Abstract
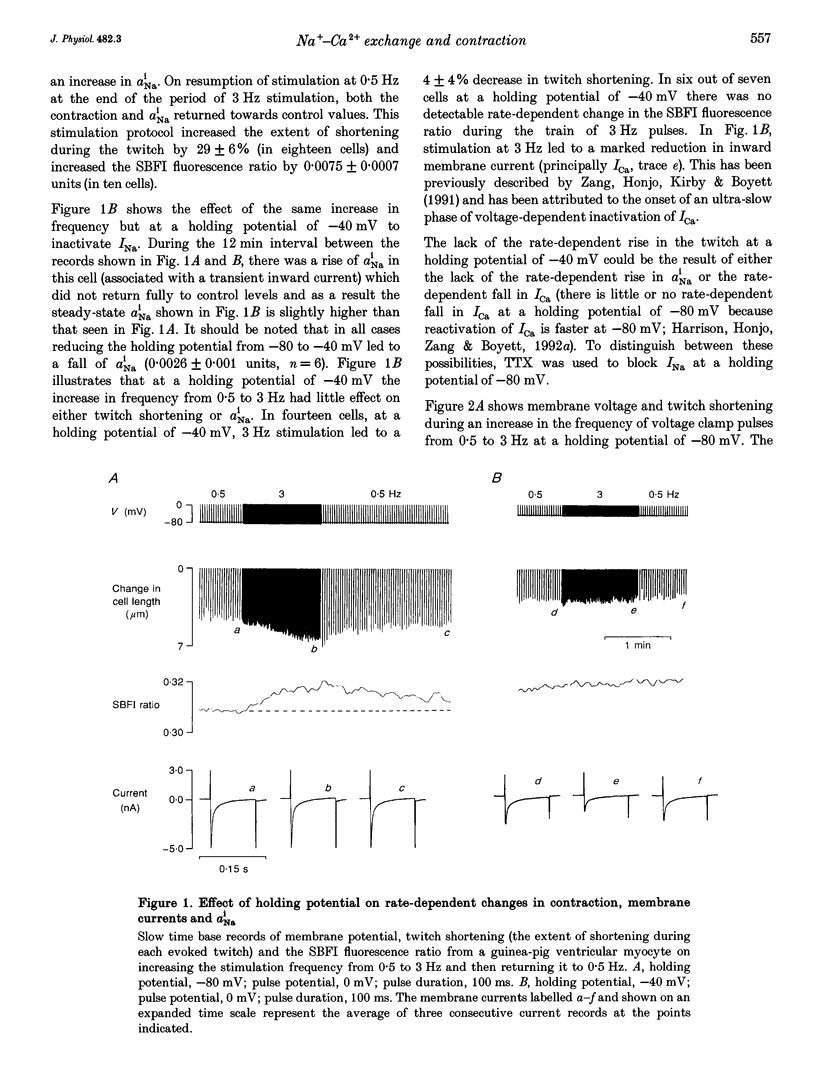
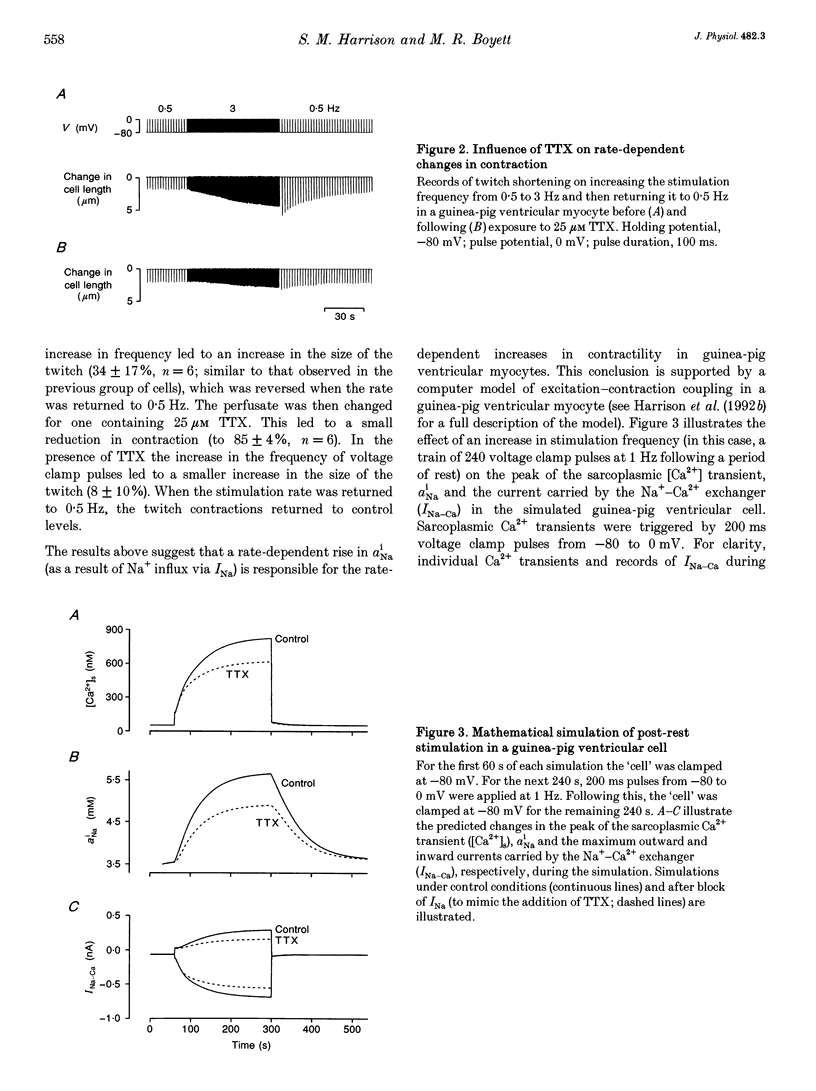
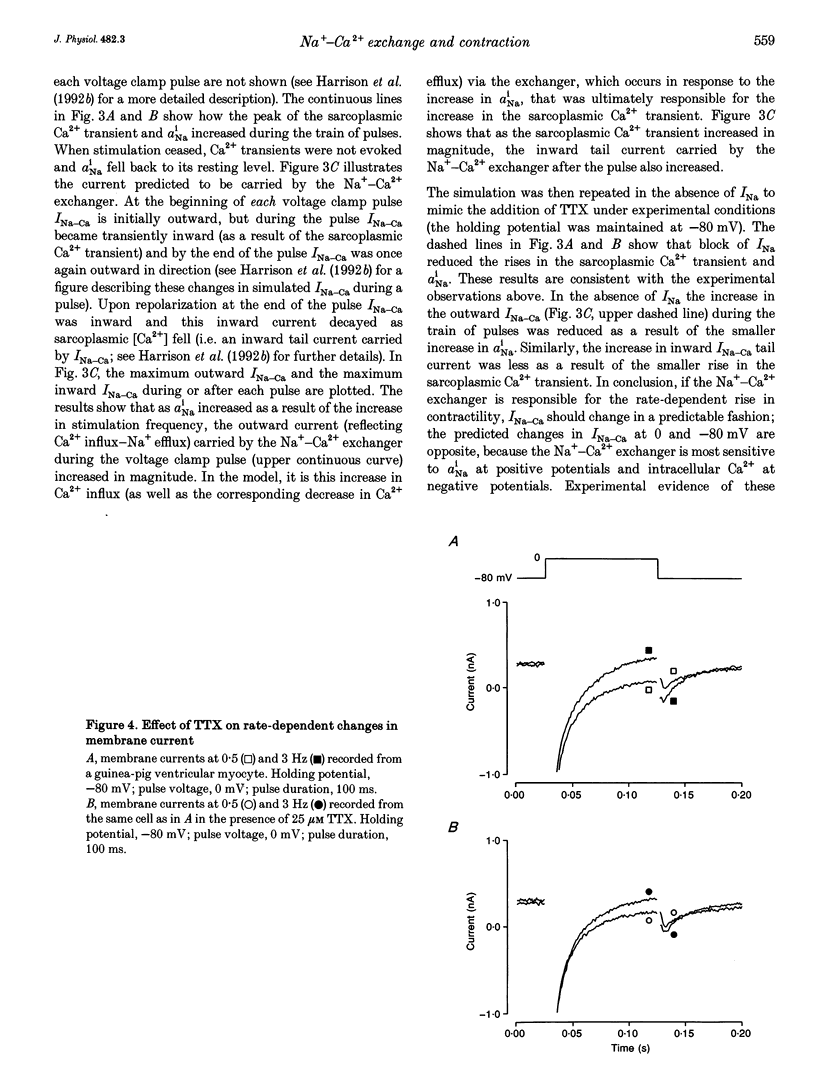
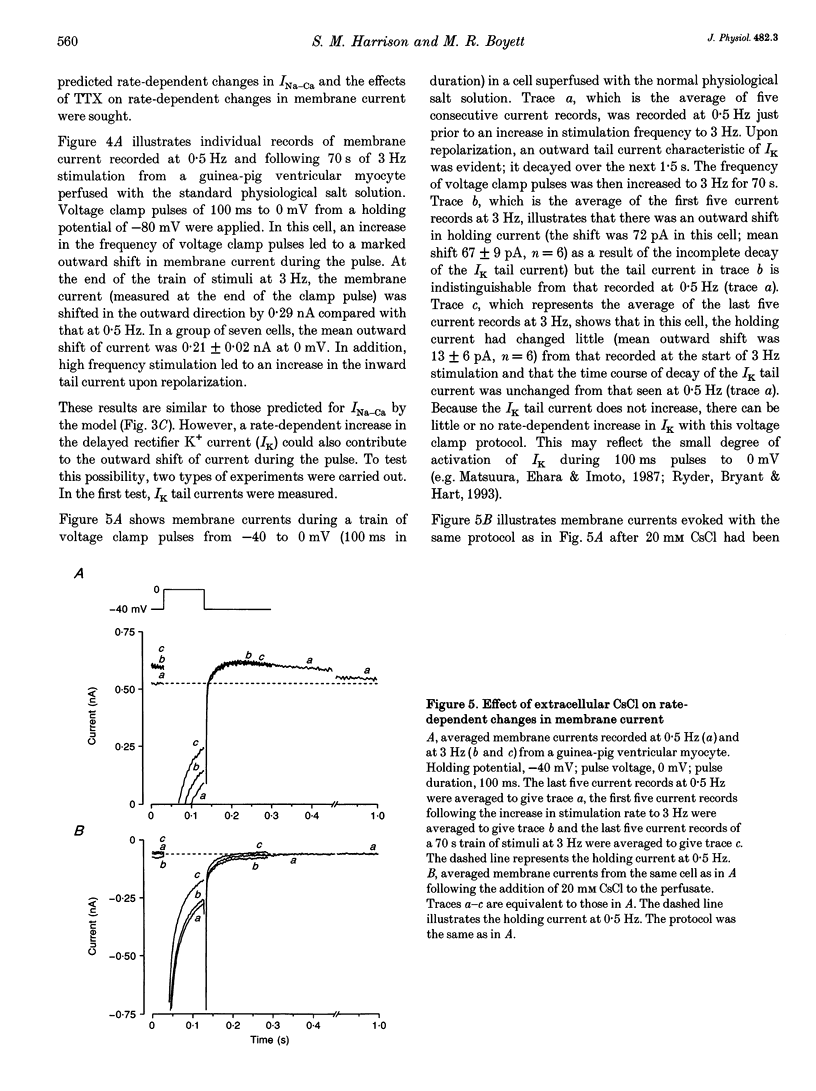
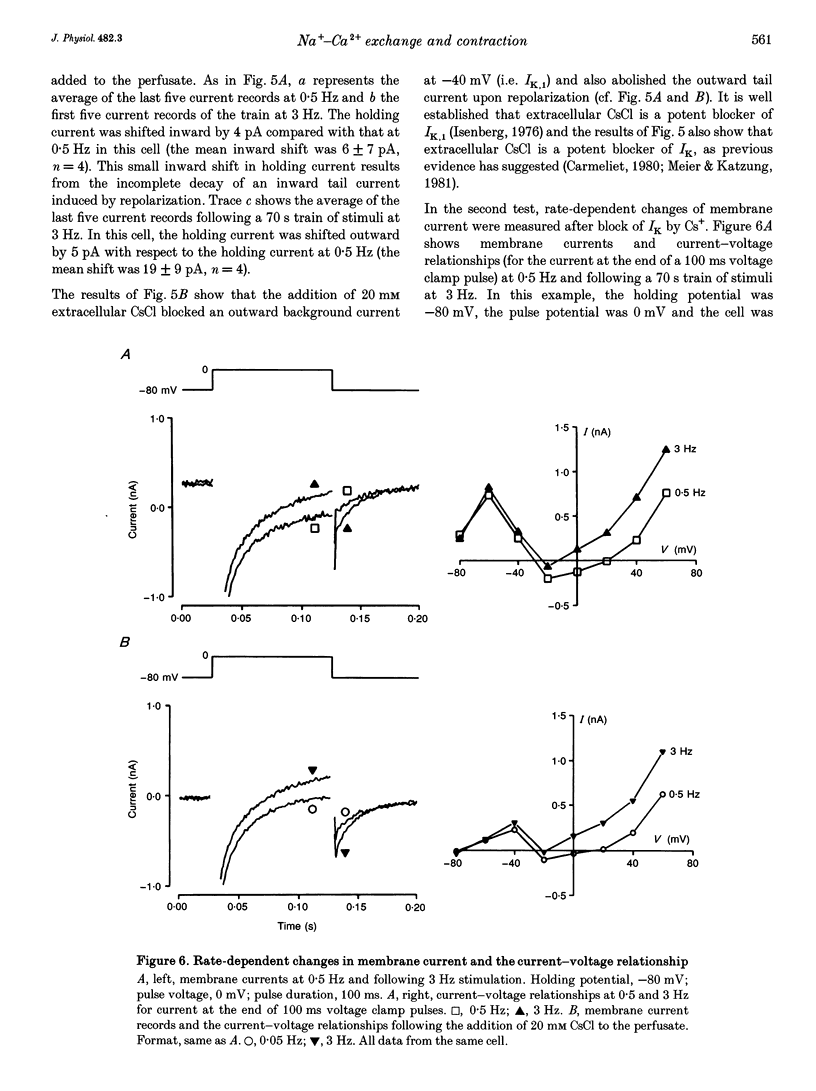
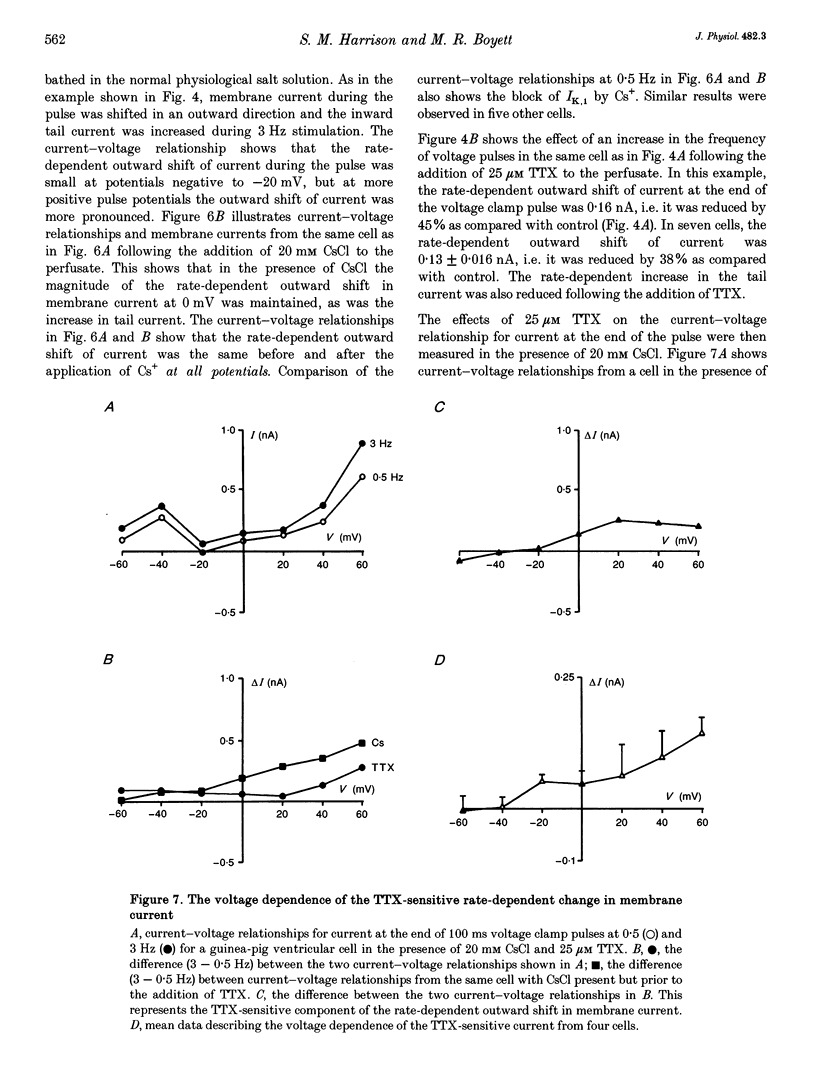
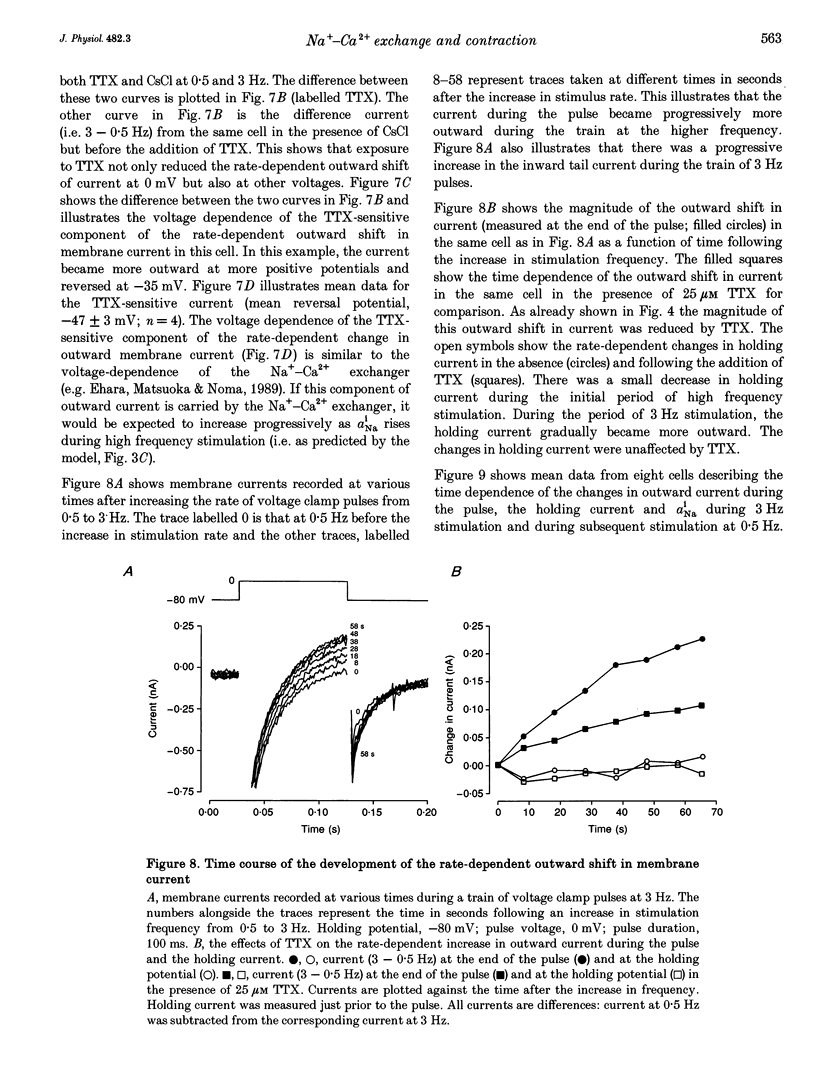
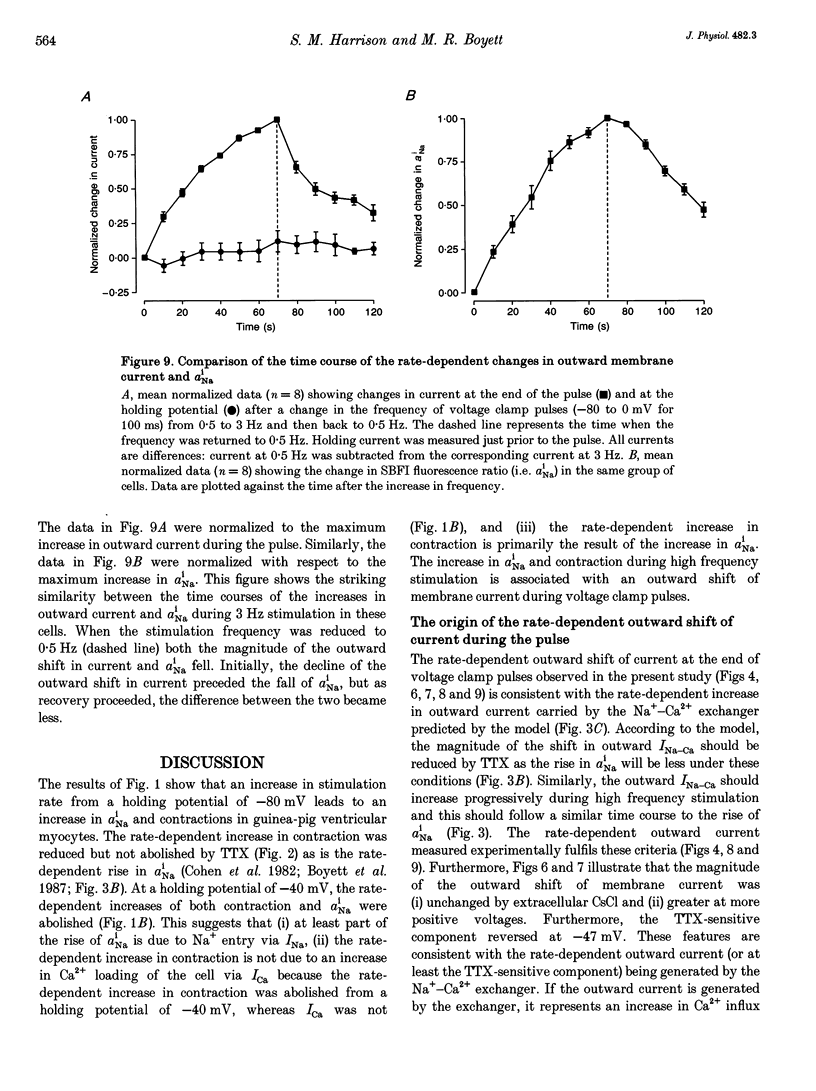
1. The intracellular sodium activity (alpha Na1), contraction and membrane current were recorded simultaneously in voltage-clamped guinea-pig ventricular myocytes. 2. Increasing the frequency (from 0.5 to 3 Hz) of voltage clamp pulses to 0 mV from a holding potential of -80 mV led to an increase in both alpha Na1 and contraction. The rate-dependent increase in contraction was reduced by 25 microM tetrodotoxin (TTX) and abolished with a holding potential of -40 mV. There was no rate-dependent rise in alpha Na1 with a holding potential of -40 mV. These results suggest an important role for alpha Na1 and in particular Na+ influx via Na+ channels during rate-dependent changes in contraction. 3. After an increase in frequency from 0.5 to 3 Hz, membrane current at the end of voltage clamp pulses became progressively more outward and the tail current upon at repolarization became progressively more inward compared with those recorded at 0.5 Hz. TTX reduced the magnitude of both the outward and inward rate-dependent shifts of current. 4. The addition of extracellular CsCl blocked the inward rectifier potassium current (IK.1) and the delayed rectifier (IK), but did not change the rate-dependent shift in current. 5. The difference between current-voltage relationships at 0.5 and 3 Hz showed that the rate-dependent outward shift of current at the end of voltage clamp pulses was small at potentials negative to -20 mV, was larger at more positive potentials and was reduced by TTX at most potentials. The TTX-sensitive component reversed at -47 mV. 6. These results are consistent with a net increase in outward Na(+)-Ca2+ exchange current during a voltage clamp pulse in response to the rise of alpha Na1. The increase in outward current (resulting from either enhanced Ca2+ influx or reduced Ca2+ efflux) will augment the Ca2+ load of the cell and contribute to the rate-dependent increase in contraction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyett M. R. An analysis of the effect of the rate of stimulation and adrenaline on the duration of the cardiac action potential. Pflugers Arch. 1978 Nov 14;377(2):155–166. doi: 10.1007/BF00582846. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Hart G., Levi A. J., Roberts A. Effects of repetitive activity on developed force and intracellular sodium in isolated sheep and dog Purkinje fibres. J Physiol. 1987 Jul;388:295–322. doi: 10.1113/jphysiol.1987.sp016616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyett M. R., Levi A. J. A simple electronic circuit for determining the twitch force and resting force of small heart muscle preparations. Pflugers Arch. 1987 Oct;410(3):340–341. doi: 10.1007/BF00580287. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Moore M., Jewell B. R., Montgomery R. A., Kirby M. S., Orchard C. H. An improved apparatus for the optical recording of contraction of single heart cells. Pflugers Arch. 1988 Dec;413(2):197–205. doi: 10.1007/BF00582531. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells? Cardiovasc Res. 1992 May;26(5):433–442. doi: 10.1093/cvr/26.5.433. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Fozzard H. A., Sheu S. S. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982 May;50(5):651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Ehara T., Matsuoka S., Noma A. Measurement of reversal potential of Na+-Ca2+ exchange current in single guinea-pig ventricular cells. J Physiol. 1989 Mar;410:227–249. doi: 10.1113/jphysiol.1989.sp017530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton J. E., Orchard C. H., Boyett M. R. Diastolic, systolic and sarcoplasmic reticulum [Ca2+] during inotropic interventions in isolated rat myocytes. J Physiol. 1991 Jun;437:351–375. doi: 10.1113/jphysiol.1991.sp018600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., McCall E., Boyett M. R. The relationship between contraction and intracellular sodium in rat and guinea-pig ventricular myocytes. J Physiol. 1992 Apr;449:517–550. doi: 10.1113/jphysiol.1992.sp019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Uehara A. "Creep currents" in single frog atrial cells may be generated by electrogenic Na/Ca exchange. J Gen Physiol. 1986 Jun;87(6):857–884. doi: 10.1085/jgp.87.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibers: cesium as a tool to block inward rectifying potassium currents. Pflugers Arch. 1976 Sep 30;365(2-3):99–106. doi: 10.1007/BF01067006. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Kakei M., Sato R., Shibasaki T., Matsuda H., Irisawa H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984 May 24;309(5966):354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Dagostino M. Effect of strophanthidin on intracellular Na ion activity and twitch tension of constantly driven canine cardiac Purkinje fibers. Biophys J. 1982 Dec;40(3):185–198. doi: 10.1016/S0006-3495(82)84474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A. J. A role for sodium/calcium exchange in the action potential shortening caused by strophanthidin in guinea pig ventricular myocytes. Cardiovasc Res. 1993 Mar;27(3):471–481. doi: 10.1093/cvr/27.3.471. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Ehara T., Imoto Y. An analysis of the delayed outward current in single ventricular cells of the guinea-pig. Pflugers Arch. 1987 Dec;410(6):596–603. doi: 10.1007/BF00581319. [DOI] [PubMed] [Google Scholar]
- Meier C. F., Jr, Katzung B. G. Cesium blockade of delayed outward currents and electrically induced pacemaker activity in mammalian ventricular myocardium. J Gen Physiol. 1981 May;77(5):531–547. doi: 10.1085/jgp.77.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minta A., Tsien R. Y. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989 Nov 15;264(32):19449–19457. [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. [Na] and [K] dependence of the Na/K pump current-voltage relationship in guinea pig ventricular myocytes. J Gen Physiol. 1989 Sep;94(3):539–565. doi: 10.1085/jgp.94.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo G. C., Chapman R. A. A sodium-activated potassium current in intact ventricular myocytes isolated from the guinea-pig heart. Exp Physiol. 1990 Nov;75(6):839–842. doi: 10.1113/expphysiol.1990.sp003465. [DOI] [PubMed] [Google Scholar]
- Ryder K. O., Bryant S. M., Hart G. Membrane current changes in left ventricular myocytes isolated from guinea pigs after abdominal aortic coarctation. Cardiovasc Res. 1993 Jul;27(7):1278–1287. doi: 10.1093/cvr/27.7.1278. [DOI] [PubMed] [Google Scholar]
- Wang D. Y., Chae S. W., Gong Q. Y., Lee C. O. Role of aiNa in positive force-frequency staircase in guinea pig papillary muscle. Am J Physiol. 1988 Dec;255(6 Pt 1):C798–C807. doi: 10.1152/ajpcell.1988.255.6.C798. [DOI] [PubMed] [Google Scholar]


