Abstract
Background
Sex is a determinant of the incidence and etiology of arrhythmia. Observational and basic studies suggest that sex hormones are essential in this process; however, the relationship between sex hormones and arrhythmia remains unclear. Mendelian randomization (MR) was used to investigate the causal relationships between sex hormone levels, arrhythmia, and electrocardiographic (ECG) indices.
Methods
Large genome-wide association studies (GWAS) data on sex hormones, stratified by sex, from the UK biobank consortium, were used as exposure data, and data on atrial fibrillation (AF), atrioventricular block, sick sinus syndrome, paroxysmal tachycardia, and ECG indices were obtained from the FinnGen consortium and summarized large GWAS data. Inverse variance weighting or wald odds was used as the primary analytical method, and weighted medians and MR-Egger regression were used for complementary analyses. The results of the MR of sex hormones and AF from different sources were analyzed using a meta-analysis. Summary-data-based MR analysis was utilized to explore the relationship between sex-hormone related drugs and arrhythmia.
Results
In men, genetically predicted higher estradiol concentrations were associated with a lower risk of AF (odds ratio: 0.908 [0.852–0.967]; p = 0.0029], whereas genetically predicted higher concentrations of total testosterone were associated with lower heart rate variability. Sex hormones showed no association with atrioventricular block, sick sinus syndrome, paroxysmal tachycardia, resting heart rate, P wave duration, P wave terminal force in lead V1 [PTFV1], PR interval, QRS duration, QTc [QT interval corrected by heart rate], ST duration, spatial [spQRSTa] and frontal [fQRSTa] QRS-T angles in males. In females, there was no significant evidence that sex hormones are associated with arrhythmias or ECG indices.
Conclusion
In this study, we identified a potential causal relationship between estradiol and the risk of AF in males. However, there was no significant association between sex hormones and either arrhythmias or ECG indices in females. These results suggested that sex hormones may play a limited role in cardiac arrhythmias, which requires further verification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04335-7.
Keywords: Arrhythmia, Sex hormones, Electrocardiography, Mendelian randomization, Reproductive factors
Introduction
Arrhythmia, a common cardiovascular disease, displays notable differences in occurrence and outcomes between sexes [1, 2]. Specifically, males have a higher prevalence of conditions such as atrial fibrillation (AF), atrioventricular block (AVB), and Brugada syndrome, whereas long QT syndrome and sick sinus syndrome (SSS) are more common in females [2]. Sex hormones, including testosterone (total testosterone [TT] and bioavailable testosterone [BT]), estradiol, and sex hormone-binding globulin (SHBG), play crucial roles in regulating human health and disease progression. Recent research has indicated a potential association between sex hormones and sex differences in the development of arrhythmias [3–9]. However, previous studies have yielded conflicting findings regarding the association between sex hormones and arrhythmias in males. In the UK database prospective study, high level of SHBG was associated with an increased risk of AF, bradycardia, and ventricular arrhythmias in men [3], where other studies provided conflicting results [10]. The incidence of arrhythmia increases in postmenopausal females; however, the effect of estradiol supplementation on AF after menopause remains unclear [11].
Differences in electrocardiographic (ECG) phenotypes and indices between males and females may also be related to sex hormones. Indeed, females have a longer QT interval than males. Moreover, with the deepening of research on transgender people, the influence of sex hormones rather than sex on the ECG phenotype has been gradually revealed, which reflects the importance of sex hormones in cardiac electrophysiology [12, 13]. However, the inferences made from observational studies have several limitations, including confusion, reverse causality, and detection bias.
Mendelian randomization (MR), based on the random allocation of genetic variation at conception, reveals the causal relationship between exposure and outcome using genetic variations strongly related to exposure factors, minimizing reverse causality and potential confounding bias [14, 15]. Therefore, to further clarify the influence of sex hormones on arrhythmias, we used MR to analyze the relationship between sex hormones (TT, BT, estradiol, and SHBG) and arrhythmias (AF, AVB, SSS, and paroxysmal tachycardia [PT]). Given the significant differences in hormone levels in females during the menstrual cycle and after menopause, age at menopause and age at menarche were included as exposure variables in females. To better understand the influence of sex hormones on cardiac electrophysiology, MR analyses of sex hormones and ECG indices were performed.
Methods
Exposure data
Instrument variants of exposure were obtained from large published genome-wide association studies (GWAS). Ruth et al. provided [16] the aggregated data for male and female TT, BT, and SHBG, while the estradiol data were acquired from a study published by Schmitz et al. [17]. Data on age at menopause were extracted from a study by Ruth et al. [18], and age at menarche was obtained from a study published by Day et al. [19]. All data sources are detailed in Supplementary Table 1. We selected the single nucleotide polymorphisms (SNPs) with the lowest p-values (p-value < 5*10 − 8). The independence of the SNPs was ensured at a linkage disequilibrium r2 < 0.001 and a distance of 10,000 kb. F-statistics was used to test the instrument strength, which was obtained according to the following formula:
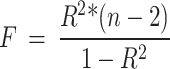 |
N represented the number of participants, and R2 represented the explained variances of each SNP, calculated according to the following equation:
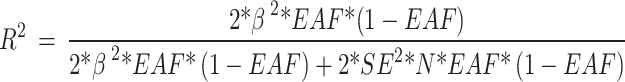 |
β is the estimated effect of the SNP, EAF is the effect allele frequency, and SE represents the standard error. All of the results are shown in Supplementary Tables 2–11.
Outcome data
The GWAS data for AF, PT, and AVB were acquired from the FinnGen Consortium. The data on SSS, ECG indices (resting heart rate, P wave duration in lead V1, P wave terminal force in lead V1 [PTFV1], PR interval, QRS duration, QTc [QT interval corrected by heart rate], ST duration, spatial [spQRSTa] and frontal [fQRSTa] QRS-T angles, heart rate variability [HRVSDNN: the standard deviation of normal-to-normal heart beat intervals, HRVRMSSD: the root mean square of the successive differences of heartbeat intervals, HRVpvRSA/HRVHF: the peak-valley respiratory sinus arrhythmia or high frequency power]) were extracted from a large, published GWAS [20–28]. Validation data for AF were obtained from a large GWAS [29], whereas other diseases and ECG indices were not validated because of insufficient GWAS data, sample overlap, and other issues.
Summary-data-based mendelian randomization (SMR) analysis
We searched for gene targets of estrogen and androgen drugs (testosterone undecanoate, testosterone, methyltestosterone, estradiol benzoate, estrone sulfate, and estradiol acetate) in the Drug Bank (https://go.drugbank.com/). We obtained expression quantitative trait loci data (eqtls) related to the target genes from the eQTLGen Consortium. SNPs with p-values < 5*10 − 8 and minor allele frequencies > 2% were screened. SMR software was used for the analysis (http://cnsgenomics.com/software/smr/). The heterogeneity in dependent instruments (HEIDI) test was employed to evaluate whether the association between gene expression and the outcome was caused by a linkage scenario. A P-HEIDI value of < 0.05 was regarded as evidence suggesting that the observed association may result from two distinct genetic variants that are in strong linkage disequilibrium with one another. A two-side p-value < 0.05 was considered statistically significant.
MR and sensitivity analysis
All MR analyses were in accordance with the following assumptions regarding the genetic instrumental variables: (a) these variables are related to the exposure, (b) they are independent of confounders, and (c) they affect the outcome only through exposure. And our MR analyses were conducted in accordance with STROBE-MR checklist (Supplementary file 1). Our MR study was an exploratory study on the relationship between sex hormones, arrhythmias, and ECG indices. The study design was shown in Fig. 1. A two-sided p-value < 0.003 (0.05/16 outcomes) was considered statistically significant, and the presence of a p-value < 0.05 but > 0.003 suggests a potential causal relationship. MR was performed using the inverse-variance weighted average approach (IVW), which has a higher test power when there is no pleiotropy in the instrumental variables. Wald ratio estimates were used when there was only one SNP. Weighted medians and MR-Egger regression were used for complementary analyses [30, 31]. MR-Egger provided an intercept to evaluate horizontal pleiotropy. Heterogeneity analyses were performed using Cochran’s Q test. Finally, we conducted a meta-analysis to synthesize the MR results of the association between sex hormones and AF, selecting either a fixed- or random-effects model based on the presence and magnitude of heterogeneity. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to present the results for arrhythmias, and beta (β) with 95% CIs for ECG-related indices. Forest plots were used to demonstrate the results of sex hormones and cardiac arrhythmias, and heat plots were used to illustrate the results of sex hormones and ECG indices. R packages TwoSampleMR, ComplexHeatmap, forestploter, and meta in R (version 4.2.1) were used to conduct the analyses.
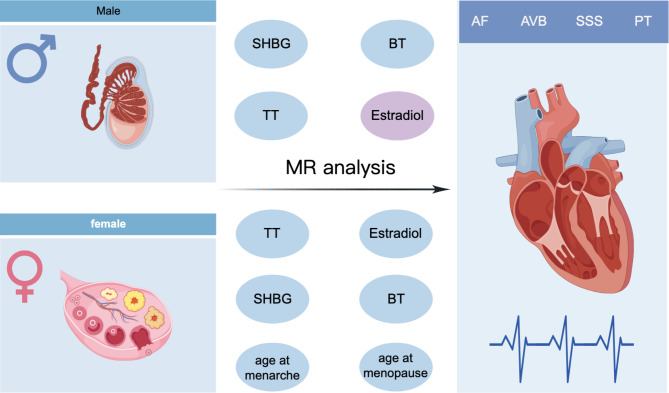
Fig. 1.
The graph for the study design (By Figdraw). AF: atrial fibrillation, AVB: atrioventricular block, SSS: sick sinus syndrome, PT: paroxysmal tachycardia, TT: total testosterone, BT: bioavailable testosterone, SHBG: sex hormone binding globulin
Results
Association between genetically predicted sex hormones and cardiac arrhythmia in male
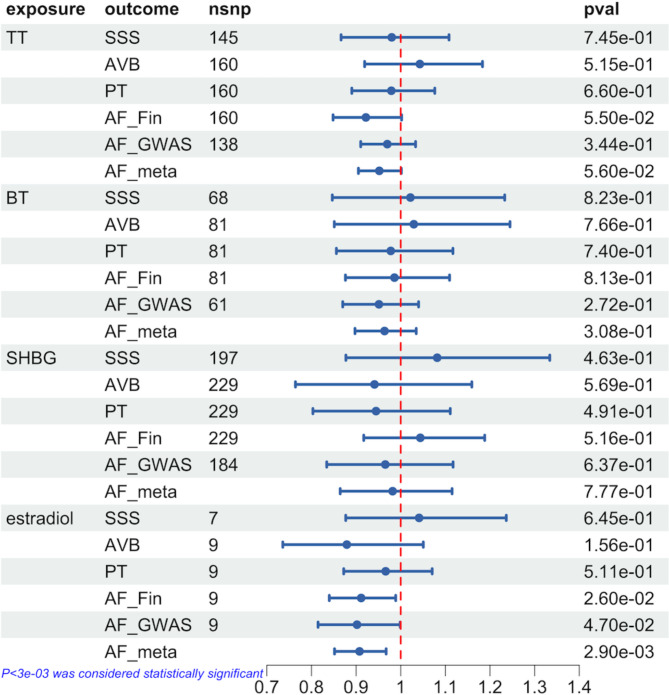
In men, genetically predicted high levels of estradiol were associated with a lower risk of AF [OR = 0.91 (0.84–0.99), p = 0.026], while no association was found with AVB, SSS, or PT. Additionally, there was no correlation between genetically predicted TT, BT, and SHBG levels and AF, AVB, SSS, and PT. In sensitivity analysis, the weight median test revealed the same direction between estradiol and AF [OR = 0.90 (0.83–0.98), p = 0.010], and we did not detect horizontal pleiotropy (p = 0.744) or heterogeneity (p = 0.147).
In the validation cohort, we found that genetically predicted high levels of estradiol were still associated with a lower risk of AF [OR = 0.90 (0.82–1.00), p = 0.047], while other sex hormones were still not associated with AF. In the meta-analysis, the correlation between high estradiol levels and AF risk was more significant [OR = 0.91 (0.85–0.97), p = 0.0029]. All of the IVW test results are presented in Fig. 2. The results of the sensitivity analysis, horizontal pleiotropy tests, and heterogeneity tests are presented in Supplementary Tables 12–13.
Fig. 2.
Genetic association between sex hormones and cardiac arrhythmia in male based on IVW-MR estimates. AF: atrial fibrillation, AVB: atrioventricular block, SSS: sick sinus syndrome, PT: paroxysmal tachycardia, TT: total testosterone, BT: bioavailable testosterone, SHBG: sex hormone binding globulin, OR: Odds ratio, 95%CI: 95% confidence intervals, AF_Fin: atrial fibrillation genome-wide association study from FinnGen Consortium, AF_GWAS: atrial fibrillation genome-wide association study from published study, AF_meta: meta-analysis of the results of atrial fibrillation, nsnp: the number of single nucleotide polymorphisms, pval: the value of p, IVW-MR: inverse-variance weighted average approach- mendelian randomization
Association between genetically predicted sex hormones and reproductive factors and cardiac arrhythmia in females
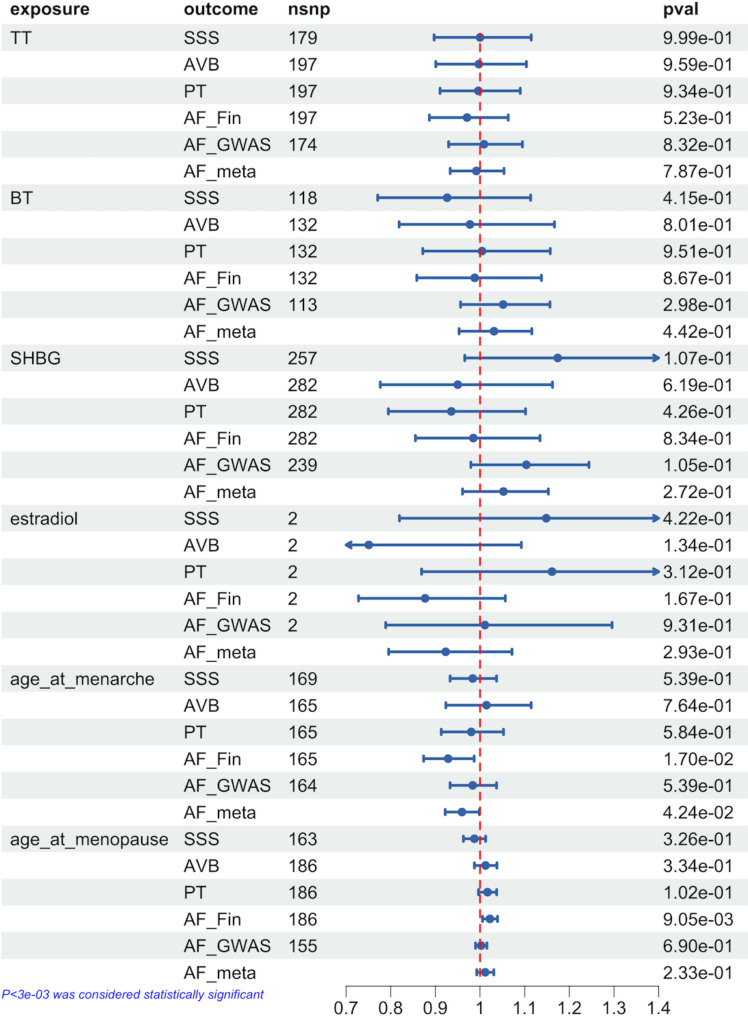
In females, we found no association between genetically predicted sex hormones and cardiac arrhythmias. Considering the fluctuation of sex hormones in women due to menopause and the menstrual cycle, we performed MR between the age of menarche or menopause and arrhythmia. No association was found between genetically predicted age at menopause and arrhythmias, whereas an earlier age at menarche predicted by genetic analysis was associated with an increased risk of AF [OR = 0.93 (0.87–0.99), p = 0.017]. The weighted median and MR-Egger test showed the same direction between age at menarche and AF. No heterogeneity (p = 9.22E-14) or horizontal pleiotropy was observed (p = 0.766).
Moreover, no significant correlation was found between genetic prediction of age at menarche and AF in the validation cohort. However, the meta-analysis verified the correlation between them [OR = 0.96 (0.92–1.00), p = 0.042]. Figure 3 shows all of the IVW test results. The results of sensitivity analysis, horizontal pleiotropy tests, and heterogeneity tests are shown in Supplementary Tables 14–15.
Fig. 3.
Genetic association between sex hormones and cardiac arrhythmia in female based on IVW-MR estimates. AF: atrial fibrillation, AVB: atrioventricular block, SSS: sick sinus syndrome, PT: paroxysmal tachycardia, TT: total testosterone, BT: bioavailable testosterone, SHBG: sex hormone binding globulin, OR: Odds ratio, 95%CI: 95% confidence intervals, AF_Fin: atrial fibrillation genome-wide association study from FinnGen Consortium, AF_GWAS: atrial fibrillation genome-wide association study from published study, AF_meta: meta-analysis of the results of atrial fibrillation, nsnp: the number of single nucleotide polymorphisms, pval: the value of p, IVW-MR: inverse-variance weighted average approach- mendelian randomization
Association between genetically predicted sex hormones and ECG indices in males
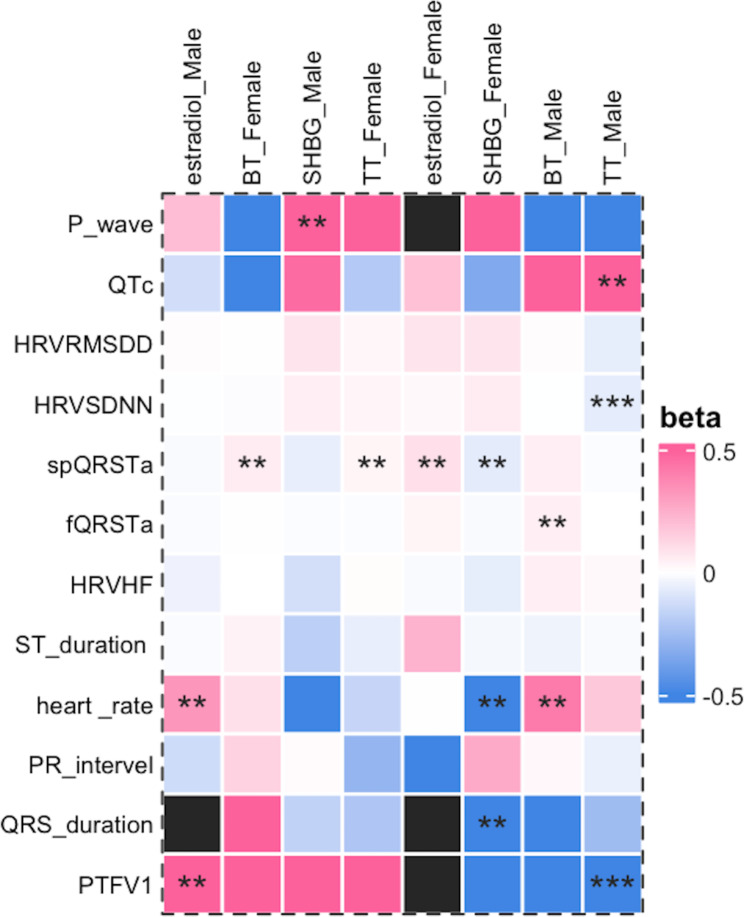
In males, genetically predicted TT was correlated with PTFV1 [β = − 1148.00 (–1742.79 to − 553.21), p = 0.0002], QTc [β = 0.71 (0.07–1.34), p = 0.029], and HRVSDNN [β = − 0.068 (–0.113 to − 0.024), p = 0.0027]. No correlation was found between the TT level and other ECG indices. Genetically predicted BT was correlated with fQRSTa [β = 0.05 (0.01–0.09), p = 0.028] and heart rate [β = 0.42 (0.04–0.80), p = 0.032]. The genetically predicted SHBG was correlated with the P wave duration [β = 6.73 (0.04–13.43), p = 0.049]. The genetically predicted estradiol was associated with PTFV1 [β = 599.39 (160.21–1038.56), p = 0.0075].
In the sensitivity analyses, the directions between TT, PTFV1, and QTc were opposite, whereas the remaining results suggested the same direction. Heterogeneity was found in most samples, while no horizontal pleiotropy was found. Figure 4 shows the results of the IVW test, and Supplementary Table 16 shows the results of the sensitivity analyses.
Fig. 4.
Genetic associations of sex hormones and ECG indices. Based on inverse-variance weighted estimates or Wald ratio estimates. Black means there was no results. ECG: electrocardiography, TT: total testosterone, BT: bioavailable testosterone, SHBG: sex hormone binding globulin, PTFV1: P wave terminal force in lead I, spQRSTa: spatial QRS-T angles, fQRSTa: frontal QRS-T angles, HRVSDNN: the standard deviation of normal-to-normal heart beat intervals, HRVRMSSD: the root mean square of the successive differences of heart beat intervals, HRVpvRSA/HRVHF: the peak-valley respiratory sinus arrhythmia or high frequency power. “***” represented p_value < 0.003, and “**” represented p-value less than 0.05 but greater than 0.003
Association between genetically predicted sex hormones and ECG indices in females
In females, genetically predicted TT was correlated with spQRSTa [β = 0.03 (0.00–0.06), p = 0.040], and genetically predicted BT was correlated with spQRSTa [β = 0.06 (0.01–0.11), p = 0.022]. SHBG was associated with QRS duration [β = − 2.20 (–4.03 to − 0.35), p = 0.019], spQRSTa [β = − 0.07 (–0.14 to − 0.01), p = 0.026], and heart rate [β = − 0.863 (–1.45 to − 0.28), p = 0.0039], while genetically predicted estradiol was correlated with spQRSTa [β = 0.10 (0.00–0.20), p = 0.040] (Fig. 4). In the sensitivity analysis, the association between SHBG and spQRSTa showed different trends in other tests; however, the remaining trends were consistent. Heterogeneity existed except for in the case of estradiol, and no horizontal pleiotropy was found. Supplementary Table 16 shows all the results.
SMR analysis of sex hormone-related drug targets and arrhythmias
Three putative loci (AR, ESR1, and ESR2) were identified by searching the databases. Only the ESR1 and ESR2 loci were found in the eqtls. We found no association between the ESR1 and ESR2 targets or arrhythmias in the SMR analysis. All of the results are shown in Supplementary Table 17.
Discussion
The sex specificity of arrhythmias remains a key research focus, and sex hormones play a critical role in this phenomenon. In this study, we found that estradiol was associated with a reduced risk of AF in men, while no relationship between sex hormones and arrhythmia was found in women, revealing the potential effect of sex hormones on cardiac electrophysiology.
Estradiol is thought to exert a cardioprotective effect, mainly in the female population, whereas males have lower estrogen levels. Previous research has suggested that the biological action of testosterone may be related to estradiol in males [32]. Appiah et al. [33] showed that a low estradiol concentration was associated with an elevated risk of cardiovascular mortality. However, few studies have investigated the association between estradiol levels and arrhythmias in men. In the Framingham Heart Study, decreased estradiol levels were associated with an increased risk of AF in middle-aged and older men [5]. In the UK database prospective study, high level of SHBG was associated with an increased risk of AF, bradycardia, and ventricular arrhythmias, while low-dose testosterone was associated with an increased risk of bradycardia and AF in men [3]. Besides, low level estradiol in men may be related to the risk of idiopathic outflow tract ventricular arrhythmias [8]. Low testosterone concentrations and high SHBG concentrations may simultaneously indicate decreased estradiol levels [34]. However, the relationship between estradiol and AF has not yet been examined. The effect of estradiol may have been overlooked because of the low estradiol levels in men.
Estradiol can improve insulin sensitivity, reduce body fat distribution, prolong action potential duration in electrical conduction, and reduce the occurrence of AF through various factors [34]. However, due to the paucity of observational studies and the limitation of confounding bias, no definitive conclusions can be drawn. Our MR further confirms the important role of estradiol in the development of AF in men while finding no evidence of horizontal pleiotropy.
In postmenopausal women, administration of exogenous estrogen alone may elevate the risk of AF [11, 35]. Moreover, case reports in transgender women have suggested that elevated estradiol and reduced testosterone levels are associated with AF development [36]. However, unlike transgender and menopausal women, who may require estradiol levels to reach premenopausal levels that are significantly higher than normal levels in men, we utilized GWAS data on estradiol levels at an average of 203.8 pmol/L in men [17]. It is plausible that slightly increased estradiol levels confer protection against the onset of AF in men, whereas excessive estradiol levels are detrimental. In women, we did not find this association, which may be related to the number of female estradiol SNPs; however, a larger sample of GWAS data may be needed in the future. We also investigated the effects of age at menopause/menarche on arrhythmias. Consistent with a previous MR study, no significant association was observed between age at menopause/menarche and AF [37].
In this MR study, we found no association between testosterone, SHBG, and AF, SSS, PT, or AVB in either men or women. In a prospective study from the UK Biobank, low-dose testosterone was associated with an increased risk of bradycardia and AF in men [3]. In patients undergoing testosterone replacement therapy, TRAVERSE study found that those receiving testosterone had a higher risk of non-fatal arrhythmias and AF compared to those receiving a placebo [38]. However, a recent meta-analysis found no significant association between testosterone replacement therapy and the risk of AF or non-fatal arrhythmias [39]. Similarly, the relationship between endogenous testosterone levels and non-fatal arrhythmias was not confirmed [39]. Our findings seem to support this lack of association, however, due to the inability to perform subgroup analyses by ethnicity or varying hormone concentrations, the potential relationship remains inconclusive. Research on SHBG and arrhythmias remains relatively limited. In a prospective study from the UK Biobank, SHBG was associated with AF, ventricular arrhythmias, and bradycardia in men [3]. In a cohort of 1,019 men (17 cases), Rosenberg et al. observed a positive correlation between SHBG levels and the incidence of AF [10]. However, the Multi-Ethnic Study of Atherosclerosis did not identify a similar association [4], which may be attributed to differences in age or geographical distribution. Tanja et al. suggested that total testosterone may have a protective effect against AF in women [9], though this was not observed in another study [40]. Based on our results, the relationship between testosterone, SHBG, and arrhythmias appeared to be insignificant. Sex hormones may have a limited role in arrhythmias, and they might influence arrhythmias through other pathways. Additionally, we performed SMR analysis between estrogen and androgen drugs and arrhythmia, and no significant association with arrhythmia was identified. Due to limitations in the data, we were unable conduct a gender-stratified analysis.
Our study suggested an effect of total testosterone on HRVSDNN in males and revealed a suggestive causal relationship between sex hormones and ECG parameters, indicating a role of sex hormones in cardiac electrophysiology. In clinical research and basic experiments, sex hormones regulate cardiac repolarization and other cellular electrophysiological processes by modulating ion channels in cardiomyocytes, which is considered the primary determinant of the disparity in ECG patterns between males and females, particularly with respect to QTc [41]. In this study, we found no direct influence of sex hormones on QTc, although this finding does not entirely negate their potential impact on QTc. A previous MR study found no correlation between testosterone levels and QTc [42]. A potential reason for this could be attributed to the fact that MR implies enduring consequences rather than temporary exposure. Additional, the effects of different sex hormones on QTc vary depending on the dose and gender [41]. Unfortunately, due to the lack of access to this data, we were unable to conduct a more in-depth exploration of this relationship.
Our study is the first to provide a comprehensive MR of sex hormones and cardiac electrophysiological arrhythmias and to explore the effects of drug targets of sex hormones on arrhythmias. We made full use of the available data and combined meta-analyses to improve confidence in the results. However, our study has some limitations. First, our study population was mainly European; therefore, our conclusions may not be generalizable to other populations. Second, the outcome data could not be stratified by sex because of the lack of existing data. Due to sample overlap and the absence of sufficient relevant data, our ability to conduct a thorough analysis of cardiac arrhythmias was constrained. This limitation prevented us from delving deeper into specific arrhythmic conditions, such as premature ventricular contractions, which require detailed exploration for more precise clinical insights. Third, owing to data limitations, we could not conduct nonlinear MR to analyze further the effects of changes in sex hormone concentrations on the outcome data in detail. Fourthly, the exposure data for women predominantly represents postmenopausal individuals. However, due to the lack of corresponding GWAS data, we were unable to perform stratified analyses between premenopausal and postmenopausal women. Finally, sex hormones may be associated with metabolic diseases such as obesity and diabetes. However, there was no obvious pleiotropy in our study, and we did not find estradiol SNPs associated with metabolic diseases. This conclusion should be confirmed in larger randomized controlled trials.
Conclusion
This study provided a comprehensive MR analysis of the relationship between sex hormones and arrhythmias/ECG indices. We identified a potential causal relationship between estradiol and the risk of AF in males. However, there was no significant association between sex hormones and either arrhythmias or ECG indices in females. These findings indicated that sex hormones may play a limited role in the development or modulation of cardiac arrhythmias. Future research is essential to validate and strengthen this conclusion, particularly through large-scale, longitudinal studies across diverse populations and clinical settings. Such investigations could provide deeper insights into the nuanced role of sex hormones in cardiac arrhythmias, helping to confirm these findings and explore potential underlying mechanisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all investigators and participants from the FinnGen consortia. And we would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- PT
Paroxysmal tachycardia
- AVB
Atrioventricular block
- SSS
Sick sinus syndrome
- TT
Total testosterone
- BT
Bioavailable testosterone
- SHBG
Sex hormone-binding globulin
- ECG
Electrocardiographic
- MR
Mendelian randomization
- PTFV1
P wave terminal force in lead I
- spQRSTa
Spatial QRS-T angles
- fQRSTa
Frontal QRS-T angles
- IVW
Inverse-variance weighted average approach
- SNPs
Single nucleotide polymorphisms
Author contributions
XW contributed to the acquisition of data, analysis and interpretation of data, and writing the article. ZW contributed to drafting the article. ZZ contributed to the acquisition of data and data analysis of this article. YS contributed to the acquisition of data and data analysis of this article. XG contributed to drafting the article. YT contributed to drafting the article. GL contributed to drafting the article. DX contributed to drafting the article. ZGZ contributed to the design of the work and revising the article. All the authors reviewed the article.
Funding
None.
Data availability
All the data can be found in the published GWAS studies and FinnGen consortia (https://r10.finngen.fi), which are detailed in Supplementary Table 1.
Declarations
Ethics approval and consent to participate
None.
Consent for publication
All authors have read and approved the final manuscript. They have provided their consent for the publication of this manuscript in BMC Cardiovascular Disorders.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grouthier V, Moey MYY, Gandjbakhch E, Waintraub X, Funck-Brentano C, Bachelot A, Salem JE. Sexual Dimorphisms, Anti-hormonal Therapy and Cardiac Arrhythmias. Int J Mol Sci 2021, 22(3). [DOI] [PMC free article] [PubMed]
- 2.Linde C, Bongiorni MG, Birgersdotter-Green U, Curtis AB, Deisenhofer I, Furokawa T, Gillis AM, Haugaa KH, Lip GYH, Van Gelder I et al. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace 2018;20(10):1565-1565ao. [DOI] [PubMed]
- 3.Xu B, Mo W, Tan X, Zhang P, Huang J, Huang C, Guo D, Wei X, Liu Y, Lei X, et al. Associations of serum testosterone and sex hormone-binding globulin with Incident Arrhythmias in men from UK Biobank. J Clin Endocrinol Metab. 2024;109(2):e745–56. [DOI] [PubMed] [Google Scholar]
- 4.Berger D, Folsom AR, Schreiner PJ, Chen LY, Michos ED, O’Neal WT, Soliman EZ, Alonso A. Plasma total testosterone and risk of incident atrial fibrillation: the atherosclerosis risk in communities (ARIC) study. Maturitas. 2019;125:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnani JW, Moser CB, Murabito JM, Sullivan LM, Wang N, Ellinor PT, Vasan RS, Benjamin EJ, Coviello AD. Association of sex hormones, aging, and atrial fibrillation in men: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2014;7(2):307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akdis D, Saguner AM, Shah K, Wei C, Medeiros-Domingo A, von Eckardstein A, Lüscher TF, Brunckhorst C, Chen HSV, Duru F. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: from a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur Heart J. 2017;38(19):1498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu P, Huang J, Lu Y, Zheng M, Li H, Duan X, Deng H, Zhao W, Liu X. Circulating sex hormones and risk of atrial fibrillation: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:952430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu X, Jiang H, Xu C, Zhou X, Cui B, Lu Z. Relationship between sex hormones and idiopathic outflow tract ventricular arrhythmias in adult male patients. Transl Res. 2009;154(5):265–8. [DOI] [PubMed] [Google Scholar]
- 9.Zeller T, Schnabel RB, Appelbaum S, Ojeda F, Berisha F, Schulte-Steinberg B, Brueckmann BE, Kuulasmaa K, Jousilahti P, Blankenberg S, et al. Low testosterone levels are predictive for incident atrial fibrillation and ischaemic stroke in men, but protective in women - results from the FINRISK study. Eur J Prev Cardiol. 2018;25(11):1133–9. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg MA, Shores MM, Matsumoto AM, Bůžková P, Lange LA, Kronmal RA, Heckbert SR, Mukamal KJ. Serum androgens and risk of atrial fibrillation in older men: the Cardiovascular Health Study. Clin Cardiol. 2018;41(6):830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong JA, Rexrode KM, Sandhu RK, Moorthy MV, Conen D, Albert CM. Menopausal age, postmenopausal hormone therapy and incident atrial fibrillation. Heart. 2017;103(24):1954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito N, Nagahara D, Ichihara K, Masumori N, Miura T, Takahashi S. Gender-affirming hormone treatment causes changes in gender phenotype in a 12-lead electrocardiogram. Heart Rhythm. 2021;18(7):1203–9. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu W. Extrinsic sex hormones rather than gender itself contribute directly to the electrocardiographic phenotype. Heart Rhythm. 2021;18(7):1210–1. [DOI] [PubMed] [Google Scholar]
- 14.Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an Approach to assess causality using Observational Data. J Am Soc Nephrol. 2016;27(11):3253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson SC, Butterworth AS, Burgess S. Mendelian randomization for cardiovascular diseases: principles and applications. Eur Heart J. 2023;44(47):4913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, Beaumont RN, Wittemans L, Martin S, Busch AS, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020;26(2):252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz D, Ek WE, Berggren E, Höglund J, Karlsson T, Johansson Å. Genome-Wide Association Study of Estradiol Levels and the Causal Effect of Estradiol on Bone Mineral density. J Clin Endocrinol Metab. 2021;106(11):e4471–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruth KS, Day FR, Hussain J, Martínez-Marchal A, Aiken CE, Azad A, Thompson DJ, Knoblochova L, Abe H, Tarry-Adkins JL, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596(7872):393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day FR, Thompson DJ, Helgason H, Chasman DI, Finucane H, Sulem P, Ruth KS, Whalen S, Sarkar AK, Albrecht E, et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49(6):834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christophersen IE, Magnani JW, Yin X, Barnard J, Weng LC, Arking DE, Niemeijer MN, Lubitz SA, Avery CL, Duan Q et al. Fifteen genetic loci Associated with the Electrocardiographic P Wave. Circ Cardiovasc Genet 2017, 10(4). [DOI] [PMC free article] [PubMed]
- 21.Dennis JK, Sealock JM, Straub P, Lee YH, Hucks D, Actkins K, Faucon A, Feng YA, Ge T, Goleva SB, et al. Clinical laboratory test-wide association scan of polygenic scores identifies biomarkers of complex disease. Genome Med. 2021;13(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann TJ, Lu M, Oni-Orisan A, Lee C, Risch N, Iribarren C. A large genome-wide association study of QT interval length utilizing electronic health records. Genetics 2022, 222(4). [DOI] [PMC free article] [PubMed]
- 23.Nolte IM, Munoz ML, Tragante V, Amare AT, Jansen R, Vaez A, von der Heyde B, Avery CL, Bis JC, Dierckx B, et al. Genetic loci associated with heart rate variability and their effects on cardiac disease risk. Nat Commun. 2017;8:15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ntalla I, Weng LC, Cartwright JH, Hall AW, Sveinbjornsson G, Tucker NR, Choi SH, Chaffin MD, Roselli C, Barnes MR, et al. Multi-ancestry GWAS of the electrocardiographic PR interval identifies 202 loci underlying cardiac conduction. Nat Commun. 2020;11(1):2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prins BP, Mead TJ, Brody JA, Sveinbjornsson G, Ntalla I, Bihlmeyer NA, van den Berg M, Bork-Jensen J, Cappellani S, Van Duijvenboden S, et al. Exome-chip meta-analysis identifies novel loci associated with cardiac conduction, including ADAMTS6. Genome Biol. 2018;19(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorolfsdottir RB, Sveinbjornsson G, Aegisdottir HM, Benonisdottir S, Stefansdottir L, Ivarsdottir EV, Halldorsson GH, Sigurdsson JK, Torp-Pedersen C, Weeke PE, et al. Genetic insight into sick sinus syndrome. Eur Heart J. 2021;42(20):1959–71. [DOI] [PubMed] [Google Scholar]
- 27.van de Vegte YJ, Eppinga RN, van der Ende MY, Hagemeijer YP, Mahendran Y, Salfati E, Smith AV, Tan VY, Arking DE, Ntalla I, et al. Genetic insights into resting heart rate and its role in cardiovascular disease. Nat Commun. 2023;14(1):4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young WJ, Haessler J, Benjamins JW, Repetto L, Yao J, Isaacs A, Harper AR, Ramirez J, Garnier S, van Duijvenboden S, et al. Genetic architecture of spatial electrical biomarkers for cardiac arrhythmia and relationship with cardiovascular disease. Nat Commun. 2023;14(1):1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, Almgren P, Alonso A, Anderson CD, Aragam KG, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. 2018;50(9):1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell N, Grossmann M. Mechanisms in endocrinology: Estradiol as a male hormone. Eur J Endocrinol. 2019;181(1):R23–43. [DOI] [PubMed] [Google Scholar]
- 33.Appiah D, Luitel S, Nwabuo CC, Ebong I, Winters SJ. Low endogenous estradiol levels are associated with elevated risk of cardiovascular disease mortality in young and middle-aged men in the United States. Atherosclerosis. 2022;361:34–40. [DOI] [PubMed] [Google Scholar]
- 34.Odening KE, Deiß S, Dilling-Boer D, Didenko M, Eriksson U, Nedios S, Ng FS, Roca Luque I, Sanchez Borque P, Vernooy K, et al. Mechanisms of sex differences in atrial fibrillation: role of hormones and differences in electrophysiology, structure, function, and remodelling. Europace. 2019;21(3):366–76. [DOI] [PubMed] [Google Scholar]
- 35.Tsai WC, Haung YB, Kuo HF, Tang WH, Hsu PC, Su HM, Lin TH, Chu CS, Jhuo SJ, Lee KT, et al. Hormone replacement therapy and risk of atrial fibrillation in Taiwanese menopause women: a nationwide cohort study. Sci Rep. 2016;6:24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wamboldt R, Haseeb S, Waddington A, Baranchuk A. Cardiac arrhythmias secondary to hormone therapy in trans women. Expert Rev Cardiovasc Ther. 2019;17(5):335–43. [DOI] [PubMed] [Google Scholar]
- 37.Ardissino M, Slob EAW, Carter P, Rogne T, Girling J, Burgess S, Ng FS. Sex-specific Reproductive factors augment Cardiovascular Disease Risk in women: a mendelian randomization study. J Am Heart Assoc. 2023;12(5):e027933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lincoff AM, Bhasin S, Flevaris P, Mitchell LM, Basaria S, Boden WE, Cunningham GR, Granger CB, Khera M, Thompson IM Jr, et al. Cardiovascular Safety of testosterone-replacement therapy. N Engl J Med. 2023;389(2):107–17. [DOI] [PubMed] [Google Scholar]
- 39.Corona G, Rastrelli G, Sparano C, Carinci V, Casella G, Vignozzi L, Sforza A, Maggi M. Cardiovascular safety of testosterone replacement therapy in men: an updated systematic review and meta-analysis. Expert Opin Drug Saf. 2024;23(5):565–79. [DOI] [PubMed] [Google Scholar]
- 40.O’Neal WT, Nazarian S, Alonso A, Heckbert SR, Vaccarino V, Soliman EZ. Sex hormones and the risk of atrial fibrillation: the multi-ethnic study of atherosclerosis (MESA). Endocrine. 2017;58(1):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salem JE, Alexandre J, Bachelot A, Funck-Brentano C. Influence of steroid hormones on ventricular repolarization. Pharmacol Ther. 2016;167:38–47. [DOI] [PubMed] [Google Scholar]
- 42.Zhao J, Jiang C, Lam TH, Liu B, Cheng KK, Xu L, Long MJ, Zhang W, Leung GM, Schooling CM. Genetically predicted testosterone and electrocardiographic QT interval duration in Chinese: a mendelian randomization analysis in the Guangzhou Biobank Cohort Study. Int J Epidemiol. 2015;44(2):613–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data can be found in the published GWAS studies and FinnGen consortia (https://r10.finngen.fi), which are detailed in Supplementary Table 1.