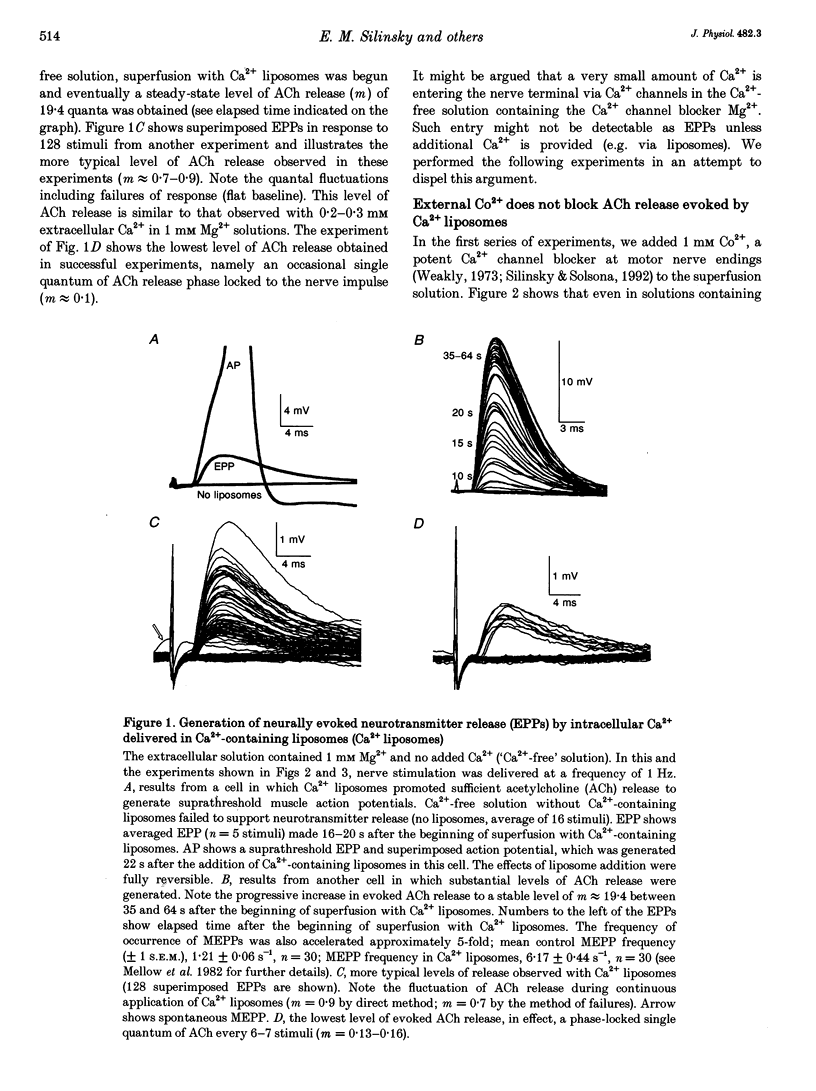
Abstract
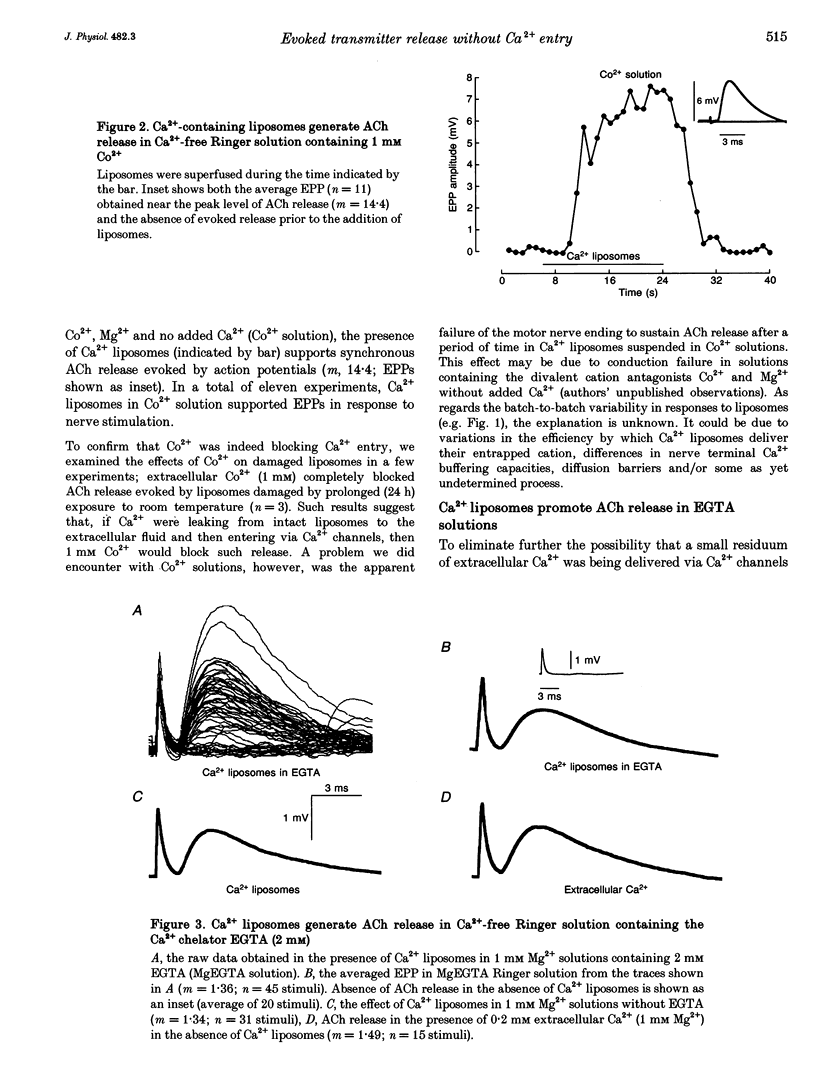
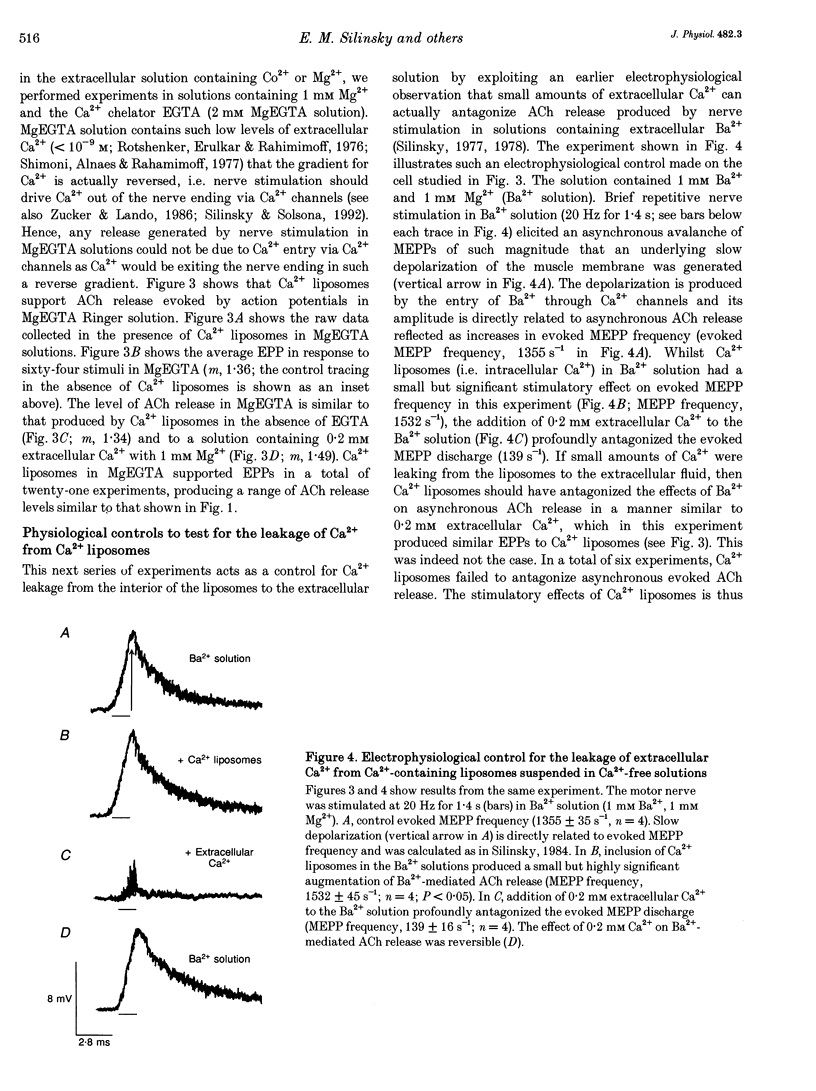
1. The requirement for extracellular Ca2+ in the process of evoked acetylcholine (ACh) release by nerve impulses was tested at endplates in frog skeletal muscle. Ca(2+)-containing lipid vesicles (Ca2+ liposomes) were used to elevate cytoplasmic Ca2+ concentrations under conditions in which Ca2+ entry from the extracellular fluid was prevented. 2. In an extracellular solution containing no added Ca2+ and 1 mM Mg2+ ('Ca(2+)-free' solution), Ca2+ liposomes promoted the synchronous release of ACh quanta, reflected electrophysiologically as endplate potentials (EPPs), in response to temporally isolated nerve impulses. 3. Motor nerve stimulation generated EPPs during superfusion with Ca2+ liposomes in Ca(2+)-free solutions containing the Ca2+ channel blocker Co2+ (1 mM), and the Ca2+ chelator EGTA (2 mM). As a physiological control for Ca2+ leakage from the liposomes to the extracellular fluid, the effect of Ca2+ liposomes on asynchronous evoked ACh release mediated by Ba2+ was examined. In contrast to the effects of 0.2-0.3 mM extracellular Ca2+, which generated EPPs but antagonized Ba(2+)-mediated asynchronous ACh release, Ca2+ liposomes generated EPPs but did not reduce asynchronous release mediated by Ba2+. The effects of Ca2+ liposomes were thus not due to leakage of Ca2+ from the liposome to the extracellular fluid. 4. Morphological studies using fluorescently labelled liposomes in conjunction with a confocal microscope demonstrate that lipid is transferred from the liposomes to nerve endings and liposomal contents are delivered to the nerve terminal cytoplasm. 5. The results suggest that when intracellular Ca2+ is elevated using liposomes as a vehicle, evoked ACh release can occur in the absence of Ca2+ entry via Ca2+ channels.
Full text
PDF





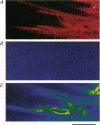
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustine G. J., Adler E. M., Charlton M. P. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Eckert R. Divalent cations differentially support transmitter release at the squid giant synapse. J Physiol. 1984 Jan;346:257–271. doi: 10.1113/jphysiol.1984.sp015020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Depolarizing pulses to neuromuscular terminals of frogs can elicit graded, phasic transmitter release in the absence of Ca influx. Neurosci Lett. 1990 Aug 14;116(1-2):94–100. doi: 10.1016/0304-3940(90)90392-m. [DOI] [PubMed] [Google Scholar]
- Gutman Y., Lichtenberg D., Cohen J., Boonyaviroj P. Increased catecholamine release from adrenal medulla by liposomes loaded with sodium or calcium ions. Biochem Pharmacol. 1979 Apr 1;28(7):1209–1211. doi: 10.1016/0006-2952(79)90330-7. [DOI] [PubMed] [Google Scholar]
- Hirsh J. K., Silinsky E. M., Solsona C. S. The role of cyclic AMP and its protein kinase in mediating acetylcholine release and the action of adenosine at frog motor nerve endings. Br J Pharmacol. 1990 Oct;101(2):311–318. doi: 10.1111/j.1476-5381.1990.tb12707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochner B., Parnas H., Parnas I. Membrane depolarization evokes neurotransmitter release in the absence of calcium entry. Nature. 1989 Nov 23;342(6248):433–435. doi: 10.1038/342433a0. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., MacDonald R. C. Characterization of a fluorescence assay to monitor changes in the aqueous volume of lipid vesicles. Anal Biochem. 1983 Oct 1;134(1):26–33. doi: 10.1016/0003-2697(83)90258-0. [DOI] [PubMed] [Google Scholar]
- Kharasch E. D., Mellow A. M., Silinsky E. M. Intracellular magnesium does not antagonize calcium-dependent acetylcholine secretion. J Physiol. 1981 May;314:255–263. doi: 10.1113/jphysiol.1981.sp013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko C. P. A lectin, peanut agglutinin, as a probe for the extracellular matrix in living neuromuscular junctions. J Neurocytol. 1987 Aug;16(4):567–576. doi: 10.1007/BF01668509. [DOI] [PubMed] [Google Scholar]
- Mellow A. M., Perry B. D., Silinsky E. M. Effects of calcium and strontium in the process of acetylcholine release from motor nerve endings. J Physiol. 1982 Jul;328:547–562. doi: 10.1113/jphysiol.1982.sp014283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey R. M., Zucker R. S. Action potentials must admit calcium to evoke transmitter release. Nature. 1991 Mar 14;350(6314):153–155. doi: 10.1038/350153a0. [DOI] [PubMed] [Google Scholar]
- Parnas I., Parnas H. The 'Ca-voltage' hypothesis for neurotransmitter release. Biophys Chem. 1988 Feb;29(1-2):85–93. doi: 10.1016/0301-4622(88)87027-3. [DOI] [PubMed] [Google Scholar]
- Perin M. S., MacDonald R. C. Fusion of synaptic vesicle membranes with planar bilayer membranes. Biophys J. 1989 May;55(5):973–986. doi: 10.1016/S0006-3495(89)82896-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Meiri H., Erulkar S. D., Barenholz Y. Changes in transmitter release induced by ion-containing liposomes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5214–5216. doi: 10.1073/pnas.75.10.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S., Erulkar S. D., Rahamimoff R. Reduction in the frequency of miniature end-plate potentials by nerve stimulation in low calcium solutions. Brain Res. 1976 Jan 16;101(2):362–365. doi: 10.1016/0006-8993(76)90277-8. [DOI] [PubMed] [Google Scholar]
- Shimoni Y., Alnaes E., Rahamimoff R. Is hyperosmotic neurosecretion from motor nerve endings a calcium-dependent process? Nature. 1977 May 12;267(5607):170–172. doi: 10.1038/267170a0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. Can barium support the release of acetylcholine by nerve impulses? Br J Pharmacol. 1977 Jan;59(1):215–217. doi: 10.1111/j.1476-5381.1977.tb06997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the calcium receptor that mediates depolarization-secretion coupling at cholinergic motor nerve terminals. Br J Pharmacol. 1981 Jun;73(2):413–429. doi: 10.1111/j.1476-5381.1981.tb10438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the role of barium in supporting the asynchronous release of acetylcholine quanta by motor nerve impulses. J Physiol. 1978 Jan;274:157–171. doi: 10.1113/jphysiol.1978.sp012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Solsona C. S. Calcium currents at motor nerve endings: absence of effects of adenosine receptor agonists in the frog. J Physiol. 1992 Nov;457:315–328. doi: 10.1113/jphysiol.1992.sp019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Struck D. K., Hoekstra D., Pagano R. E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981 Jul 7;20(14):4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- Theoharides T. C., Douglas W. W. Secretion in mast cells induced by calcium entrapped within phospholipid vesicles. Science. 1978 Sep 22;201(4361):1143–1145. doi: 10.1126/science.684435. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Delaney K. R., Mulkey R., Tank D. W. Presynaptic calcium in transmitter release and posttetanic potentiation. Ann N Y Acad Sci. 1991;635:191–207. doi: 10.1111/j.1749-6632.1991.tb36492.x. [DOI] [PubMed] [Google Scholar]
- Zucker R. S., Landò L. Mechanism of transmitter release: voltage hypothesis and calcium hypothesis. Science. 1986 Feb 7;231(4738):574–579. doi: 10.1126/science.2868525. [DOI] [PubMed] [Google Scholar]