Abstract
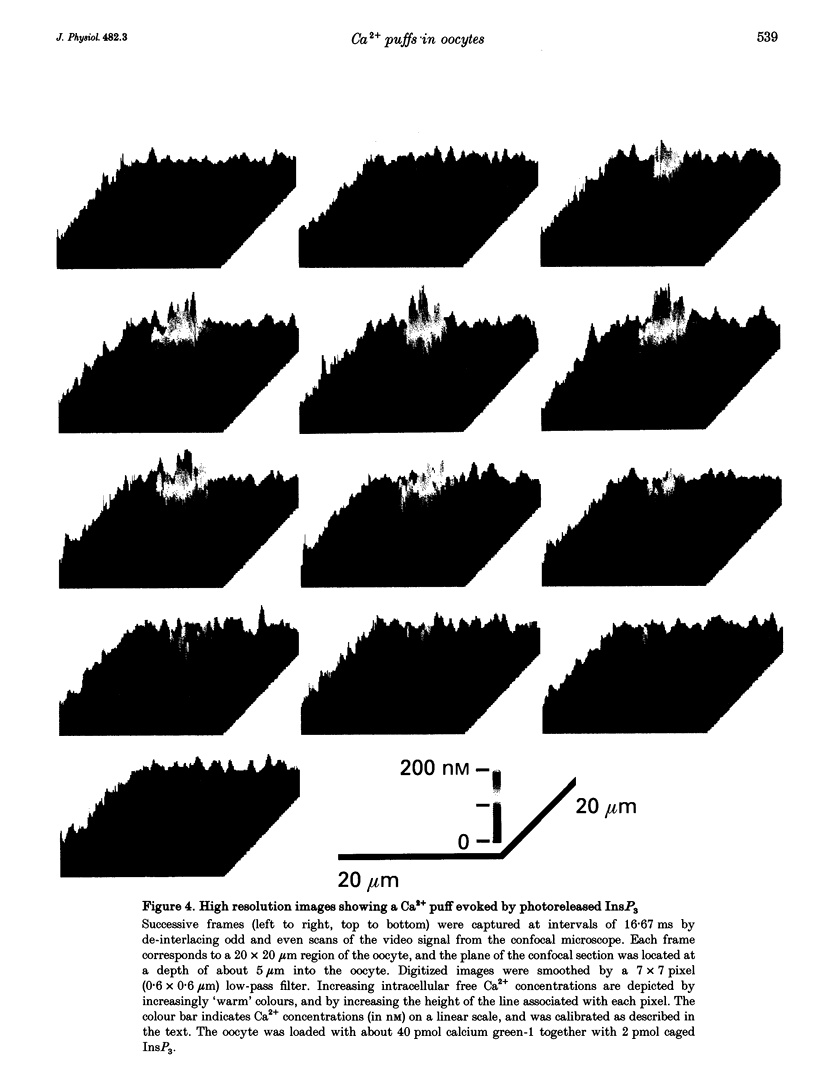
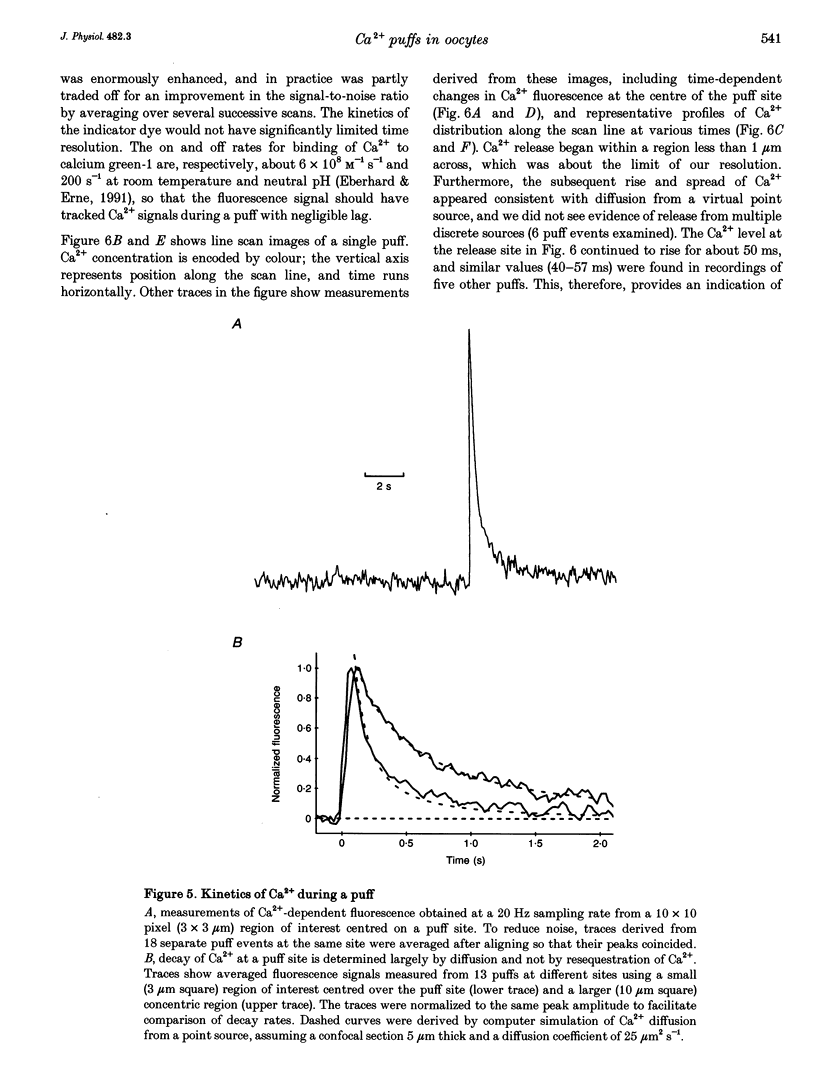
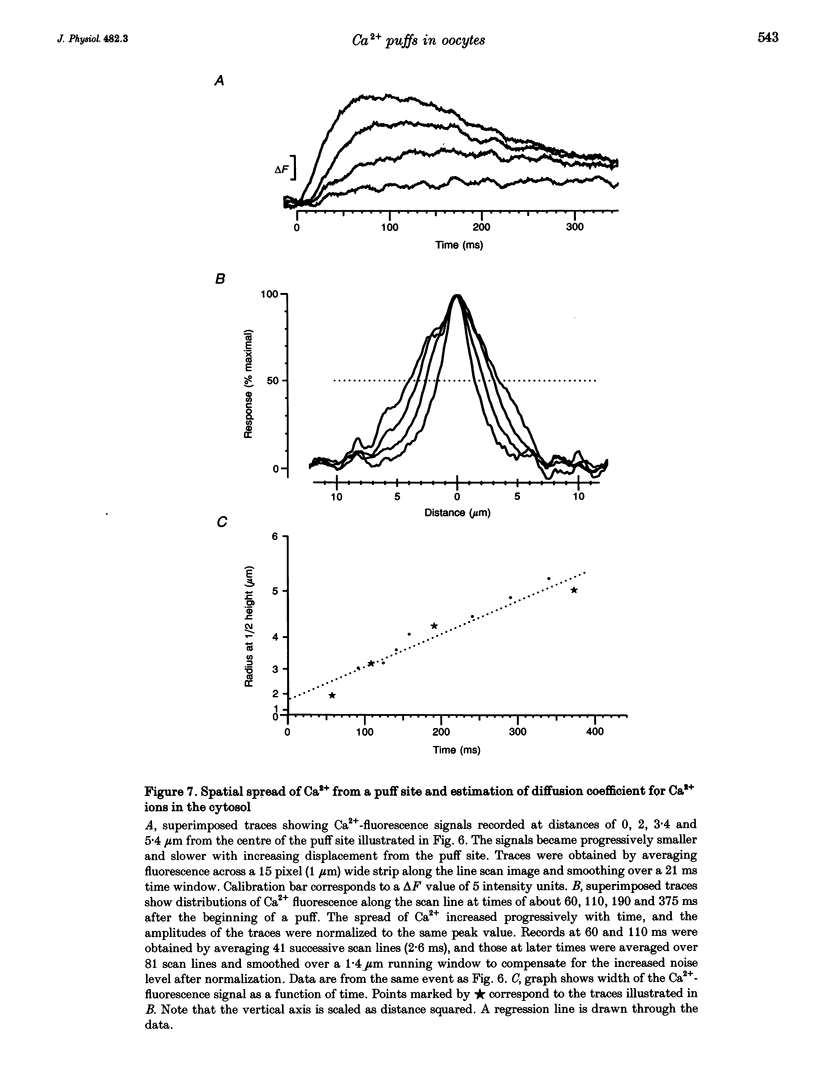
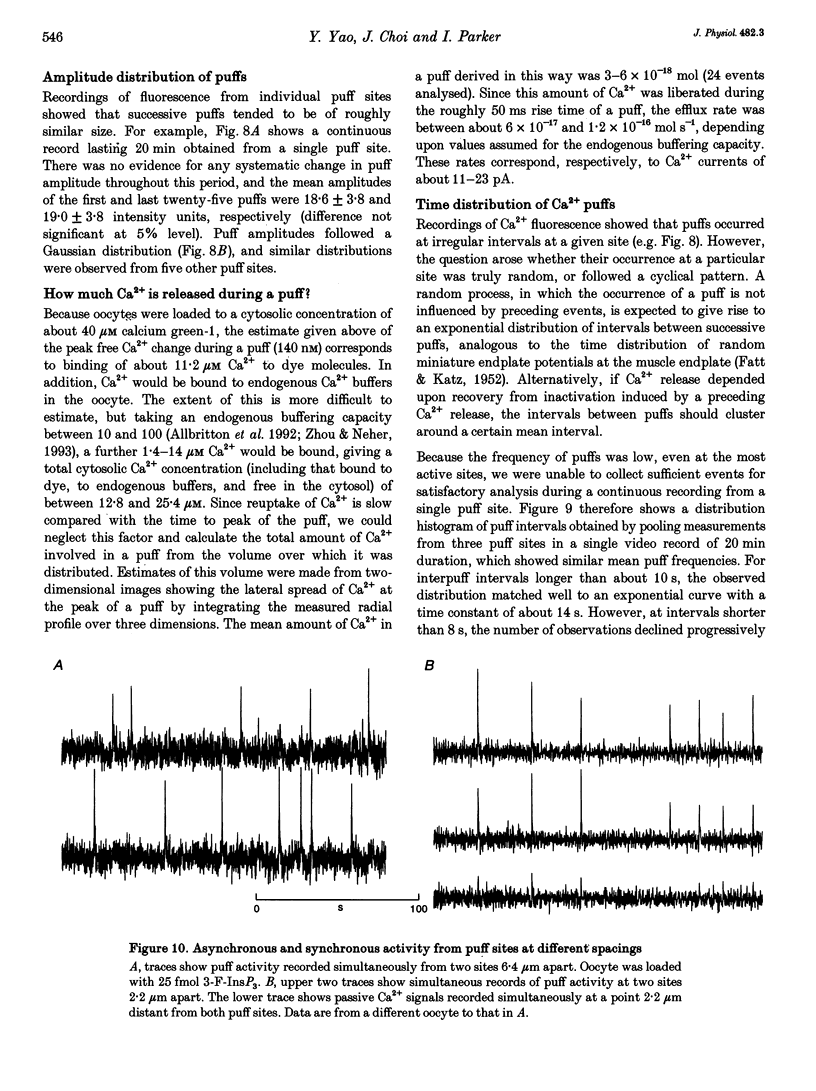
1. Ca2+ liberation induced in Xenopus oocytes by a poorly metabolized derivative of inositol 1,4,5-trisphosphate (3-deoxy-3-fluoro-D-myo-inositol 1,4,5-trisphosphate; 3-F-InsP3) was visualized using a video-rate confocal microscope to image fluorescence signals reported by the indicator dye calcium green-1. 2. Low (10-30 nM) intracellular concentrations of 3-F-InsP3 evoked Ca2+ release as localized transient 'puffs'. Progressively higher concentrations (30-60 nM) gave rise to abortive Ca2+ waves triggered by puffs, and then (> 60 nM) to a sustained elevation of Ca2+ followed by the appearance of propagating Ca2+ waves. At concentrations up to that giving waves, the frequency of puffs increased as about the third power of [InsP3], whereas their amplitudes increased only slightly. 3. The rise of cytosolic Ca2+ during a puff began abruptly, and peaked within about 50 ms. The peak free Ca2+ level was about 180 nM, and the total amount of Ca2+ liberated was several attomoles (10(-18) mol), too much to be accounted for by opening of a single InsP3-gated channel. The subsequent decline of Ca2+ occurred over a few hundred milliseconds, determined largely by diffusion of Ca2+ away from the release site, rather than by resequestration. Lateral spread of Ca2+ was restricted to a few micrometres, consistent with an effective diffusion coefficient for Ca2+ ions of about 27 microns2 s-1. 4. The peak amplitudes of puffs recorded at a given site were distributed in a roughly Gaussian manner, and a small proportion of sites consistently gave puffs much larger than the main population. Intervals between successive puffs at a single site were exponentially distributed, except for a progressive fall-off in puffs seen at intervals shorter than about 10 s. Thus, triggering of puffs appeared to be stochastically determined after recovery from a refractory period. 5. There was little correlation between the occurrence of puffs at sites more than a few micrometres apart, indicating that puff sites can function autonomously, but closely (ca 2 microns) adjacent sites showed highly correlated behaviour. 6. Puffs arose from sites-present at a density of about 1 per 30 microns2 in the animal hemisphere, located within a narrow band about 5-7 microns below the plasma membrane. 7. We conclude that Ca2+ puffs represent a 'quantal' unit of InsP3-evoked Ca2+ liberation, which may arise because local regenerative feedback by cytosolic Ca2+ ions causes the concerted opening of several closely clustered InsP3 receptor channels.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Atri A., Amundson J., Clapham D., Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993 Oct;65(4):1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Eberhard M., Erne P. Calcium binding to fluorescent calcium indicators: calcium green, calcium orange and calcium crimson. Biochem Biophys Res Commun. 1991 Oct 15;180(1):209–215. doi: 10.1016/s0006-291x(05)81278-1. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Paranjape S., Adams S. R., Potter B. V., Tsien R. Y. Cytosolic Ca2+ oscillations in REF52 fibroblasts: Ca(2+)-stimulated IP3 production or voltage-dependent Ca2+ channels as key positive feedback elements. Cell Calcium. 1991 Feb-Mar;12(2-3):153–164. doi: 10.1016/0143-4160(91)90017-9. [DOI] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Kasai H., Li Y. X., Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993 Aug 27;74(4):669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J Physiol. 1991 Feb;433:495–517. doi: 10.1113/jphysiol.1991.sp018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S., Muto A., Aruga J., Nakagawa T., Michikawa T., Furuichi T., Nakade S., Okano H., Mikoshiba K. The Xenopus IP3 receptor: structure, function, and localization in oocytes and eggs. Cell. 1993 May 7;73(3):555–570. doi: 10.1016/0092-8674(93)90142-d. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Trentham D. R. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- Meyer T. Cell signaling by second messenger waves. Cell. 1991 Feb 22;64(4):675–678. doi: 10.1016/0092-8674(91)90496-l. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Taylor C. W., Berridge M. J. Luminal Ca2+ promoting spontaneous Ca2+ release from inositol trisphosphate-sensitive stores in rat hepatocytes. J Physiol. 1992 Sep;455:623–640. doi: 10.1113/jphysiol.1992.sp019319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem. 1989 Jan 5;264(1):205–212. [PubMed] [Google Scholar]
- Parker I., Ivorra I. Characteristics of membrane currents evoked by photoreleased inositol trisphosphate in Xenopus oocytes. Am J Physiol. 1992 Jul;263(1 Pt 1):C154–C165. doi: 10.1152/ajpcell.1992.263.1.C154. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Confocal microfluorimetry of Ca2+ signals evoked in Xenopus oocytes by photoreleased inositol trisphosphate. J Physiol. 1993 Feb;461:133–165. doi: 10.1113/jphysiol.1993.sp019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Localized all-or-none calcium liberation by inositol trisphosphate. Science. 1990 Nov 16;250(4983):977–979. doi: 10.1126/science.2237441. [DOI] [PubMed] [Google Scholar]
- Parker I., Yao Y. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc Biol Sci. 1991 Dec 23;246(1317):269–274. doi: 10.1098/rspb.1991.0154. [DOI] [PubMed] [Google Scholar]
- Parys J. B., Sernett S. W., DeLisle S., Snyder P. M., Welsh M. J., Campbell K. P. Isolation, characterization, and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. J Biol Chem. 1992 Sep 15;267(26):18776–18782. [PubMed] [Google Scholar]
- Taylor C. W., Potter B. V. The size of inositol 1,4,5-trisphosphate-sensitive Ca2+ stores depends on inositol 1,4,5-trisphosphate concentration. Biochem J. 1990 Feb 15;266(1):189–194. doi: 10.1042/bj2660189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P., Lawrie A. M., Smith P. M., Gallacher D. V., Petersen O. H. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993 Aug 27;74(4):661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- Tigyi G., Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992 Oct 25;267(30):21360–21367. [PubMed] [Google Scholar]
- Yao Y., Parker I. Ca2+ influx modulation of temporal and spatial patterns of inositol trisphosphate-mediated Ca2+ liberation in Xenopus oocytes. J Physiol. 1994 Apr 1;476(1):17–28. [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Parker I. Inositol trisphosphate-mediated Ca2+ influx into Xenopus oocytes triggers Ca2+ liberation from intracellular stores. J Physiol. 1993 Aug;468:275–295. doi: 10.1113/jphysiol.1993.sp019771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Parker I. Potentiation of inositol trisphosphate-induced Ca2+ mobilization in Xenopus oocytes by cytosolic Ca2+. J Physiol. 1992 Dec;458:319–338. doi: 10.1113/jphysiol.1992.sp019420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]







