Abstract
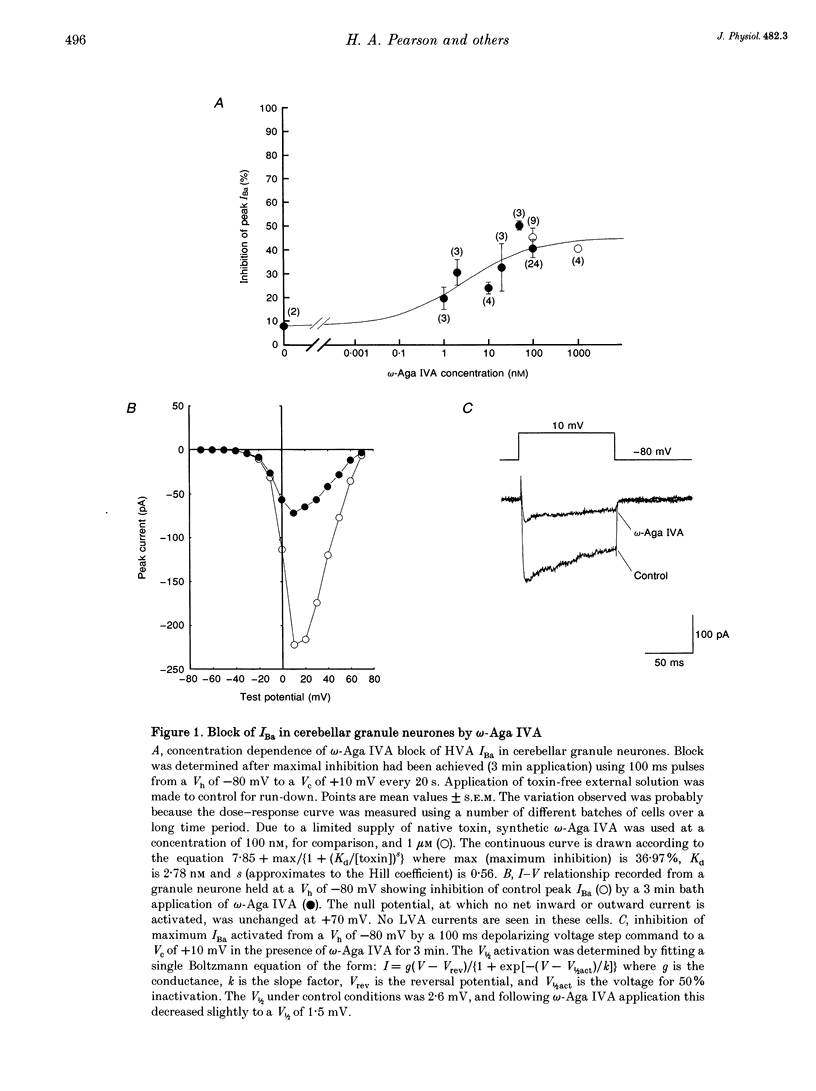
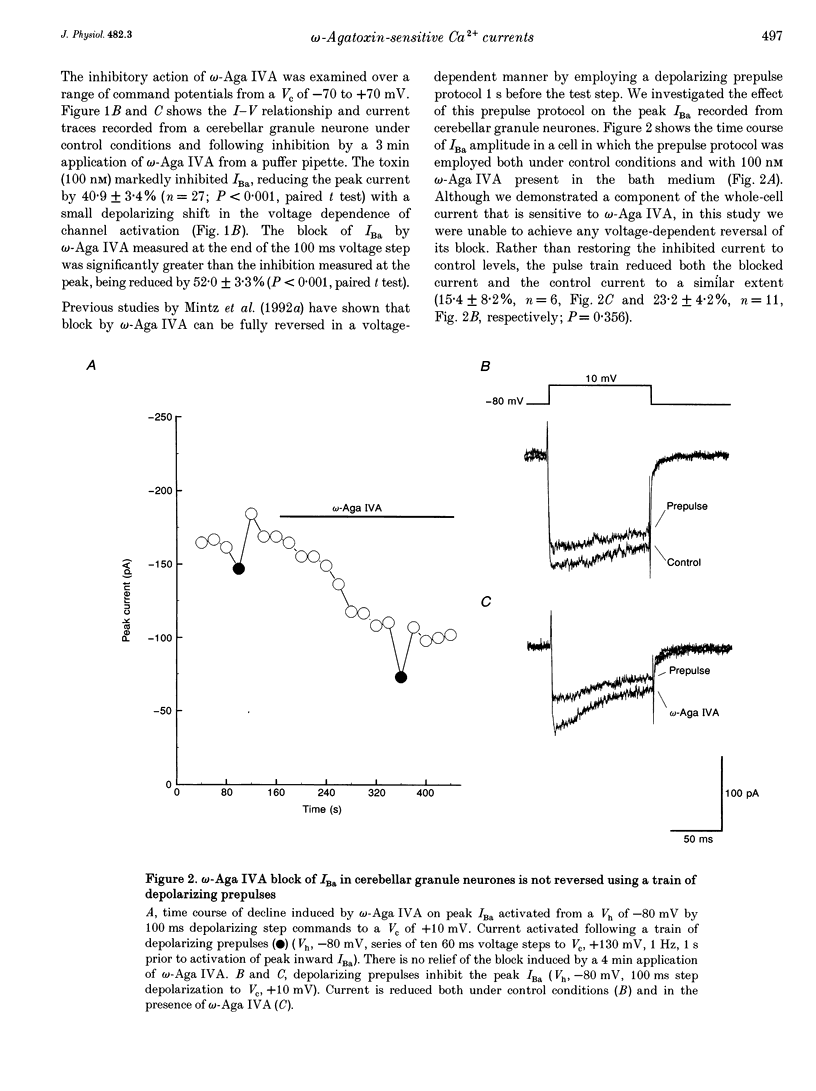
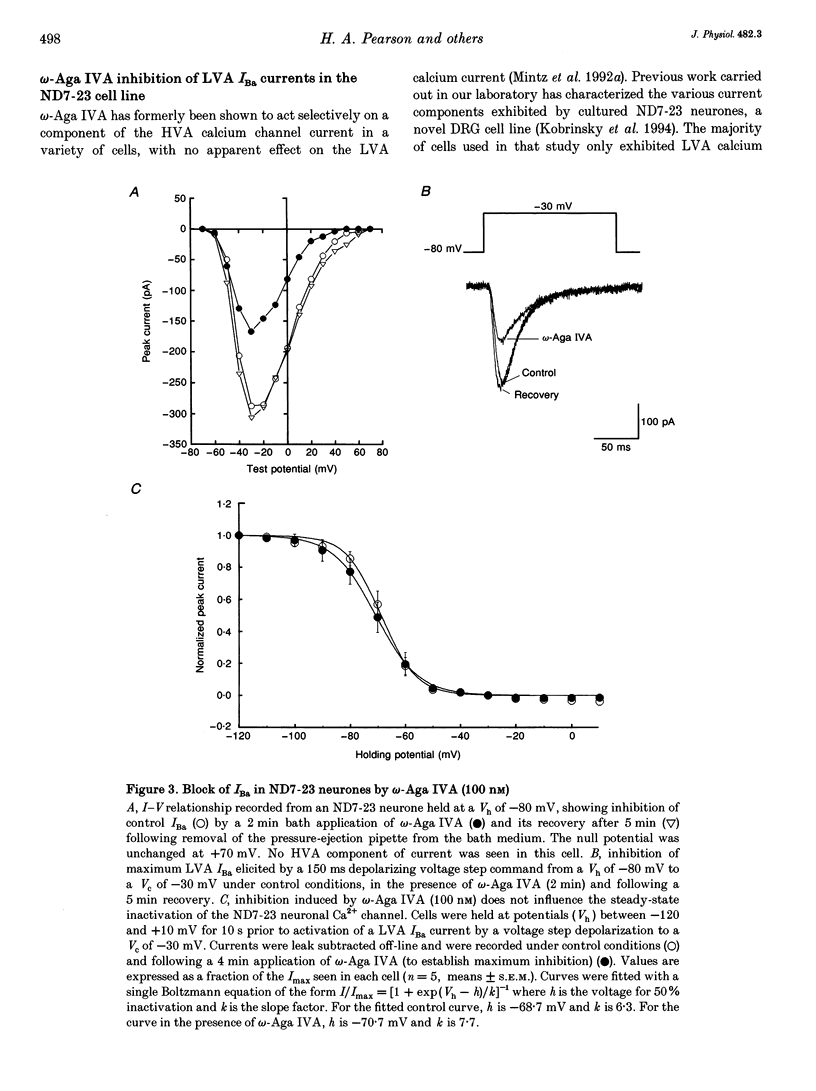
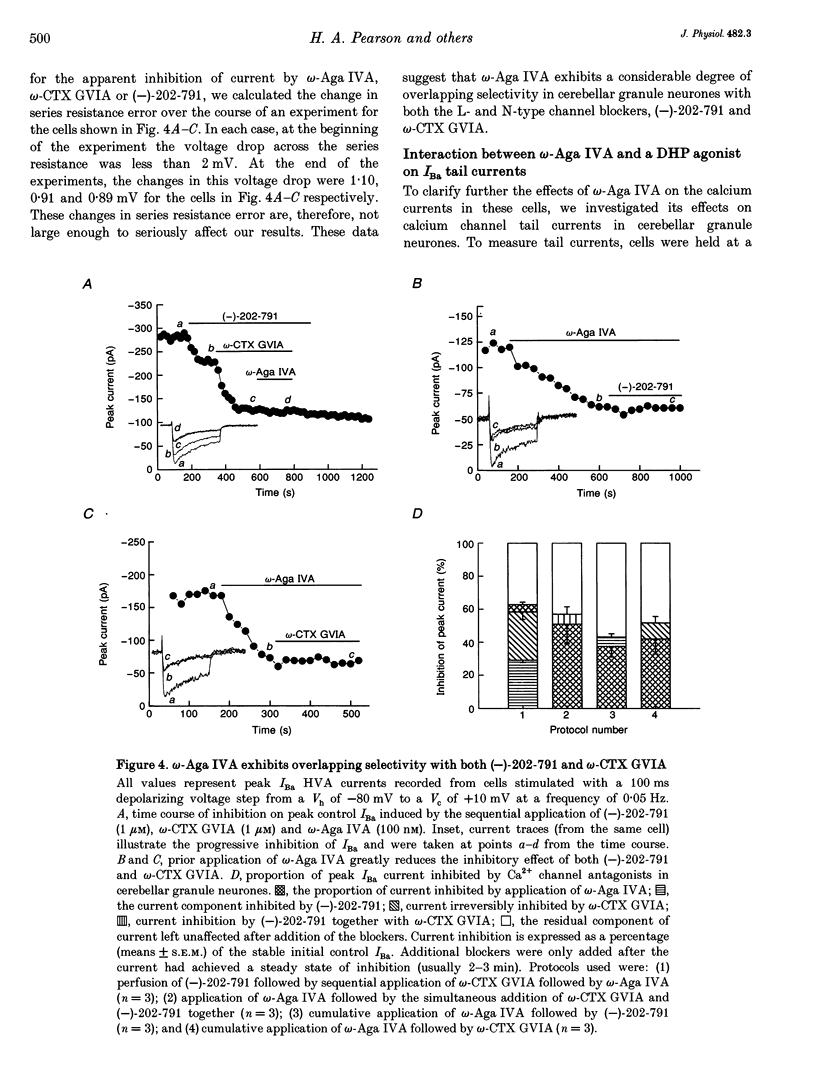
1. High-threshold voltage-gated calcium channel currents (IBa) were studied in cultured rat cerebellar granule neurones using the whole-cell patch clamp technique with 10 mM Ba2+ as the charge carrier. The putative P-type component of whole-cell current was characterized by utilizing the toxin omega-agatoxin IVA (omega-Aga IVA) in combination with other blockers. 2. omega-Aga IVA (100 nM) inhibited the high voltage-activated (HVA) IBa by 40.9 +/- 3.4% (n = 27), and the dissociation constant Kd was 2.7 nM. Maximal inhibition occurred within a 2-3 min time course, and was irreversible. The isolated omega-Aga IVA-sensitive current was non-inactivating. 3. omega-Aga IVA exhibited overlapping selectivity with both N- and L-channel blockers; omega-conotoxin GVIA (omega-CTX GVIA) (1 microM) and the dihydropyridine (-)-202-709 (1 microM), respectively. Together these toxins reduced the omega-Aga IVA-sensitive component to just 4.5 +/- 1.4% (n = 3). Thus only a small proportion of the current can be unequivocally attributed to P-type current. Inhibition of the HVA IBa by omega-Aga IA also reduced the proportion of omega-Aga IVA-sensitive current to 28.0 +/- 3.2% (n = 3). 4. Application of omega-Aga IVA and a synthetic form of funnel-web toxin, N-(7-amino-4-azaheptyl)-L-argininamide (sFTX-3.3; 10 microM), produced an additive block of the HVA IBa. Consequently these two toxins do not act on the same channel in cerebellar granule neurones. 5. omega-Aga IVA inhibition of low voltage-activated (LVA) IBa was studied in the ND7-23 neuronal cell line. omega-Aga IVA (100 nM) reduced the LVA current by 41.3 +/- 3.2% (n = 17) in a fully reversible manner with no shift in the steady-state inactivation of the channel. 6. A component of current insensitive to N-, L- and P-channel blockers remained unclassified in all our studies. This component, and also that remaining following block by omega-Aga IVA and omega-Aga IA, exhibited relatively rapid, although incomplete, inactivation compared to the other currents isolated in this study. 7. In conclusion, omega-Aga IVA inhibits a component of current in cultured cerebellar granule neurones which overlaps almost completely with that inhibited by L- and N-channel blockers. In addition, a large component of whole-cell current in these neurones still remains unclassified.
Full text
PDF








Selected References
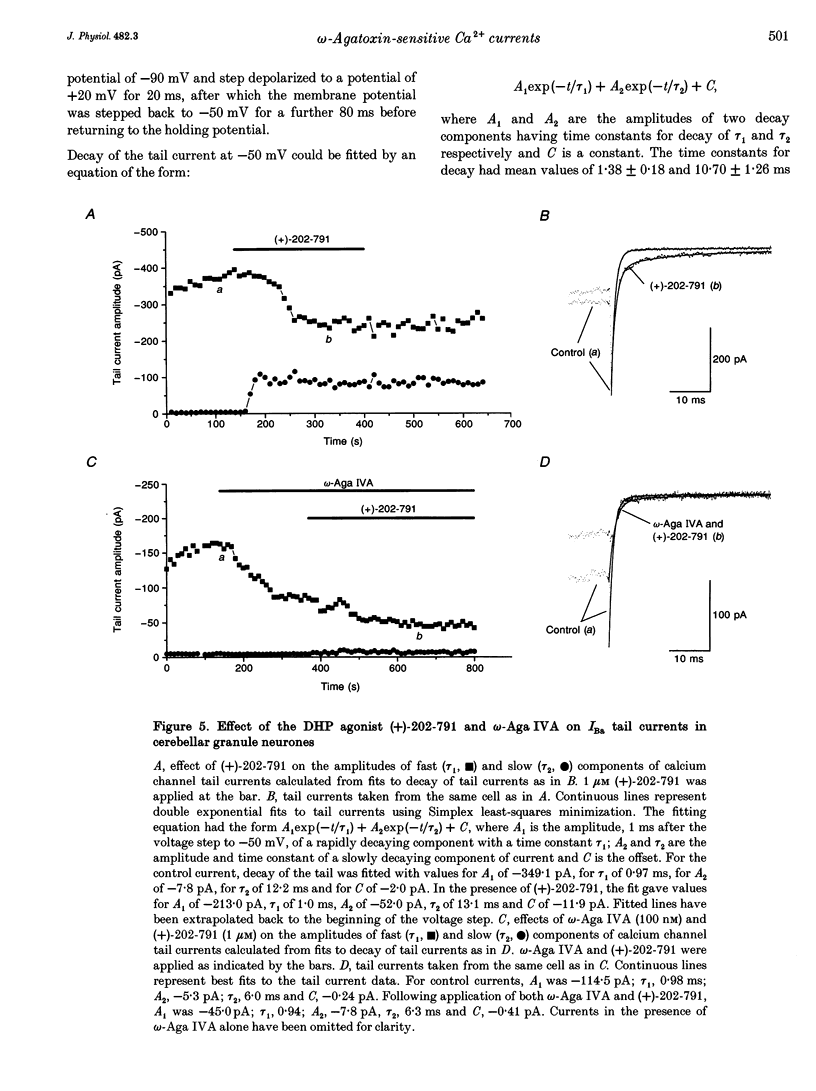
These references are in PubMed. This may not be the complete list of references from this article.
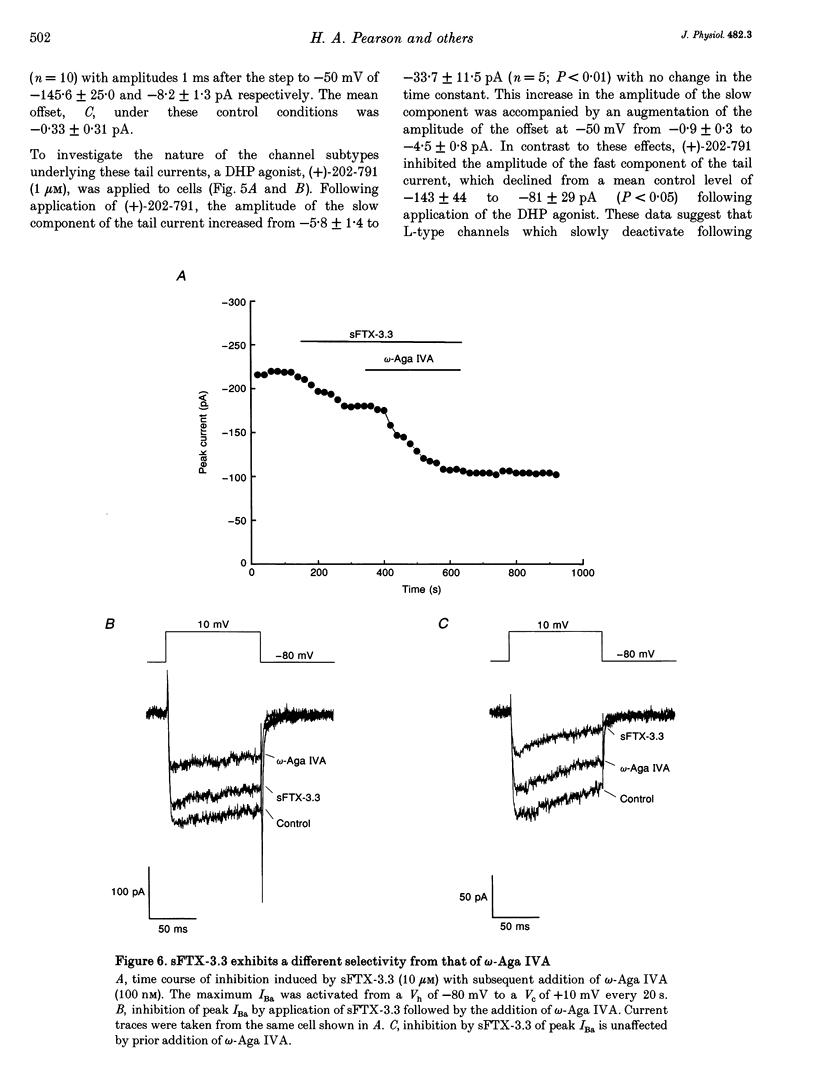
- Adams M. E., Bindokas V. P., Hasegawa L., Venema V. J. Omega-agatoxins: novel calcium channel antagonists of two subtypes from funnel web spider (Agelenopsis aperta) venom. J Biol Chem. 1990 Jan 15;265(2):861–867. [PubMed] [Google Scholar]
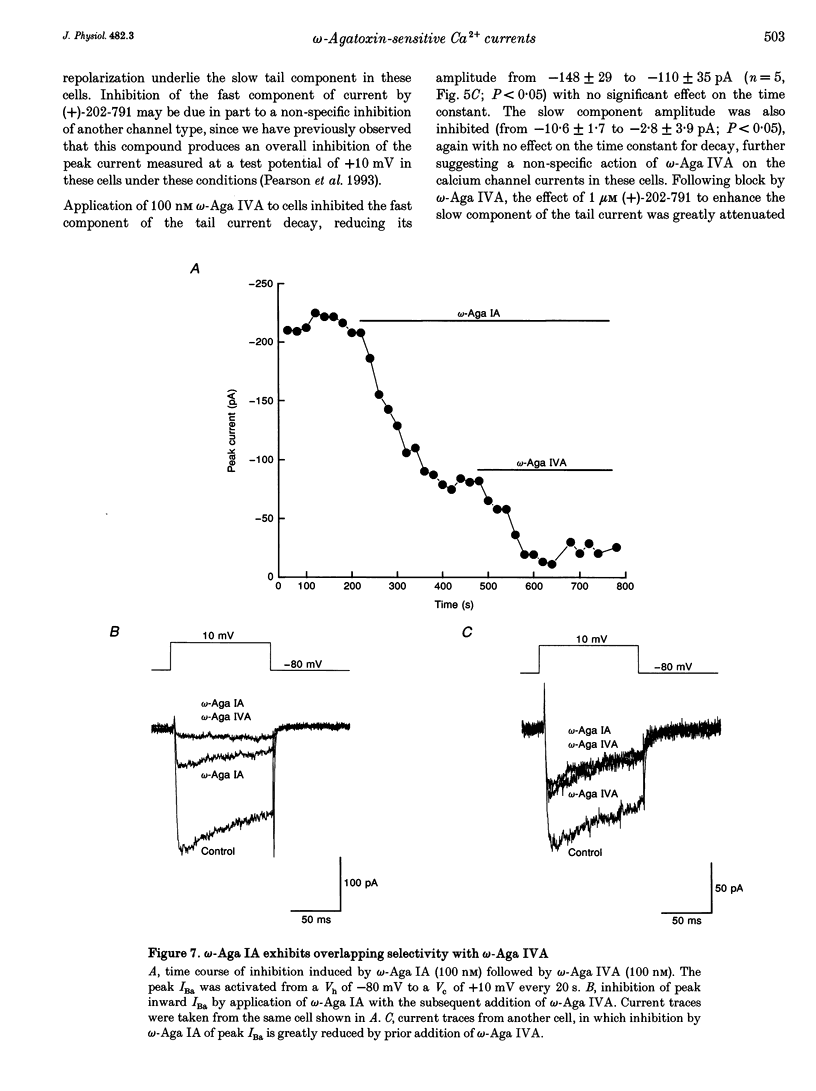
- Brown A. M., Sayer R. J., Schwindt P. C., Crill W. E. P-type calcium channels in rat neocortical neurones. J Physiol. 1994 Mar 1;475(2):197–205. doi: 10.1113/jphysiol.1994.sp020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
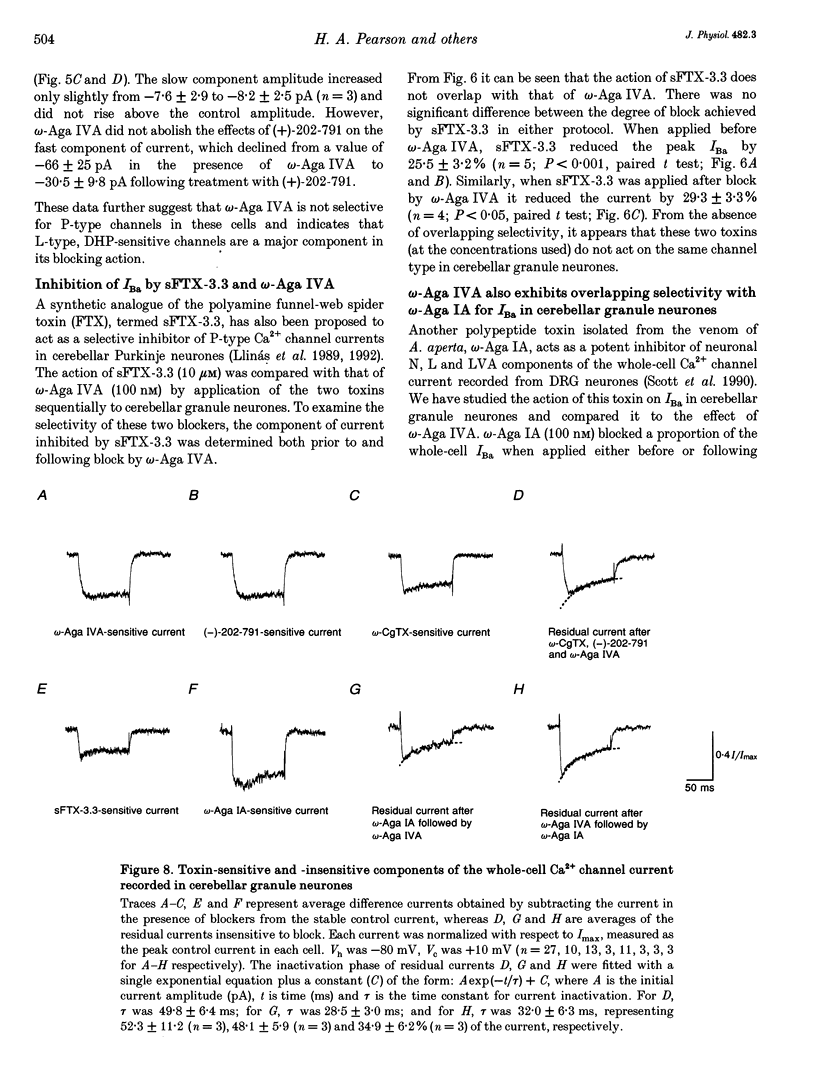
- Cherksey B. D., Sugimori M., Llinás R. R. Properties of calcium channels isolated with spider toxin, FTX. Ann N Y Acad Sci. 1991;635:80–89. doi: 10.1111/j.1749-6632.1991.tb36483.x. [DOI] [PubMed] [Google Scholar]
- De Waard M., Feltz A., Bossu J. L. Properties of a High-threshold Voltage-activated Calcium Current in Rat Cerebellar Granule Cells. Eur J Neurosci. 1991;3(8):771–777. doi: 10.1111/j.1460-9568.1991.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Ellinor P. T., Zhang J. F., Randall A. D., Zhou M., Schwarz T. L., Tsien R. W., Horne W. A. Functional expression of a rapidly inactivating neuronal calcium channel. Nature. 1993 Jun 3;363(6428):455–458. doi: 10.1038/363455a0. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hillyard D. R., Monje V. D., Mintz I. M., Bean B. P., Nadasdi L., Ramachandran J., Miljanich G., Azimi-Zoonooz A., McIntosh J. M., Cruz L. J. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992 Jul;9(1):69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- Hof R. P., Rüegg U. T., Hof A., Vogel A. Stereoselectivity at the calcium channel: opposite action of the enantiomers of a 1,4-dihydropyridine. J Cardiovasc Pharmacol. 1985 Jul-Aug;7(4):689–693. doi: 10.1097/00005344-198507000-00012. [DOI] [PubMed] [Google Scholar]
- Huston E., Cullen G., Sweeney M. I., Pearson H., Fazeli M. S., Dolphin A. C. Pertussis toxin treatment increases glutamate release and dihydropyridine binding sites in cultured rat cerebellar granule neurons. Neuroscience. 1993 Feb;52(4):787–798. doi: 10.1016/0306-4522(93)90529-o. [DOI] [PubMed] [Google Scholar]
- Huston E., Scott R. H., Dolphin A. C. A comparison of the effect of calcium channel ligands and GABAB agonists and antagonists on transmitter release and somatic calcium channel currents in cultured neurons. Neuroscience. 1990;38(3):721–729. doi: 10.1016/0306-4522(90)90065-c. [DOI] [PubMed] [Google Scholar]
- Kasai H., Aosaki T., Fukuda J. Presynaptic Ca-antagonist omega-conotoxin irreversibly blocks N-type Ca-channels in chick sensory neurons. Neurosci Res. 1987 Feb;4(3):228–235. doi: 10.1016/0168-0102(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Kobrinsky E. M., Pearson H. A., Dolphin A. C. Low- and high-voltage-activated calcium channel currents and their modulation in the dorsal root ganglion cell line ND7-23. Neuroscience. 1994 Feb;58(3):539–552. doi: 10.1016/0306-4522(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Lin J. W., Rudy B., Llinás R. Funnel-web spider venom and a toxin fraction block calcium current expressed from rat brain mRNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4538–4542. doi: 10.1073/pnas.87.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Hillman D. E., Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992 Sep;15(9):351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Venema V. J., Swiderek K. M., Lee T. D., Bean B. P., Adams M. E. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992 Feb 27;355(6363):827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Pearson H. A., Dolphin A. C. Inhibition of omega-conotoxin-sensitive Ca2+ channel currents by internal Mg2+ in cultured rat cerebellar granule neurones. Pflugers Arch. 1993 Dec;425(5-6):518–527. doi: 10.1007/BF00374880. [DOI] [PubMed] [Google Scholar]
- Pearson H. A., Sutton K. G., Scott R. H., Dolphin A. C. Ca2+ currents in cerebellar granule neurones: role of internal Mg2+ in altering characteristics and antagonist effects. Neuropharmacology. 1993 Nov;32(11):1171–1183. doi: 10.1016/0028-3908(93)90011-q. [DOI] [PubMed] [Google Scholar]
- Sather W. A., Tanabe T., Zhang J. F., Mori Y., Adams M. E., Tsien R. W. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel alpha 1 subunits. Neuron. 1993 Aug;11(2):291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Dolphin A. C., Bindokas V. P., Adams M. E. Inhibition of neuronal Ca2+ channel currents by the funnel web spider toxin omega-Aga-IA. Mol Pharmacol. 1990 Nov;38(5):711–718. [PubMed] [Google Scholar]
- Scott R. H., Pearson H. A., Dolphin A. C. Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Prog Neurobiol. 1991;36(6):485–520. doi: 10.1016/0301-0082(91)90014-r. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Sweeney M. I., Kobrinsky E. M., Pearson H. A., Timms G. H., Pullar I. A., Wedley S., Dolphin A. C. Actions of arginine polyamine on voltage and ligand-activated whole cell currents recorded from cultured neurones. Br J Pharmacol. 1992 May;106(1):199–207. doi: 10.1111/j.1476-5381.1992.tb14315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slesinger P. A., Lansman J. B. Inactivating and non-inactivating dihydropyridine-sensitive Ca2+ channels in mouse cerebellar granule cells. J Physiol. 1991 Aug;439:301–323. doi: 10.1113/jphysiol.1991.sp018668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong T. W., Stea A., Hodson C. D., Dubel S. J., Vincent S. R., Snutch T. P. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993 May 21;260(5111):1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- Suburo A. M., Wheatley S. C., Horn D. A., Gibson S. J., Jahn R., Fischer-Colbrie R., Wood J. N., Latchman D. S., Polak J. M. Intracellular redistribution of neuropeptides and secretory proteins during differentiation of neuronal cell lines. Neuroscience. 1992;46(4):881–889. doi: 10.1016/0306-4522(92)90191-4. [DOI] [PubMed] [Google Scholar]
- Usowicz M. M., Sugimori M., Cherksey B., Llinás R. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron. 1992 Dec;9(6):1185–1199. doi: 10.1016/0896-6273(92)90076-p. [DOI] [PubMed] [Google Scholar]
- Williams M. E., Feldman D. H., McCue A. F., Brenner R., Velicelebi G., Ellis S. B., Harpold M. M. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992 Jan;8(1):71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Bevan S. J., Coote P. R., Dunn P. M., Harmar A., Hogan P., Latchman D. S., Morrison C., Rougon G., Theveniau M. Novel cell lines display properties of nociceptive sensory neurons. Proc Biol Sci. 1990 Sep 22;241(1302):187–194. doi: 10.1098/rspb.1990.0084. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993 Nov;32(11):1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]



