Abstract
Background
The COVID-19 pandemic has significantly impacted public health, with emerging evidence suggesting substantial effects on maternal and neonatal health. This systematic review and meta-analysis aimed to quantify the prevalence and risk of respiratory distress syndrome (RDS) in newborns born to mothers infected with SARS-CoV-2, the virus responsible for COVID-19.
Methods
We conducted a literature search in Embase, PubMed, and Web of Science up to April 20, without language or date restrictions. Observational studies reporting on the prevalence or risk of RDS among newborns from mothers with confirmed SARS-CoV-2 infection were included. Quality assessment was performed using the JBI tool. Statistical analysis was performed by using R software version 4.3.
Results
Twenty-two studies met the inclusion criteria. The pooled prevalence of RDS among newborns born to COVID-19-infected mothers was 11.5% (95% CI: 7.4–17.3%), with significant heterogeneity (I² = 93%). Newborns from infected mothers had a significantly higher risk of developing RDS, with a pooled risk ratio (RR) of 2.69 (95% CI: 1.77 to 4.17).
Conclusion
Newborns born to mothers with COVID-19 have a substantially increased risk of developing RDS. These findings emphasize the need for vigilant monitoring and appropriate management of pregnant women with COVID-19 to mitigate adverse neonatal outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10161-1.
Keywords: COVID-19, Respiratory distress syndrome, Meta-analysis, Good health and well-being
Introduction
The global outbreak of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a significant and far-reaching impact on both public health and healthcare systems worldwide. The primary clinical symptoms of COVID-19 primarily involve respiratory issues and complications, emerging evidence suggests that the virus may also have significant implications for maternal and neonatal health [1]. As a novel pathogen, there remain many uncertainties related to the potential vertical transmission of COVID-19 from infected mothers to their infants and the associated risk of adverse perinatal outcomes [2].
A concerning potential consequence of COVID-19 infection in pregnant women has been associated with an increased risk of respiratory distress syndrome (RDS) in their newborn. RDS is a respiratory disorder primarily affecting preterm infants, characterized by a deficiency of pulmonary surfactant and subsequent impaired gas exchange, leading to respiratory distress, hypoxemia, and the need for respiratory support [3]. In term infants, RDS can also occur due to various factors, including perinatal asphyxia, meconium aspiration syndrome, and genetic disorders affecting surfactant production or function [4].
The potential mechanisms by which maternal COVID-19 infection may contribute to the development of RDS in newborns are not fully understood. However, several plausible pathways have been proposed. First, the inflammatory response induced by SARS-CoV-2 infection may lead to placental dysfunction and impaired gas exchange, potentially resulting in fetal hypoxia and the subsequent development of RDS [5]. Additionally, maternal COVID-19 infection has been linked to a heightened risk of preterm birth [6], which is a well-established risk factor for RDS due to the immaturity of the fetal lungs and inadequate surfactant production [7].
Furthermore, there exists a potential for vertical transmission of SARS-CoV-2 from infected mothers to their infants, either through transplacental transmission or during delivery [8]. While the precise mechanisms of vertical transmission are not fully elucidated, and the risk appears to be relatively low [9], neonatal SARS-CoV-2 infection could potentially contribute to the development of RDS through direct viral injury to the immature lungs or through the induction of an inflammatory response [5].
Given the possible impact of maternal COVID-19 infection on neonatal respiratory health, it is crucial to understand the prevalence and risk of RDS among babies born to women with COVID-19 infection during pregnancy. Early reports from case series and cohort studies have provided valuable insights into this issue, but the findings have been inconsistent, and the overall risk estimates have varied widely across studies. To address this knowledge gap and synthesize the available evidence, a systematic review and meta-analysis are needed to provide a comprehensive and quantitative assessment of the prevalence and risk of RDS in newborns born to mothers with COVID-19. Such a synthesis would not only enhance our knowledge of the possible consequences of maternal SARS-CoV-2 infection on neonatal respiratory health but also inform health policies and clinical practice to optimize the management and care of mothers and newborns during the pandemic. The primary objectives of this systematic review and meta-analysis are to estimate the pooled prevalence of RDS among newborns born to mothers with confirmed SARS-CoV-2 infection during pregnancy and to assess the risk of developing RDS in newborns born to mothers with COVID-19 compared to those born to mothers without SARS-CoV-2 infection.
Methods
This systematic review and meta-analysis was conducted strictly in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, as detailed in Table S1. Additionally, the protocol for this study was officially registered with the PROSPERO database, ensuring adherence to established research standards.
Eligibility criteria
Studies qualified for inclusion based on the following criteria: The study design needed to be observational, including case-control, cohort, or cross-sectional studies that reported on the prevalence or risk of RDS among newborns whose mothers had confirmed SARS-CoV-2 infection during pregnancy. The population focus was on pregnant women diagnosed with SARS-CoV-2 infection, verified either by RT-PCR or serological testing, and their newborns. Additionally, eligible studies were required to report on the number of newborns diagnosed with RDS or provide enough information to calculate the prevalence or risk of RDS. No language restrictions were imposed. Studies were excluded if they only involved mothers without a confirmed SARS-CoV-2 infection, did not report data on RDS in newborns, or were types of publications such as reviews, editorials, commentaries, and case reports.
Literature search
A thorough literature search was carried out across the following electronic databases from inception to April 20 in Embase, PubMed, and Web of Science. The search strategy employed a mix of pertinent keywords related to “COVID-19,” “SARS-CoV-2,” “pregnancy,” “maternal,” “newborn,” “infant,” and “respiratory distress syndrome.” No restrictions were placed on language or publication date. The search strategy is detailed in Table S2.
Screening
The process of selecting studies involved two independent reviewers who screened the titles and abstracts of identified records according to the eligibility criteria. Difference in opinion between reviewers were resolved by discussion or by consulting a third reviewer. Articles that potentially met the inclusion criteria were retrieved in full-text and further evaluated for inclusion. A semiautomated web software (Nested-Knowledge, MN, USA) was used for screening.
Data extraction and quality assessment
Data extraction was carried out independently by two reviewers utilizing a uniform data collection template. Any conflicts were resolved through discussion or by involving a third reviewer for consultation. Extracted data from each study comprised the first author’s name, the year of publication, the study design, the country where the study was conducted, and the sample size, the number of newborns diagnosed with RDS, and the odds ratios (OR) and risk ratios (RR) for RDS. The included studies’ methodological quality and risk of bias were assessed using the JBI tool. Quality assessments were independently conducted by two reviewers. In cases of disagreement, issues were resolved by discussing them or consulting a third reviewer for a final decision.
Data synthesis and analysis
The outcomes of interest in this study were two-fold: first, the pooled prevalence of RDS among newborns born to mothers with confirmed SARS-CoV-2 infection during pregnancy; and second, the comparative risk of developing RDS between newborns born to mothers with COVID-19 and those born to mothers without SARS-CoV-2 infection. To calculate the pooled prevalence estimates and their 95% confidence intervals (CIs), a random effect model was employed. Additionally, RRs with 95% CIs were derived from the included studies for the risk analysis. Heterogeneity across the studies was assessed using Cochran’s Q test and quantified using the I2 statistic, with an I2 value greater than 50% indicating substantial heterogeneity. Publication bias was evaluated using the Doi plot and the LFK index. Meta-regression has been performed to explore the impact of sample size on the pooled prevalence. All statistical analyses were performed using R statistical software (version 4.3, R Foundation for Statistical Computing, Vienna, Austria), utilizing the ‘metafor’ and ‘meta’ package.
Results
Literature search
The search identified a total of 997 records. After the initial removal of 324 duplicate records, 673 records were screened for relevance. Out of these, 364 records were excluded, leaving 46 reports that were sought for detailed retrieval. All 46 reports were subsequently retrieved and assessed for eligibility based on the study’s inclusion criteria. Of these, 24 full-text articles were excluded for reasons such as not reporting the outcome of interest (21 reports) or the exposure of interest not being relevant (3 reports). Ultimately, 22 [10–31] studies met the inclusion criteria and were included in the final analysis (Fig. 1).
Fig. 1.
PRISMA flow diagram depicting article selection process
Characteristics of included studies
The characteristics of the studies included are presented in Table 1. Research was carried out in various countries such as Iran [18, 19, 26], India [22, 24, 27, 28], China [31], Poland [29], Italy [15, 30], USA [16, 21], Canada [13], Turkey [12, 14, 23], Egypt [10], Kazakhstan [10], Taiwan [25], and Brazil [20]. Predominantly observational in nature, these studies utilize a variety of designs, including cohort studies, prospective and retrospective observational studies, and cross-sectional studies, with a few employing case-control methodologies to provide comparative insights between infected and non-infected groups. Focusing on pregnant women confirmed to have COVID-19 through RT-PCR or serological testing, these studies reported the primary outcome of RDS incidence in newborns. Sample sizes vary widely, ranging from as few as 7 to as many as 4,707 participants, reflecting the varied scale and scope of research interests. Most studies concentrate solely on infants born to infected mothers, with a few providing comparative RR or OR for RDS between infants born to COVID-19 positive and negative mothers. The quality assessment of studies is presented in Table S3.
Table 1.
Characteristics of included studies
| Study | Country | Study design | Population | Total sample | Mothers with COVID-19 | Mothers without COVID-19 | Newborns with respiratory distress in COVID-19 mothers/Cases | Newborns with respiratory distress in non-COVID-19 mothers | RR/OR (95% CI) and CI for respiratory distress |
|---|---|---|---|---|---|---|---|---|---|
| Anter 2023 [10] | Egypt | Retrospective study | Pregnant women admitted to the quarantine hospital due to SARS-CoV-2 infection | 35 | 35 | NIL | 4 | NA | NA |
| Barros 2024 [11] | Multicounty study | Prospective observational study | Women diagnosed with laboratory-confirmed COVID-19 during pregnancy | 4707 | 1577 | 3130 | 81 | 166 | NA |
| Beykara 2024 [12] | Turkey | Retrospective study | SARS-CoV-2-infected pregnant/puerperal women | 341 | 341 | NA | 21 | NA | NA |
| Brandt 2021 [13] | Canada | Matched case control study | Pregnant patients with confirmed coronavirus disease | 183 | 61 | 122 | 5 | 6 | NA |
| Ciplak 2023 [14] | Turkey | Cohort study | Preterm infants < 37 gestational weeks with maternal COVID-19 | 254 | 127 | 127 | 51 | NA | NA |
| Costa 2022 [15] | Italy | Prospective cohort study | Women who contracted COVID-19 during pregnancy | 96 | 96 | NA | 2 | NA | NA |
| Dumitriu 2021 [16] | USA | Cohort study | Mothers positive for or with suspected SARS-CoV-2 | 101 | 100 | NA | 3 | NA | NA |
| Dzhaynakbaev 2023 [17] | Kazakhstan | Retrospective study | Pregnant women with COVID-19 | 65 | 65 | NA | 10 | NA | NA |
| Farhadi 2022 [18] | Iran | Cross sectional study | Neonates delivered bySARS-CoV-2 infected pregnant women | 60 | 58 | NA | 2 | NA | NA |
| Gargani 2022 [19] | Iran | Cross sectional study | Pregnant women infected with COVID-19 | 182 | 136 | 24 | 5 | NA | NA |
| Guida 2022 [20] | Brazil | Nested Case control | Pregnant women with COVID 19 | 203 | 203 | NA | 41 | NA | NA |
| Hamidi 2022 [21] | USA | Retrospective observational cohort study | COVID-19 patients | 441 | 441 | NA | 25 | NA | NA |
| Kapadia 2021 [22] | India | Prospective observational study | 50 pregnant patients who were COVID-19 positive | 50 | 50 | NA | 1 | NA | NA |
| Kilic 2021 [23] | Turkey | Prospective observational study | Neonates borne from COVID 19 infected mothers | 16 | 15 | NIL | 2 | NA | NA |
| Kumar 2021 [24] | India | Prospective cohort study | Neonates born to women with SARS-CoV-2 infection within two weeks before or two days after birth | 1713 | 143 (IM), 39 (EM) | 1187 (IM), 65 (EM) | 13 (IM), 13 (EM) | 12 (IM), 8 (EM) | IM: RR = 7.5 (3.4–16.8), EM: 2.7(1.2–5.9) |
| Liao 2022 [25] | Taiwan | Retrospective Cohort Study | Positive COVID-19 cases delivered in NPIDRs and COVID-19-negative mothers delivered in conventional delivery rooms (CDRs) | 213 | 41 | 168 | 18 | 18 | NA |
| Mosayebi 2021 [26] | Iran | Cohort study | Neonates born to COVID-19 infected mothers | 44 | 44 | NA | 21 | NA | NA |
| Nayak 2021 [27] | India | Prospective observational study | Neonates borne from COVID 19 infected mothers | 165 | 165 | NA | 22 | NA | NA |
| Pandey 2021 [28] | India | Prospective Observational Study | Neonates borne from COVID 19 infected mothers | 16 | 16 | NA | 2 | NA | NA |
| Seniuk 2021 [29] | Poland | Retrospective case control study | SARS- Co V -2 infected mothers | 101 | 101 | 101 | 16 | 6 | OR = 3.016 (1.128–8.063) |
| Vimercati 2022 [30] | Italy | Cross sectional study | Un-vaccinated pregnant women with COVID-19 infection tested by RT-PCR nasopharyngeal swab | 122 | 122 | NIL | 7 | NA | NA |
| Yang 2020 [31] | China | Prospective observational study | Newborns delivered by SARS-CoV-2 infected pregnant women | 7 | 7 | NIL | 2 | NIL | NA |
Abbreviations: COVID-19 Coronavirus Disease 2019, EM Extramural, IM Intramural, NA Not Available, NPIDRs Not Provided in Data Received, OR Odds Ratio, RR Risk Ratio, RT-PCR Reverse Transcription Polymerase Chain Reaction, SARS-CoV-2 Severe Acute Respiratory Syndrome Coronavirus 2
Prevalence of RDS among newborn born to COVID-19 infected mothers
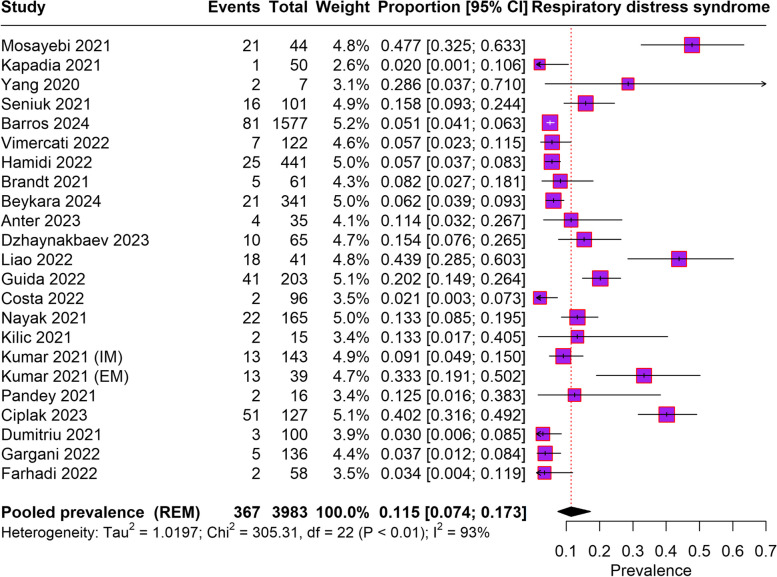
From 23 studies that reported on RDS among babies born to COVID-19-infected mothers, the total pooled prevalence of RDS was found to be 11.5% (95% CI: 7.4–17.3%). A high level of heterogeneity was observed across the studies, as indicated by an I2 value of 93%. The findings are visually represented in the forest plot provided in Fig. 2.
Fig. 2.
Forest plot depicting prevalence of Respiratiory distress syndrome among newborn born to COVID-19 infected mothers
Risk of RDS for newborn born due to COVID-19 infection of mothers
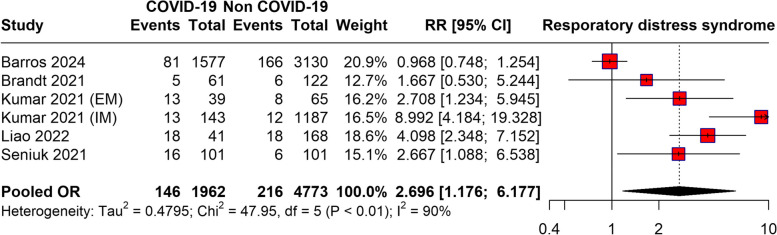
The meta-analysis evaluating the risk of RDS in newborns born to women with COVID-19 compared to those born to non-infected mothers reveals a pooled RR of 2.69 (95%CI: 1.77 to 4.17), indicating a significantly higher likelihood of RDS in newborns from COVID-19-infected mothers (p = 0.027). High heterogeneity was observed as evidenced by an I2 statistic of 90%. The forest plot is presented in Fig. 3.
Fig. 3.
Risk of RDS for newborn born due to COVID-19 infection of mothers
Meta-regression
We performed a meta-regression to assess the effect of sample size on the pooled prevalence of RDS. However, the analysis showed a non-significant association between sample size and the pooled prevalence (p = 0.2513). Figure 4 presents the corresponding bubble plot.
Fig. 4.
Bubble plot based on the meta-regression of association of sample size on pooled RDS
Publication bias
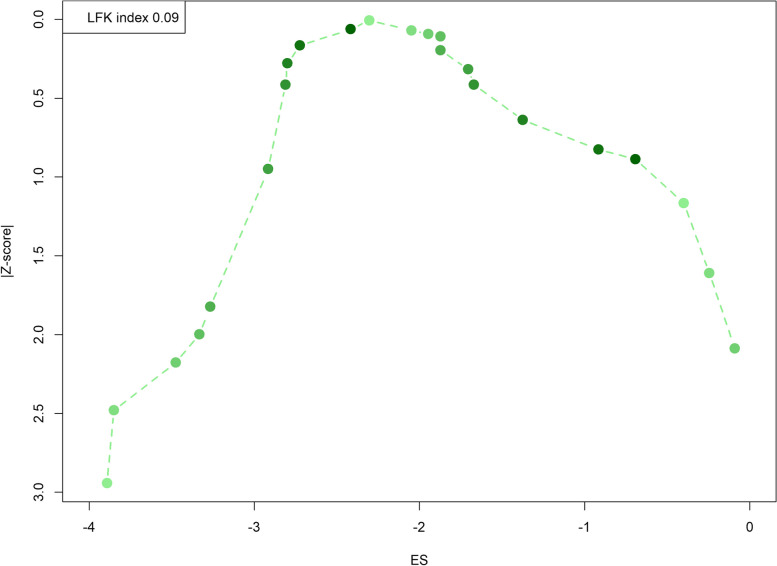
The Doi plot provided is instrumental in assessing publication bias in meta-analyses, showing a visualization of the dispersion of studies around the effect size (Fig. 5). In this specific Doi plot, the LFK index, a diagnostic tool used to quantify the asymmetry of the Doi plot, is reported as 0.09. This value indicates minimal asymmetry, suggesting that there is little to no publication bias present in this meta-analysis. Typically, an LFK index value less than 1 suggests no significant publication bias, which is confirmed here by the smooth, almost symmetrical arc of the plot from the higher effect sizes on the left to the lower effect sizes on the right. The plot, by not displaying a tail on one side or the other, supports the conclusion that the meta-analysis results are robust and not significantly influenced by unpublished or selectively reported studies.
Fig. 5.
Doi plot for publication bias assessment
Discussion
The present study provides important insights into the impact of maternal SARS-CoV-2 infection on RDS in newborns. The pooled prevalence of RDS among newborns born to mothers with COVID-19 was found to be 11.5%, which is significantly greater than the reported prevalence of RDS in the general newborn population (around 1%) [32]. This elevated prevalence highlights the potential respiratory complications that newborns may face when their mothers are infected with SARS-CoV-2 during pregnancy. Furthermore, the meta-analysis demonstrated a nearly three-fold increased risk of developing RDS in newborns born to mothers with COVID-19 compared to those born to mothers without SARS-CoV-2 infection (pooled RR = 2.69, 95% CI: 1.77–4.17). This finding is consistent with the proposed mechanisms by which maternal SARS-CoV-2 infection could contribute to the development of RDS in newborns, as outlined in the introduction.
The inflammatory response initiated by SARS-CoV-2 infection may lead to placental dysfunction and impaired gas exchange, potentially resulting in fetal hypoxia and subsequent RDS development [33]. Additionally, the increased risk of preterm birth associated with maternal COVID-19 is a well-established risk factor for RDS due to the immaturity of the fetal lungs and inadequate surfactant production [34, 35]. Moreover, the potential for vertical transmission of SARS-CoV-2 from infected individuals mothers to their infants could also play a role in the development of RDS through direct viral injury to the immature lungs or the induction of an inflammatory response [18, 36]. While the precise mechanisms require further investigation, the findings from this meta-analysis indicate the need for heightened vigilance and appropriate respiratory support for newborns born to mothers with COVID-19.
It is important to note that high heterogeneity was observed among the included studies for both the prevalence and risk analyses. This heterogeneity may be attributed to several factors, such as variations in study design, population characteristics, disease severity, and treatment protocols. Additionally, the included studies were conducted across multiple countries, which may reflect differences in healthcare systems, resources, and management strategies for COVID-19 during pregnancy. One of the key issues raised in the review process concerns the high heterogeneity observed in our meta-analysis, which is indeed a common phenomenon in observational studies. The observed heterogeneity may be attributed to various factors, such as differences in study design, population characteristics, disease severity, and treatment protocols. Additionally, the included studies were conducted across multiple countries with different healthcare systems, management strategies, and available resources for handling COVID-19 in pregnant women. Despite these variations, the overall findings are consistent with biological plausibility, reinforcing the association between maternal COVID-19 infection and the increased risk of RDS in newborns. The width of the confidence interval (1.77 to 4.17) reflects variability in the existing data, emphasizing the need for further research to refine these estimates. Larger, well-powered studies are crucial to provide more precise risk estimates and to better understand the full spectrum of neonatal outcomes associated with maternal SARS-CoV-2 infection. These findings highlight the necessity for tailored clinical protocols to optimize neonatal outcomes in this population.
Gestational age is a well-known risk factor for the development of RDS, especially in preterm infants due to the immaturity of the lungs and insufficient surfactant production. In our analysis, we recognized the importance of gestational age and made efforts to extract and analyze data related to this variable. Unfortunately, the majority of the included studies did not provide sufficient detail on gestational age to perform a comprehensive analysis. We recommend that future studies focus on reporting gestational age more consistently to allow for better understanding of its role in the development of RDS in the context of maternal COVID-19 infection. Addressing this gap in the literature will be crucial for refining risk estimates and tailoring clinical interventions.
A previous systematic review aimed to clarify the transmission route, clinical features, and outcomes of neonatal COVID-19 infections [37]. It found that 70% of infections were attributed to environmental transmission, while 30% were due to vertical transmission. Of the infected neonates, 55% developed COVID-19, with the most common symptoms being fever (44%), gastrointestinal issues (36%), respiratory symptoms (52%), and neurological manifestations (18%) [37]. Additionally, 64% had abnormal lung imaging. The review identified a significant association between the lack of mother-neonate separation at birth and a higher risk of late SARS-CoV-2 infection (OR 4.94, p = 0.0002; adjusted OR 6.6, p < 0.0001). However, breastfeeding was not significantly associated with infection risk (OR 0.35, p = 0.10; adjusted OR 2.2, p = 0.148). These findings contribute to the growing body of knowledge on neonatal SARS-CoV-2 infections.
The potential mechanisms by which maternal SARS-CoV-2 infection contributes to the development of RDS in newborns remain an area of ongoing investigation. Current evidence suggests that maternal COVID-19 could lead to placental dysfunction, resulting in fetal hypoxia, which in turn increases the risk of RDS. Moreover, maternal infection has been linked to an increased risk of preterm birth, a major risk factor for RDS. While the possibility of vertical transmission of SARS-CoV-2 remains low, it cannot be completely ruled out as a contributing factor, as direct viral injury to the fetal lungs or the induction of an inflammatory response could also play a role [38–40]. Further research is needed to elucidate these mechanisms and to better understand the pathophysiology of RDS in newborns exposed to maternal COVID-19.
The findings suggest a need for enhanced monitoring and proactive management of pregnant women who are diagnosed with COVID-19. Given the increased risk of RDS among newborns born to COVID-19-infected mothers, healthcare providers, including obstetricians and neonatologists, should be prepared for potential complications. There may be a need to adjust delivery plans and respiratory support strategies to optimize neonatal outcomes and manage the elevated risk of RDS effectively. Public health policies should prioritize vaccinations and protective measures for pregnant women to mitigate the risks associated with COVID-19 infection. This is particularly important given the demonstrated vulnerability of this group and the direct implications for neonatal health. Policies aimed at reducing the incidence of COVID-19 among pregnant women could significantly decrease the prevalence of RDS in newborns, thereby easing the burden on healthcare systems and improving overall public health outcomes during the pandemic. The results of this meta-analysis highlight the importance of clear and effective communication strategies targeting pregnant women about the risks of COVID-19 and the potential health implications for their newborns. Public health campaigns should focus on educating pregnant women on the importance of following preventive measures, seeking timely medical advice, and adhering to vaccination schedules to protect both their health and that of their infants.
There is a need for further research to elucidate the precise mechanisms by which maternal SARS-CoV-2 infection leads to RDS in newborns. Such studies could explore the role of placental dysfunction, fetal hypoxia, and direct viral effects on the fetal lungs. Understanding these mechanisms in greater detail will aid in the development of targeted interventions to prevent RDS in newborns of infected mothers. Future studies should consider longitudinal designs to track the long-term respiratory and developmental outcomes of newborns exposed to SARS-CoV-2 in utero. This will provide critical insights into the lasting impacts of maternal COVID-19 infection and inform long-term care strategies for affected individuals. Conducting studies in diverse geographical and healthcare settings can help understand how different management strategies and healthcare infrastructures impact the prevalence and outcomes of RDS among newborns born to COVID-19-infected mothers. These comparative studies will be valuable in tailoring public health interventions to specific regional needs and challenges. Research on the effectiveness of COVID-19 vaccination during pregnancy in reducing the incidence of RDS in newborns could provide compelling evidence to support public health recommendations for vaccination. Studies could focus on the timing of vaccination during pregnancy and its correlation with neonatal health outcomes to optimize vaccination strategies. By addressing these research gaps, the scientific community can better understand and mitigate the impacts of COVID-19 on maternal and neonatal health, contributing to more effective healthcare responses in current and future pandemics.
The strengths of this study include the comprehensive literature search, the inclusion of studies from various countries and settings, and the rigorous methodology employed in study selection, data extraction, and quality assessment. However, several limitations should be acknowledged. The included studies were primarily observational in nature, which inherently carries a risk of bias and confounding factors that may influence the reported associations. Additionally, the severity of COVID-19 infection in pregnant women, gestational age at delivery, and other potential risk factors for RDS were not consistently reported across studies, limiting the ability to perform subgroup analyses or meta-regression to explore these factors as sources of heterogeneity. The high level of heterogeneity observed across the included studies further limits the reliability of pooled estimates, as it reflects significant variability in study design, populations, and outcomes. Despite our efforts to explore the sources of this heterogeneity, the lack of consistent data prevented us from fully addressing this issue through statistical means. Studies did not report the adjusted ORs for the outcome. While some studies provided comparative data between infants born to mothers with SARS-CoV-2 infection and those without, others reported only on infants born to infected mothers. Many of the studies included in our meta-analysis were retrospective, which introduces limitations such as potential recall bias and inconsistencies in data reporting. Furthermore, the varied definitions of RDS across studies may have influenced the results, making it challenging to draw definitive conclusions. Future research should focus on prospective cohort studies with rigorous data collection and standardized reporting to further elucidate the connection between maternal SARS-CoV-2 infection and neonatal respiratory outcomes, including RDS. Investigation of potential modifying factors, such as disease severity, gestational age, mode of delivery, and maternal comorbidities, would provide valuable insights into risk stratification and targeted interventions.
Conclusion
The increased prevalence and risk of RDS among newborns born to mothers with COVID-19. These findings indicate the importance of close monitoring and appropriate respiratory support for these infants and the need for continued research to further elucidate the underlying mechanisms and identify potential preventive or therapeutic strategies. Effective management of maternal COVID-19 infection and optimization of neonatal care are crucial to mitigate the potential adverse respiratory consequences for newborns during the ongoing pandemic and potential future outbreaks of emerging respiratory viruses.
Supplementary Information
Acknowledgements
The authors acknowledge the Nested-Knowledge, MN, USA for providing the access to the software.
Authors’ contributions
Conceptualization: Mahalaqua Nazli Khatib, Rachana Mehta, Methodology: Nishant Rai, Mahakshit Bhat, Sanjit Sah, Software: Muhammed Shabil, Manvinder Brar, Laksmi Thangavelu, Validation: Shilpa Gaidhane, Suhas Ballal, Ganesh Bushi, Formal analysis: Shilpa Sharma, M Ravi Kumar, Gajendra Sharma, Investigation: Sanjay Kumar, Sarvesh Rustagi, Rukshar Syed, Resources: Mohammed Garout, Nabiha A. Bouafia, Amer Alshengeti, Data Curation: Muhammed Shabil, Ali Hazazi, Ali A. Rabaan, Writing - Original Draft Preparation: Mahalaqua Nazli Khatib, Rachana Mehta, Writing - Review & Editing: Mahalaqua Nazli Khatib, Sakshi Pandey, Ashok Kumar Balaraman, Visualization: Sanjit Sah, Sorabh Lakhanpal, Nagavalli Chilakam, Supervision: Nawal A. Al Kaabi, Mubarak Alfaresi, Ashok Kumar Balaraman, Project Administration: Hayam A Alrasheed, Mubarak Alfaresi, Ali A. Rabaan
Funding
This study received no funding.
Data availability
Data availability: The data is with the authors and available on request
Declarations
Ethics approval and consent to participate
Not required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Muhammed Shabil and Shilpa Gaidhane contributed equally to this work.
Contributor Information
Mahalaqua Nazli Khatib, Email: nazlikhatib@dmiher.edu.in.
Sanjit Sah, Email: sanjitsahnepal561@gmail.com.
References
- 1.Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127: 104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phua J, Weng L, Ling L, Egi M, Lim C-M, Divatia JV, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Luca D, Tingay DG, Van Kaam AH, Courtney SE, Kneyber MC, Tissieres P, et al. Epidemiology of neonatal acute respiratory distress syndrome: prospective, multicenter, international cohort study. Pediatr Crit Care Med. 2022;23(7):524–34. [DOI] [PubMed] [Google Scholar]
- 4.Jain L, Eaton DC, editors. Physiology of fetal lung fluid clearance and the effect of labor. In: Seminars in perinatology. Elsevier; 2006. [DOI] [PubMed]
- 5.Man OM, Azamor T, Cambou MC, Fuller TL, Kerin T, Paiola SG, et al. Respiratory distress in SARS-CoV-2 exposed uninfected neonates followed in the COVID outcomes in mother-infant pairs (COMP) study. Nat Commun. 2024;15(1):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allotey J, Fernandez S, Bonet M, Stallings E, Yap M, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bircher C, Wilkes M, Zahradka N, Wells E, Prosser-Snelling E. Remote care and triage of obstetric patients with COVID-19 in the community: operational considerations. BMC Pregnancy Childbirth. 2022;22(1):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huntley BJ, Huntley ES, Di Mascio D, Chen T, Berghella V, Chauhan SP. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstet Gynecol. 2020;136(2):303–12. [DOI] [PubMed] [Google Scholar]
- 10.Anter ME, Abd El-Aal NK, Rezk MAA, Moawad HF, Abudakika AT. Impact of COVID-19 infection during pregnancy on maternal and fetal outcomes. Reprod Dev Med. 2023;7(2).
- 11.Barros FC, Gunier RB, Rego A, Sentilhes L, Rauch S, Gandino S et al. Maternal vaccination against COVID-19 and neonatal outcomes during Omicron: INTERCOVID-2022 study. Am J Obstet Gynecol. 2024. [DOI] [PubMed]
- 12.Baykara N. Clinical characteristics, outcomes, and risk factors for mortality in Pregnant/Puerperal women with COVID-19 admitted to ICU in Turkey: a Multicenter, Retrospective Study from a Middle-Income Country. J Intensive Care Med. 2024;39(6):577–94. [DOI] [PubMed] [Google Scholar]
- 13.Brandt JS, Hill J, Reddy A, Schuster M, Patrick HS, Rosen T, et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am J Obstet Gynecol. 2021;224(4):389 e1-. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Çıplak G, Becerir C, Sarı FN, Alyamaç Dizdar E. Effect of maternal coronavirus disease on Preterm Morbidities. Am J Perinatol. 2023. [DOI] [PubMed]
- 15.Costa S, Giordano L, Bottoni A, Tiberi E, Fattore S, Pastorino R, et al. Vertical transmission of SARS-CoV-2 during pregnancy: a prospective Italian cohort study. Am J Perinatol. 2022. [DOI] [PubMed]
- 16.Dumitriu D, Emeruwa UN, Hanft E, Liao GV, Ludwig E, Walzer L, et al. Outcomes of neonates born to mothers with severe Acute Respiratory Syndrome Coronavirus 2 infection at a Large Medical Center in New York City. JAMA Pediatr. 2021;175(2):157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dzhaynakbaev N, Aldangarova G, Aumoldaeva Z, Toreyeva SM, Suleimenova A. Features of the course and outcome of pregnancy in women with COVID-19. New Armen Med J. 2023;17(1).
- 18.Farhadi R, Ghaffari V, Mehrpisheh S, Moosazadeh M, Haghshenas M, Ebadi A. Characteristics and outcome of infants born to mothers with SARS-CoV-2 infection during the first three waves of COVID-19 pandemic in northern Iran: a prospective cross-sectional study. Ann Med Surg. 2022;78:103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saleh Gargari S, Rahmati N, Fateh R, Khandani A, Nikfar S, Ghafouri-Fard S. Investigation of maternal and perinatal outcome in a population of Iranian pregnant women infected with COVID-19. Sci Rep. 2022;12(1):9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guida JP, Cecatti JG, Souza RT, Pacagnella RC, Ribeiro-do-Valle CC, Luz AG, et al. Preeclampsia among women with COVID-19 during pregnancy and its impact on maternal and perinatal outcomes: results from a national multicenter study on COVID in Brazil, the REBRACO initiative. Pregnancy Hypertens. 2022;28:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamidi OP, Lijewski V, Sheeder J, Powell K, Dolph E, Quayson D, Reeves S. Adverse perinatal outcomes in pregnancies affected by severe COVID-19 infection. AJOG Glob Rep. 2022;2(4):100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapadia SN, Mehta A, Mehta CR, Soni ST, Joharwal N, Dixit M, Vaishnav JG. Study of pregnancy with COVID-19 and its clinical outcomes in a tertiary care teaching hospital in Western India. J South Asian Federation Obstet Gynecol. 2021;13(2):125–30. [Google Scholar]
- 23.Kilic T, Kilic S, Berber NK, Gunduz A, Ersoy Y. Investigation of SARS-CoV‐2 RNA in milk produced by women with COVID‐19 and follow‐up of their infants: a preliminary study. Int J Clin Pract. 2021;75(7):e14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar P, More K, Chawla D, Murki S, Tandur B, NNFC-R Group, et al. Outcomes of neonates born to mothers with coronavirus disease 2019 (COVID-19)—National Neonatology Forum (NNF) India COVID-19 Registry. Indian Pediatr. 2021;58:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Y-C, Wu P-C, Chiu L-C, Chueh H-Y, Chen Y-N, Lee Y-C, et al. Maternal–neonatal outcomes of Obstetric Deliveries Performed in negative pressure isolation rooms during the COVID-19 omicron variant pandemic in Taiwan: a retrospective cohort study of a single Institution. J Clin Med. 2022;11(18): 5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosayebi Z, Esmaeilnia T, Sabagh VG, Naddaf A, Sagheb S, Shariat M et al. Clinical findings, laboratory assessment, and outcomes of 44 infants born to mothers with confirmed or suspected covid-19: a multicenter cohort study. Iran J Pediatr. 2021;31(4).
- 27.Nayak MK, Panda SK, Panda SS, Rath S, Ghosh A, Mohakud NK. Neonatal outcomes of pregnant women with COVID-19 in a developing country setup. Pediatr Neonatol. 2021;62(5):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey AK, Shukla A, Lal P. SARS-CoV-2 transmission risk through expressed breast milk feeding in neonates born to COVID 19 positive mothers: a prospective observational study. Iran J Neonatol. 2021;12:53–7. [Google Scholar]
- 29.Wróblewska-Seniuk K, Basiukajć A, Wojciechowska D, Telge M, Miechowicz I, Mazela J. Clinical characteristics of newborns born to mothers with COVID-19. J Clin Med. 2021;10(19): 4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vimercati A, De Nola R, Trerotoli P, Metta ME, Cazzato G, Resta L, et al. COVID-19 infection in pregnancy: obstetrical risk factors and neonatal outcomes—a monocentric, single-cohort study. Vaccines. 2022;10(2):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P, Wang X, Liu P, Wei C, He B, Zheng J, Zhao D. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020;127: 104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith PB, Ambalavanan N, Li L, Cotten CM, Laughon M, Walsh MC, et al. Approach to infants born at 22 to 24 weeks’ gestation: relationship to outcomes of more-mature infants. Pediatrics. 2012;129(6):e1508-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Argueta LB, Lacko LA, Bram Y, Tada T, Carrau L, Rendeiro AF, et al. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. Iscience. 2022;25(5):104223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SH, Jin JH, Yoo JH, Yoon SW. Association between maternal coronavirus disease 2019 and transient tachypnea of the newborn: a single-center study. Clin Exp Pediatr. 2023;66(11):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasa B, Memur S, Ozturk DY, Bagci O, Uslu SI, Cetinkaya M. Neonatal outcomes of premature infants born to women with the novel coronavirus (SARS-CoV-2) infection: a case control study. Am J Perinatol. 2023;40(15):1715–24. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz DA. Vertical transmission of severe acute respiratory syndrome coronavirus 2 from the mother to the infant. JAMA Pediatr. 2020;174(10):1004–5. [DOI] [PubMed] [Google Scholar]
- 37.Raschetti R, Vivanti AJ, Vauloup-Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11(1):5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz DA, Mulkey SB, Roberts DJ. SARS-CoV-2 placentitis, stillbirth, and maternal COVID-19 vaccination: clinical-pathologic correlations. Am J Obstet Gynecol. 2023;228(3):261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Surekha M, Suneetha N, Balakrishna N, Putcha UK, Satyanarayana K, Geddam JB, et al. Impact of COVID-19 during pregnancy on placental pathology, maternal and neonatal outcome–A cross-sectional study on anemic term pregnant women from a tertiary care hospital in southern India. Front Endocrinol. 2023;14:1092104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magawa S, Nii M, Enomoto N, Tamaishi Y, Takakura S, Maki S, et al. COVID-19 during pregnancy could potentially affect placental function. J Maternal-Fetal Neonatal Med. 2023;36(2):2265021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data availability: The data is with the authors and available on request