n (%) kg/m2 | 3340 (4.7) | 2016 (4.1) | 2054 (3.1) | ||
| BMI: > 40 n (%) kg/m2 | 2529 (3.5) | 1517 (3.1) | 1091 (1.6) | ||
| BMI: unknown n (%) kg/m2 | 4286 (6) | 2773 (5.6) | 4993 (7.5) | ||
| BMI: mean (SD) kg/m2 | 26.74 (5.98) | 26.23 (5.89) | 0 | 26.10 (4.86) | 0 |
| BMI: median (IQR) kg/m2 | 25.712 (22.918–29.385) | 25.209 (22.498–28.732) | 25.249 (23.148–28.028) | ||
| Admission type: 1. Medical n (%) | 55,534 (77.7) | 35,650 (72.6) | < 0.001 | 54,857 (81.9) | < 0.001 |
| Admission type: 2. Surgery urgent n (%) | 12,747 (17.8) | 10,478 (21.3) | 8612 (12.9) | ||
| Admission type: 3.Surgery elective n (%) | 3196 (4.5) | 2997 (6.1) | 3499 (5.2) | ||
| APACHE III APS: quintile < 20% n (%) | 10,487 (14.7) | 12,587 (25.6) | < 0.001 | 23,715 (35.4) | < 0.001 |
| APACHE III APS: quintile 20–40% n (%) | 12,283 (17.2) | 11,144 (22.7) | 8037 (12) | ||
| APACHE III APS: quintile 40–60% n (%) | 14,939 (20.9) | 10,847 (22.1) | 6094 (9.1) | ||
| APACHE III APS: quintile 60–80% n (%) | 15,670 (21.9) | 8368 (17) | 6849 (10.2) | ||
| APACHE III APS: quintile 80–100% n (%) | 18,098 (25.3) | 6179 (12.6) | 22,273 (33.3) | ||
| APACHE III APS: Mean (SD) | 66.76 (29.88) | 54.89 (24.5) | 0 | 63.89 (39.17) | 0 |
| APACHE III APS: Median (IQR) | 62 (46–82) | 51 (38–67) | 54 (31–95) | ||
| CPR: yes n (%) | 1612 (2.3) | 1012 (2.1) | 0.0236 | 26,712 (39.9) | < 0.001 |
| Gastro-intestinal bleeding: yes n (%) | 863 (1.2) | 444 (0.9) | < 0.001 | 1040 (1.6) | < 0.001 |
| Mechanical ventilation: yes n (%) | 35,701 (49.9) | 30,173 (61.4) | < 0.001 | 40,919 (61.1) | < 0.001 |
| Acute renal failure: yes n (%) | 23,047 (32.2) | 6986 (14.2) | < 0.001 | 6622 (9.9) | < 0.001 |
| Vaso-active drugs: yes n (%) | 48,683 (68.1) | 21,637 (44) | < 0.001 | 31,959 (47.7) | < 0.001 |
| Diabetes: yes n (%) | 16,137 (22.6) | 8969 (18.3) | < 0.001 | 7994 (11.9) | < 0.001 |
| Immuno-deficiency: yes n (%) | 12,094 (16.9) | 7600 (15.5) | < 0.001 | 2289 (3.4) | < 0.001 |
| Renal insufficiency: yes n (%) | 7917 (11.1) | 3723 (7.6) | < 0.001 | 2980 (4.4) | < 0.001 |
| Chronic respiratory insufficiency: yes n (%) | 13,464 (18.8) | 14,714 (30) | < 0.001 | 7042 (10.5) | < 0.001 |
| Malignancy: yes n (%) | 7940 (11.1) | 4361 (8.9) | < 0.001 | 1534 (2.3) | < 0.001 |
| Cardiovascular insufficiency: yes n (%) | 3205 (4.5) | 1870 (3.8) | < 0.001 | 3152 (4.7) | 0.0493 |
| Cirrhosis: yes n (%) | 1706 (2.4) | 686 (1.4) | < 0.001 | 1329 (2) | < 0.001 |
| 12,094 (16.9) | |||||
| Variables included in matching | |||||
| Age: quintile < 20% n (%) | 13,803 (19.3) | 11,268 (22.9) | < 0.001 | 24,275 (36.2) | < 0.001 |
| Age: quintile 20–40% n (%) | 14,684 (20.5) | 10,717 (21.8) | 12,728 (19) | ||
| Age: quintile 40–60% n (%) | 12,607 (17.6) | 8507 (17.3) | 8949 (13.4) | ||
| Age: quintile 60–80% n (%) | 15,479 (21.7) | 9769 (19.9) | 9796 (14.6) | ||
| Age: quintile 80–100% n (%) | 14,904 (20.9) | 8864 (18) | 11,220 (16.8) | ||
| Age: mean (SD) years | 66.64 (14.35) | 64.91 (15.09) | 0 | 60.03 (18.72) | 0 |
| Age: median (IQR) years | 69 (59–77) | 67 (57–76) | 63 (48–74) | ||
| Sex: female n (%) | 30,871 (43.2) | 20,147 (41) | < 0.001 | 21,785 (32.5) | < 0.001 |
| Sex: male n (%) | 40,606 (56.8) | 28,978 (59) | 45,183 (67.5) | ||
| LOS ICU: quintile < 33% n (%) | 23,817 (33.3) | 14,761 (30) | < 0.001 | 27,734 (41.4) | < 0.001 |
| LOS ICU: quintile 33–66% n (%) | 23,834 (33.3) | 16,205 (33) | 22,555 (33.7) | ||
| LOS ICU: quintile > 66% n (%) | 23,826 (33.3) | 18,159 (37) | 16,679 (24.9) | ||
| LOS ICU: mean (SD) days | 6.36 (10.5) | 6.59 (10.52) | 0 | 4.63 (8.36) | 0 |
| LOS ICU: median (IQR) days | 2.781 (1.212–6.894) | 3.133 (1.399–7.475) | 2.107 (0.896–4.872) |
BMI, body mass index; APS, acute physiological score; LOS, length of stay; ICU, intensive care unit
Table 2.
Variables included in matching of the three cohorts (sepsis, infection, inflammatory illness)
| Group 1 sepsis | Group 2 infection | Group 2 versus 1 p value |
Group 3 inflammatory illness | Group 3 versus 1 p value |
|
|---|---|---|---|---|---|
| Number of patients n | 10,000 | 10,000 | 9997 | ||
| Variables included in the matching: | |||||
| Age: quintile < 20% n (%) years | 1970 (19.7) | 1970 (19.7) | 1 | 1970 (19.7) | 1 |
| Age: quintile 20–40% n (%) years | 1897 (19) | 1897 (19) | 1897 (19) | ||
| Age: quintile 40–60% n (%) years | 1992 (19.9) | 1992 (19.9) | 1989 (19.9) | ||
| Age: quintile 60–80% n (%) years | 2112 (21.1) | 2112 (21.1) | 2112 (21.1) | ||
| Age: quintile 80–100% n (%) years | 2029 (20.3) | 2029 (20.3) | 2029 (20.3) | ||
| Age: mean (SD) years | 65.01 (14.72) | 64.89 (14.98) | 0.9172 | 64.38 (16.42) | 0.5243 |
| Age: median (IQR) years | 68 (57–76) | 68 (57–76) | 68 (56–76) | ||
| Sex: female n (%) | 4203 (42) | 4203 (42) | 1 | 4200 (42) | 1 |
| Sex: male n (%) | 5797 (58) | 5797 (58) | 5797 (58) | ||
| Prognostic score: quintile < 20% n (%) | 1996 (20) | 1996 (20) | 1 | 1996 (20) | 1 |
| Prognostic score: quintile 20–40% n (%) | 1962 (19.6) | 1962 (19.6) | 1962 (19.6) | ||
| Prognostic score: quintile 40–60% n (%) | 2025 (20.2) | 2025 (20.2) | 2022 (20.2) | ||
| Prognostic score: quintile 60–80% n (%) | 2019 (20.2) | 2019 (20.2) | 2019 (20.2) | ||
| Prognostic score: quintile 80–100% n (%) | 1998 (20) | 1998 (20) | 1998 (20) | ||
| Prognostic score: mean (SD) | 0.21 (0.18) | 0.21 (0.17) | 0.5148 | 0.23 (0.2) | 0.7745 |
| Prognostic score: median (IQR) | 0.158 (0.085–0.286) | 0.157 (0.085–0.284) | 0.157 (0.084–0.298) | ||
| LOS ICU: quintile < 33% n (%) | 3262 (32.6) | 3262 (32.6) | 1 | 3262 (32.6) | 1 |
| LOS ICU: quintile 33–66% n (%) | 3330 (33.3) | 3330 (33.3) | 3330 (33.3) | ||
| LOS ICU: quintile > 66% n (%) | 3408 (34.1) | 3408 (34.1) | 3405 (34.1) | ||
| LOS ICU: mean (SD) days | 6.38 (10.34) | 6.29 (10.49) | 0.9684 | 5.99 (9.69) | 0.0704 |
| LOS ICU: median (IQR) days | 2.849 (1.449–6.867) | 2.941 (1.37–6.775) | 2.892 (1.253–6.499) |
LOS, length of stay; ICU, intensive care unit
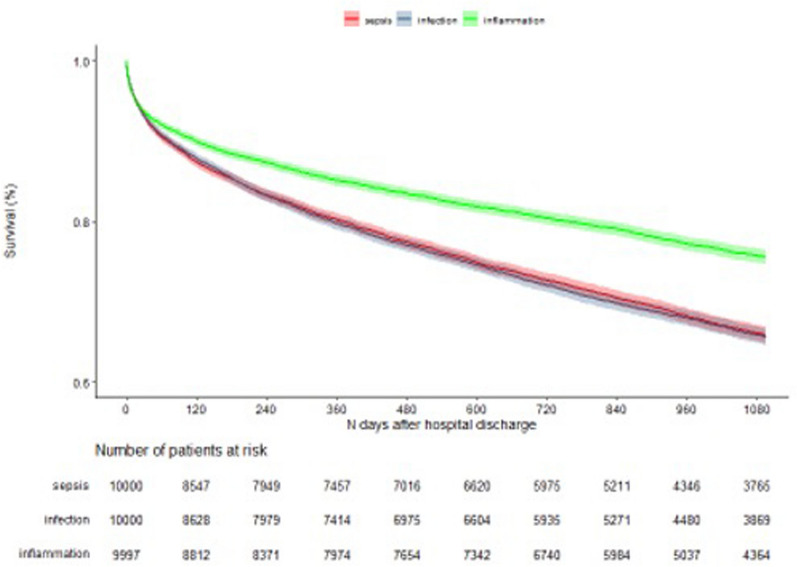
Three-year mortality was 32.7% in the sepsis, 33.6% in the infection and 23.8% in the inflammatory illness cohort. Compared with sepsis patients, the crude HR of death during the 3 years after hospital discharge for infection patients was 1.01 (95% CI 0.97–1.07) and for inflammatory illness patients 0.68 (95% CI 0.65–0.72). Compared with sepsis patients, the adjusted HR for death within 3 years after hospital discharge for infection patients was 1.00 (95% CI 0.95–1.05) and for inflammatory illness patients 0.88 (95% CI 0.83–0.94) (Fig. 1, Table 3).
Fig. 1.
Kaplan Meier 3-year survival of sepsis, infection and inflammatory condition patients
Table 3.
Hazard ratio of 3-year mortality of sepsis, infection and inflammatory condition patients
| Group 1: sepsis = reference | Group 2: infection HR [95% CI] | Group 2 versus 1: Wald p value | Group 3: inflammatory condition HR [95% CI] | Group 3 versus 1: Wald p value | |
|---|---|---|---|---|---|
| Cox regression of 3-year mortality | |||||
| Crude | 1 [1–1] | 1.01 [0.97–1.07] | 0.5710073 | 0.68 [0.65–0.72] | < 0.001 |
| Cox regression of 3-year mortality | |||||
| Adjusted for chronic comorbidity | 1 [1–1] | 0.97 [0.92–1.02] | 0.2538482 | 0.83 [0.79–0.88] | < 0.001 |
| Cox regression of 3-year mortality | |||||
| Adjusted for APACHE-IV probability | 1 [1–1] | 1.03 [0.98–1.08] | 0.3251795 | 0.68 [0.65–0.72] | < 0.001 |
| Cox regression of 3-year mortality | |||||
| Adjusted for chronic comorbidities and APACHE-IV probability | 1 [1–1] | 0.98 [0.93–1.03] | 0.3616948 | 0.83 [0.78–0.88] | < 0.001 |
| Cox regression of 3-year mortality | |||||
| Adjusted for chronic comorbidities, APACHE-IV probability, mechanical ventilation, acute renal failure, vasoactive drugs | 1 [1–1] | 1 [0.95–1.05] | 1 | 0.88 [0.83–0.94] | < 0.001 |
Chronic comorbidities includes: cardiovascular insufficiency, chronic respiratory insufficiency, renal insufficiency, malignancy, immuno-deficiency, cirrhosis
Second reason for ICU admission and organ failure in the three study cohorts
For the three cohorts of 10,000, 10,000 and 9997 patients, 36.5% of the sepsis patients, 51.7% of the infection patients, and 57.7% of the inflammatory condition patients did not have a second diagnosis. The most common second reasons for ICU admission are shown in Supplement Table 3 and Supplement Table 4.
Sensitivity analyses
Compared with infection patients, sepsis patients had a higher incidence of use of vasoactive drugs within 24 h of admission, 68.1% versus 44% (p < 0.001), and a higher incidence of acute renal failure within 24 h of admission, 32.2% versus 14.2% (p < 0.001). Also, sepsis patients had a higher mean [SD] SOFA score at admission, compared to patients with an infection, 8.5 [3.9] versus 6.7 [3.59], p < 0.001 and had a higher incidence of two or more failing organ systems, 78.8% versus 59.2% (p < 0.001), respectively. Patients with an inflammatory illness had a mean [SD] SOFA score at admission of 8.2 [4.6] and had an incidence of two or more failing organ systems of 68.7%. The level of organ failure in the three groups for the initial population was comparable to the matched cohorts (Supplement Table 5 and Supplement Table 6).
(1) Sepsis patients compared with patients with an infection and delta SOFA < 2
To check whether the diagnosis ‘infection without sepsis’, i.e. without organ failure, was true for patients admitted to the ICU, we compared sepsis patients (n = 1925) with patients with an infection and a delta SOFA < 2 over the ICU period. Compared to their individually matched sepsis patients, the adjusted HR of death for patients with infection with a delta SOFA < 2 (N = 1,925) was 0.98 (95% CI 0.87–1.10) (Supplement Fig. 2, Supplement Table 7).
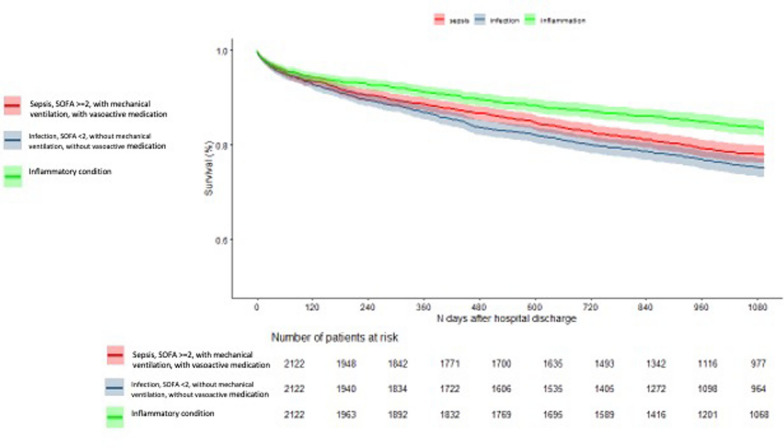
(2) Sepsis patients with SOFAfirst24 > = 2, mechanical ventilation and use of vasoactive medication compared with infection patients with SOFAfirst24 < 2, without mechanical ventilation and without vasoactive medication in the first 24 h of admission.
We matched 2122 sepsis patients with SOFAfirst24 > = 2, with mechanical ventilation and with vasoactive medication in the first 24 h of ICU admission with 2122 patients with an infection and SOFAfirst24 < 2, without mechanical ventilation, and without vasoactive medication in the first 24 h of admission, and with 2212 inflammatory condition patients. The inflammatory illness patients had a better 3-year survival, compared with the other two groups, consistent with the main analysis. Patients with an infection with SOFAfirst24 < 2, without mechanical ventilation, and without vasoactive medication in the first 24 h of admission, had worse 3-year survival compared with sepsis patients with SOFAfirst24 > = 2, with mechanical ventilation and with vasoactive medication (Fig. 2, Supplement Table 8).
Fig. 2.
Kaplan Meier 3-year survival
(3) Sepsis patients with > = 2 failing organ systems compared with infection patients with < 2 failing organ systems
We matched 990 patients with sepsis and 2 or more failing organ systems with 990 patients with an infection and one or no failing organ systems and with 1000 inflammatory condition patients.
Patients with an infection and SOFAfirst24 < 2, without mechanical ventilation, and without vasoactive medication in the first 24 h) had comparable outcomes to sepsis patients with SOFAfirst24 > = 2, with mechanical ventilation and with vasoactive medication. Consistent with the main analyses, this additional analyses showed that sepsis and infection both had equally worse outcome compared to the patients with an inflammatory illness (Supplement Fig. 3, Supplement Table 9).
(4) Sepsis patients compared with subgroups of patients with an inflammatory illness
Patients with a universally accepted diagnosis of severe inflammatory illness (n = 1108) were individually matched to sepsis patients (n = 1108). Compared to sepsis patients, the inflammatory illness patients had an increased mortality rate in the 3 years of follow-up (Supplement Fig. 4).
Patients with a severe trauma diagnosis (n = 618) were individually matched to sepsis patients (n = 618). The trauma patients had a lower mortality rate in the 3 years of follow-up, although in the first period their mortality rate seemed comparable to the sepsis patients (Supplement Fig. 5).
Discussion
In this multicenter cohort study of critically ill patients admitted to the ICU, we assessed the 3-year mortality in survivors admitted to the ICU with sepsis and compared this to individually matched cohorts of non-sepsis infection patients and patients with an inflammatory illness. We found that both sepsis and non-sepsis infection patients had a significantly increased HR of death in the 3 years after hospital discharge compared to patients with an inflammatory condition. Among sepsis and infection patients one third died in the next 3 years, approximately 10% more than patients with an inflammatory condition. However, the subgroup of patients with a severe inflammatory illness had a higher mortality rate in the 3 years of follow-up, compared with sepsis patients.
Findings in context
Our results are consistent with previous studies in sepsis patients that have shown excess long-term mortality after sepsis compared to hospitalized non-sepsis patients [5, 6, 8, 9, 11, 28]. However, past studies, particularly a 2016 systematic review by Shankar-Hari et al., showed that, as the severity of illness in the comparison group increased, this association waned, and sepsis ceased to be an independent predictor of long-term outcome [6]. This was confirmed by the study of Thompson et al. who compared critically ill ICU sepsis and non-sepsis patients and showed that both groups had a similar 2-year survival [10].
Our study advances the existing literature by comparing critically ill sepsis patients with carefully selected and individually matched critically ill patients admitted to the ICU for a non-sepsis infection or an inflammatory illness, the rationale of which makes sense as infection and inflammation are two fundamental features of sepsis. Only one earlier study by Prescott et al. executed this comparison and concluded the independent role of sepsis in long-term mortality after matching and control for confounding [5]. However, this study focused on hospitalized patients, not specifically on ICU patients.
Secondly, our study advances the existing literature by examining a very large, multicenter cohort with extensive information available on demographic, index admission, acute physiological disturbance and acute diagnoses, and comorbidity factors. Also, we calculated a prognostic score estimating the risk of hospital mortality for the three study cohorts and matched rigorously on an individual patient level on important clinical determinants, including this prognostic score, to create comparable cohorts in terms of predicted risk of hospital death among those who survived their hospital stay. Furthermore, we adjusted long-term risk of death in the three matched cohorts for factors that are, according to current understanding, related to long-term outcome, i.e. APACHE IV probability, comorbidities, need for mechanical ventilation, acute renal failure and use of vasoactive drugs.
A striking outcome in our study was the fact that the long-term outcome of patients with sepsis and with an infection did not differ, and that both had worse long-term outcome compared to patients with an inflammatory illness, after matching and controlling for all available confounders. It could have been that infection patients developed sepsis during their ICU stay. However, even when comparing sepsis patients with infection patients with a delta SOFA score < 2 (i.e. without significant organ failure), we did not find a difference in long-term mortality. Our sub analysis on organ failure showed that, although the septic patients had a significant higher incidence of multi-organ failure compared to infection patients, the infection patients were also quite sick, and were also experiencing organ failure, so one could argue that these infection patients were actually already sepsis patients at ICU admission. However, in our sensitivity analyses, where we compared very sick sepsis patients (SOFA > = 2, mechanical ventilation, vasoactive medication within 24 h of admission) with less sick infection patients (SOFA < 2, without mechanical ventilation, without vasoactive medication), it was shown that sepsis and infection patients both still had a worse outcome compared to patients with an inflammatory condition. This was also the case when we focused on the number of failing organ systems of sepsis and infection patients, respectively > = 2 versus < 2 failing organ systems). Both results are consistent with the results of the main analysis. Combining these findings it might be suggested that it is the infection component, with more or less intense organ failure, that is an important determinant of impaired long-term outcome, but future research is needed.
With respect to the analysis of second diagnoses, the high incidence of use of mechanical ventilation and vasoactive medication in the 10,000 infection patients could be compatible with the seven non-infectious second diagnoses. Furthermore, the second diagnoses in the 2122 infection patients (SOFA < 2, without mechanical ventilation and without vasoactive medication) were mostly related to respiratory/pulmonary and cardiac conditions, which could explain the impaired long-term outcome, even worse compared to sepsis patients.
Another striking outcome were the results of the population with inflammatory illnesses. The overall group had a high mean SOFA score and a high incidence of two or more failing organ systems, comparable to sepsis patients, but had better long-term outcome. However, the subgroup of patients with a severe inflammatory condition had higher mortality compared with sepsis patients. Apparently, a severe inflammatory reaction, not caused by an infection, for which a patient needs ICU admission, has an even worse effect on long-term outcome than an inflammatory reaction caused by an infection. Furthermore, we assessed heterogeneity in the inflammatory condition population. Patients with a severe trauma diagnosis, matched on prognostic score, age and comorbidity load, known to cause inflammation, had a lower mortality rate in the 3 years of follow-up, compared with sepsis patients. The inflammatory diagnoses used in our study were based on the diagnoses used in the study by Prescott et al. [5] and reflect the concept of a non-infectious inflammatory response described in the 2001consensus definitions of sepsis. However, this study was performed in hospitalized patients, of whom the level of inflammation could have been less compared to ICU patients. Our results illustrate that we need better biochemical, pathophysiology-based markers of the inflammatory response, both in infectious and non-infectious patients.
Limitations and strengths of the study
There are limitations to our study. First, we used data from a national registry. Although there is an institutionalized, national training program for coding (e.g. admission diagnoses and comorbidities) and meticulous quality control of the data [20, 22, 23, 29], misclassification might have occurred in reason for admission and in recording of comorbidities. Secondly, related to the misclassification, there might have been overlap between the sepsis and infection patients. And although we performed sub analyses to increase our insight in the overlap, our sub analyses, based on SOFA scores and some measures of need for life support, only crudely differentiated the two populations. Thirdly, in this study, ICU readmissions during the same hospitalization episode were not considered while the readmission of patients across different hospitalization episodes were included as, due to legal regulations, we cannot reliably identify these in our data. Fourthly, we had quite extensive information on potential confounders, such as premorbid state and acute physiological disturbance. However, in terms of a complete picture of the patient, i.e. functional status, socioeconomic characteristics, and ethnic origin, we were limited, leaving room for residual confounding. Fifthly, although long-term mortality is a patient-important outcome, other endpoints, such as quality of life and health care utilization, would have been as important, both in terms of burden for the patient as in terms of consistency in our findings. Unfortunately, these other outcomes are not available in our data. Finally, we had no measure for the severity of the systemic inflammatory response which would have made it possible to dissect the three groups on the level of inflammation. On the other hand, we matched on acute physiological disturbance, thus, making the cohorts comparable with respect to this important factor. And we endeavored to increase our insight in the severity of the disturbances caused by the inflammatory response, by performing sub analyses based on SOFA scores, and on patients with universally accepted inflammatory diagnoses and trauma patients.
Strengths of our study are the large study population (with a broad age range compared to the study of Prescott et al. [5]) and the relatively high granular data on acute and chronic comorbidities and many other potential confounders which allowed for robust adjustment and for sub analyses. Also, we compared long-term outcome of sepsis with two relevant comparators, allowing to disentangle the effect of sepsis itself in contrast to two other cardinal features associated with sepsis. Furthermore, although a small country, the Netherlands is up to date with respect to care for the septic patient and countrywide all hospitals adhere to current surviving sepsis guidelines. In our study all types of hospitals were included, as during the study period over 90% of the ICUs provided data to the NICE registry. The latter two factors contribute to the generalizability of the results beyond the Dutch study population and included calendar years.
Interpretation of the results and future research
Our study is adding knowledge to the current dilemma of the contribution of sepsis as opposed to premorbid state, acute physiological disturbances, and the inflammatory response to the impaired long-term outcome of sepsis survivors. One of the more insidious outcomes of patients who survive sepsis is profound immunosuppression and a disbalance between pro- and anti-inflammation [30–35]. This might contribute to the high rates of deaths related to infection [33, 36, 37] cardiovascular disease and cancer that have been described in studies of sepsis survivors [32, 38–40]. We stress that future research should aim at identifying point of care biomarkers of the inflammatory response. And we confirm that future research should aim at unmasking mechanisms by which sepsis and infection, as opposed to inflammation caused by other diseases, complicate the post-sepsis course of ICU survivors, leading to increased long-term mortality; and at increasing insight in what precisely the role of the infectious cause, multi-organ failure and the role of particular organ systems is in critically ill patients with sepsis or an infection. Future studies should focus on the increased incidence of cardiovascular diseases, cancer, and infections in sepsis survivors, e.g. by record linkage of national registries and health care data bases [18]. Finally, our results might have value to patients and their families in terms of expectations about life after ICU admission for sepsis, an infection, or a severe inflammatory illness. They might guide post-ICU care trajectories for ICU survivors of sepsis, non-sepsis infections and severe inflammatory illnesses.
Conclusion
Using a large national cohort of Dutch ICU survivors, we found that patients with sepsis and patients with a non-sepsis infection had a high mortality, persisting over 3 years of follow-up and that this mortality was considerably higher compared to patients with an inflammatory illness. However, patients with a severe inflammatory illness had higher mortality rate in the 3 years of follow-up, compared with sepsis patients. The fact that we did not find a difference in long term outcome between patients with sepsis and a non-sepsis infection, suggests that needing ICU admission with an infection, being it defined as sepsis or as a non-sepsis infection, specifically increases the risk of long-term mortality. Our results also suggest a need for greater attention to the post-discharge management of ICU survivors of sepsis, infections and severe inflammatory illnesses. The mechanisms of this increased long-term mortality remain unclear and warrant future studies as insight in the mechanisms might lead to interventions to prevent deterioration after discharge in these patients.
Supplementary Information
Acknowledgements
We sincerely thank J. van Paassen (Department of Intensive Care, Leiden University Medical Center, The Netherlands) for her contribution to the categorization of the three cohorts.
Author contributions
Concept and design: SMA. Categorization of study population: SMA, DWdL. Drafting of the manuscript: SMA. Critical revision of the manuscript: SMA, FT, SB, DWdL, RJB, OMD, NFdK. Statistical analysis: FT. Interpretation of data: SMA, FT, SB, DWdL, NFdK.
Funding
No funding was received for the research reported.
Availability of data and materials
The data that support the findings of this study are not publicly available due to legal restrictions between the participating hospitals and the NICE registry. They are available according the regulations descripted at: https://www.stichting-nice.nl/extractieverzoeken.jsp (In Dutch) Data are located under controlled data storage at the processor of the NICE foundation; the Department of Medical Informatics, AUMC, Amsterdam, The Netherlands.
Declarations
Ethics approval and consent to participate
The Institutional Research Board of the Academic Medical Center, AMC, Amsterdam, The Netherlands, reviewed the research proposal and concluded that the anonymized data were not subject to the Dutch Research on Human Subjects Act (in Dutch “WMO”) and waived the need for informed consent (IRB protocol W18_318.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kadri SS, et al. Estimating ten-year trends in septic shock incidence and mortality in United States Academic Medical Centers using clinical data. Chest. 2017;151(2):278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer N, et al. Temporal trends in incidence, sepsis-related mortality, and hospital-based acute care after sepsis. Crit Care Med. 2018;46(3):354–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott HC, Kepreos KM, Wiitala WL, Iwashyna TJ. Temporal changes in the influence of hospitals and regional healthcare networks on severe sepsis mortality. Crit Care Med. 2015;43(7):1368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaukonen KM, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–16. [DOI] [PubMed] [Google Scholar]
- 5.Prescott HC, et al. Late mortality after sepsis: propensity matched cohort study. BMJ. 2016;353:i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shankar-Hari M, et al. Evidence for a causal link between sepsis and long-term mortality: a systematic review of epidemiologic studies. Crit Care. 2016;20:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson EK, et al. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Crit Care Med. 2014;42(3):625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winters BD, et al. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38(5):1276–83. [DOI] [PubMed] [Google Scholar]
- 9.Prescott HC, et al. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190(1):62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson K, et al. Health-related outcomes of critically ill patients with and without sepsis. Intensive Care Med. 2018;44(8):1249–57. [DOI] [PubMed] [Google Scholar]
- 11.Farrah K, et al. Sepsis-associated mortality, resource use, and healthcare costs: a propensity-matched cohort study. Crit Care Med. 2021;49(2):215–27. [DOI] [PubMed] [Google Scholar]
- 12.Iwashyna TJ, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4(7):566–73. [DOI] [PubMed] [Google Scholar]
- 13.Garland A, et al. Distinct determinants of long-term and short-term survival in critical illness. Intensive Care Med. 2014;40(8):1097–105. [DOI] [PubMed] [Google Scholar]
- 14.Linder A, et al. Long-term (10-year) mortality of younger previously healthy patients with severe sepsis/septic shock is worse than that of patients with nonseptic critical illness and of the general population. Crit Care Med. 2014;42(10):2211–8. [DOI] [PubMed] [Google Scholar]
- 15.Quartin AA, Schein RM, Kett DH, Peduzzi PN. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277(13):1058–63. [PubMed] [Google Scholar]
- 16.Schuler A, et al. The impact of acute organ dysfunction on long-term survival in sepsis. Crit Care Med. 2018;46(6):843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304(16):1833–4. [DOI] [PubMed] [Google Scholar]
- 18.Munroe E, Prescott HC. Late mortality from sepsis: what we know and what it means. Crit Care Med. 2021;49(2):353–5. 10.1097/CCM.0000000000004795. [DOI] [PubMed] [Google Scholar]
- 19.Dutch National Intensive Care Evaluation (NICE) Foundation. Cited 1 Oct 2019.
- 20.van de Klundert N, Holman R, Dongelmans DA, de Keizer NF. Data resource profile: the Dutch National Intensive Care Evaluation (NICE) Registry of admissions to adult intensive care units. Int J Epidemiol. 2015;44(6):1850–1850h. [DOI] [PubMed] [Google Scholar]
- 21.Levy MM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003;29(4):530–8. [DOI] [PubMed] [Google Scholar]
- 22.Arts DG, Bosman RJ, de Jonge E, Joore JC, de Keizer NF. Training in data definitions improves quality of intensive care data. Crit Care. 2003;7(2):179–84. 10.1186/cc1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arts D, de Keizer N, Scheffer GJ, de Jonge E. Quality of data collected for severity of illness scores in the Dutch National Intensive Care Evaluation (NICE) registry. Intensive Care Med. 2002;28(5):656–9. 10.1007/s00134-002-1272-z. [DOI] [PubMed] [Google Scholar]
- 24.Vektis National Database: Dutch health care information center of national insurance companies (Vektis BV. Zeist, The Netherlands). Dutch Health Care Information Center of the National Insurance Companies (Vektis BV, Zeist, The Netherlands). Cited 1 Oct 2019.
- 25.Roos LL, Wajda A. Record linkage strategies. Part I: estimating information and evaluating approaches. Methods Inf Med. 1991;30(2):117–23. [PubMed] [Google Scholar]
- 26.Arts DG, de Keizer NF, Vroom MB, de Jonge E. Reliability and accuracy of Sequential Organ Failure Assessment (SOFA) scoring. Crit Care Med. 2005;33(9):1988–93. 10.1097/01.ccm.0000178178.02574.ab. [DOI] [PubMed] [Google Scholar]
- 27.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10. 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lone NI, et al. Five-year mortality and hospital costs associated with surviving intensive care. Am J Respir Crit Care Med. 2016;194(2):198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koetsier A, et al. Reliability of in-hospital mortality as a quality indicator in clinical quality registries. A case study in an intensive care quality register. Methods Inf Med. 2013;52(5):432–40. [DOI] [PubMed] [Google Scholar]
- 30.Cavassani KA, et al. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood. 2010;115(22):4403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arens C, et al. Sepsis-induced long-term immune paralysis–results of a descriptive, explorative study. Crit Care. 2016;20:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yende S, et al. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177(11):1242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamim CF, Hogaboam CM, Kunkel SL. The chronic consequences of severe sepsis. J Leukoc Biol. 2004;75(3):408–12. [DOI] [PubMed] [Google Scholar]
- 34.van Vught LA, et al. Comparative analysis of the host response to community-acquired and hospital-acquired pneumonia in critically ill patients. Am J Respir Crit Care Med. 2016;194(11):1366–74. [DOI] [PubMed] [Google Scholar]
- 35.Yende S, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2(8):e198686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313(10):1055–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeMerle KM, Royer SC, Mikkelsen ME, Prescott HC. Readmissions for recurrent sepsis: new or relapsed infection? Crit Care Med. 2017;45(10):1702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mankowski RT, Yende S, Angus DC. Long-term impact of sepsis on cardiovascular health. Intensive Care Med. 2019;45(1):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yende S, et al. Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;189(9):1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ou SM, et al. Long-term mortality and major adverse cardiovascular events in sepsis survivors. A nationwide population-based study. Am J Respir Crit Care Med. 2016;194(2):209–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to legal restrictions between the participating hospitals and the NICE registry. They are available according the regulations descripted at: https://www.stichting-nice.nl/extractieverzoeken.jsp (In Dutch) Data are located under controlled data storage at the processor of the NICE foundation; the Department of Medical Informatics, AUMC, Amsterdam, The Netherlands.