Abstract
Background
Current clinical guidelines support use of parenteral prostacyclin therapy for patients with pulmonary arterial hypertension (PAH) at intermediate risk. The objective of this study was to assess parenteral prostacyclin therapy use among patients at intermediate risk according to the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) 2.0 four-strata risk assessment model.
Methods
This was a retrospective chart review and cross-sectional online survey of healthcare professionals (HCPs). Included patients were classified as intermediate-low or intermediate-high risk per COMPERA 2.0 between 2016 and 2020 (index visit), initiated on a parenteral prostacyclin any time following intermediate risk assessment, and had World Health Organization (WHO) Functional Class (FC), 6-minute walk distance (6MWD), and B-type natriuretic peptide/N-terminal pro B-type natriuretic peptide (BNP/NT-proBNP) assessments at index and first comprehensive follow-up visits (follow-up).
Results
A total of 139 HCPs (53% community-based, 47% Pulmonary Hypertension Care Center-based) participated in the survey and provided 350 patient records; among these, mean age (SD) was 54.1 (15.3) years and 52% were female. Median (IQR) time from parenteral prostacyclin initiation to follow-up was 3.0 months (2.0, 7.0). At parenteral prostacyclin initiation for the 280 patient records with available COMPERA 2.0 assessments, 62% of patients were intermediate-high risk, 33% were intermediate-low risk and 3% were low risk, improving to 38%, 53%, and 8%, respectively, at follow-up.
Conclusions
Improvements were seen for the individual COMPERA 2.0 risk calculator parameters and for several other clinical parameters. Findings from this study substantiate recent guidelines suggesting earlier use of this treatment in intermediate-risk patients with PAH.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-024-03388-w.
Keywords: Retrospective studies, Prostacyclins, Prostaglandins, Risk assessment, Pulmonary hypertension
Background
Pulmonary arterial hypertension (PAH) is a serious disease associated with a broad range of medical conditions involving the pulmonary circulation [1]. PAH is progressive, leading to right heart failure and is typically fatal [2, 3]. Managing PAH can be complex, as risk assessments to predict patient outcomes include various clinical, laboratory, right heart imaging and catheterization hemodynamic parameters as well as exercise and functional status [2, 4]. Risk stratification tools can aid healthcare professionals in managing and treating their patients with PAH by formally evaluating their risk of death in one year, but the number of available tools and lack of consensus on the best approach adds to the complexity of PAH management. Risk assessment tools for PAH have evolved over the past decade and now also include the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) [5], REVEAL 2.0 [6], and REVEAL Lite 2 [7]. As with previous risk stratification methods, the 2022 European Society of Cardiology (ESC) and the European Respiratory Society (ERS) guidelines [2] include a three-stratum baseline risk score calculator, derived from the Swedish PAH Registry [8, 9]. Because intermediate clinical risk represents a majority of patients (approximately 70%) [9], and many patients in the European registries remained in the intermediate-risk stratum at follow-up, a method differentiating the intermediate-risk category into intermediate-low and intermediate-high risk was developed to guide effective treatment approaches and understand delineation within the broader risk category [10]. The Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) 2.0 is a four-stratum risk assessment model using the three non-invasive parameters identified from the French PH Registry [9–11].
Parenteral prostacyclin therapy is an effective treatment option for PAH [12–17] but many patients never receive this therapy [18–22]. Until recently, clinical guidelines only recommended treatment with prostacyclin analogue therapy as initial treatment for patients with more severe and rapidly progressing PAH [23, 24]. However, recent guidelines from ESC/ERS in 2022 support use of parenteral prostacyclin therapy as an adjunct therapy for patients with PAH who present at high risk status or stay at intermediate-high risk status after an initial therapy is instituted [2]. In an editorial discussing upfront combination therapy for PAH, Cascino and McLaughlin suggest that parenteral prostacyclin therapy be considered as initial therapy for appropriate patients at intermediate risk, as well as at three or six-month follow-up [25].
The primary goal of this research was to determine the use of parenteral prostacyclin therapy among patients with PAH at intermediate risk of death in one year according to the COMPERA 2.0 four-strata risk assessment model. Specifically, we sought to (1) understand the impact of timing of escalation to parenteral prostacyclin therapy on clinical outcomes in an intermediate risk patient population, (2) differentiate and understand utilization and clinical outcomes in patients who receive parenteral prostacyclin therapy as intermediate-low or intermediate-high risk, (3) assess healthcare professionals’ (HCP) decisions regarding escalation of therapy to include a parenteral prostacyclin, (4) quantify HCPs’ perceptions regarding utilization of parenteral prostacyclin for intermediate-risk patients, and (5) understand HCPs’ attitudes and treatment decisions regarding PAH management.
Methods
Study design
This study consisted of a cross-sectional survey and a retrospective chart review, conducted in the United States between September 27, 2022, and March 30, 2023. HCPs completed an online survey and provided anonymized patient chart data for their patients with PAH. The study instruments were developed by the authors and a third-party vendor (KJT Group, Inc., Rochester, NY, USA). The survey was conducted by KJT Group, Inc. The survey and chart review form were pretested among six HCPs (three pulmonologists, two cardiologists, and one nurse practitioner with a specialty in cardiology) using web-assisted telephone interviews to assess clarity and face validity. Minor revisions were made to the survey and chart review form based on feedback from the pretests.
Study participants were recruited via email or postal mail across the United States. Potential respondents contacted via email were recruited through online panel companies (SampleNinja LLC and Dynata LLC) with which they provided permission to be contacted for research purposes. Additionally, a postal mailing with a letter from KJT Group, Inc. was sent to a list of HCPs from 74 accredited PH Care Centers. Follow-up reminders were sent to non-responders via postal mail, email, and/or telephone to maximize the response rate. The study invitations described the nature of the study and included a link to Decipher, a secure online survey platform, to self-administer the survey and complete the chart reviews. Respondents who electronically consented to participate in the study were allowed to enter the screening portion of the survey. Eligible participants completing the entire survey received a modest monetary incentive; they also received an incentive for completing the chart forms. Sample sizes were determined based on estimated feasibility and prior research with HCPs who treat patients with PAH.
The study protocol was submitted to WCG Institutional Review Board for ethical review and was determined to be exempt because the research included survey procedures with adequate provisions to protect the privacy of subjects and maintain data confidentiality.
Healthcare professional selection criteria
HCPs were included in the study if they met the following criteria: were a physician, nurse practitioner, physician assistant, or advanced practice registered nurse; specialized in pulmonology, cardiology, or rheumatology, practiced in the United States but not Vermont (to comply with Sunshine Act reporting requirements); in practice for at least two years; currently treating at least 20 patients with PAH (Group 1 PH); initiated treatment for at least ten patients with PAH in the past 12 months; currently treating at least five patients with PAH at intermediate risk; and personally initiated or oversaw the initiation of parenteral prostacyclin therapy.
Healthcare professional survey
The 20-minute survey (Supplementary Material 1) consisted of questions regarding HCPs’ patient population; PAH diagnosis, risk assessment, monitoring practices; and attitude and approach to PAH treatment. The questions included open text (unaided response), numeric entry (proportion of patients) multiple-choice, five-point Likert-scales from one (very unlikely/no influence at all/strongly disagree) to five (very likely/great deal of influence/strongly agree), and 11-point Likert-scales from 0 (not at all important/do not at all meet my needs) to ten (extremely important/completely meets my needs). A maximum difference scaling exercise was also included in the survey. This exercise consisted of 11 attributes (including factors related to survival, clinical efficacy, risk status, safety profile, compliance, insurance coverage, and dosing flexibility) that might influence HCPs’ decision making when prescribing a therapy for the treatment of PAH to their intermediate-risk patients. A total of ten choice tasks were presented to each participant in which they were asked to choose the most and least important attribute.
Patient chart review
Participating HCPs were asked to complete chart review forms for a minimum of two and up to ten patients with Group 1 PH. Patients were included in the chart review if they (1) were categorized as intermediate-low (score = 2) or intermediate-high risk (score = 3) per COMPERA 2.0 risk stratification model between 2016 and 2020 (index visit); (2) were initiated on a parenteral prostacyclin any time following the index visit; (3) did not receive an experimental therapy at any point after the index visit; (4) received a comprehensive follow-up visit (follow-up) after parenteral prostacyclin was initiated; and (5) had the following assessments at index and follow-up: World Health Organization (WHO)/New York Heart Association (NYHA) Functional Class (FC), 6-minute walk distance (6MWD), and B-type natriuretic peptide/N-terminal pro B-type natriuretic peptide (BNP/NT-proBNP).
After an initial sample patient charts, an oversample of patient charts were collected to obtain additional data, which included the requirement of a follow-up visit date and COMPERA 2.0 parameters at parenteral prostacyclin initiation. Additionally, the time period during which the follow-up visit occurred was modified to be 6–12 months following parenteral prostacyclin initiation.
Patient demographic information was collected at the time of patients’ intermediate risk classification. Clinical characteristics were captured at the time of patients’ intermediate risk classification and at the follow-up visit. Details of patients’ parenteral prostacyclin therapy history following their intermediate risk classification were captured, including treatment type, timing, and reasons for initiation. PAH treatment history from diagnosis prior to index date to follow-up was also collected. Risk status per COMPERA 2.0 from parenteral prostacyclin therapy initiation to follow-up was assessed. Participating HCPs entered clinical values directly into the chart forms or were asked to select from categorial response options. The only information calculated from the inputted data was intermediate risk status per COMPERA 2.0.
Qualitative interviews
Following the initial quantitative survey, 45-minute qualitative interviews were conducted between October 23, 2023 and November 3, 2023. Respondents were recruited from the HCPs who completed the survey and patient chart portion of the study. At the end of the survey, HCPs were asked about their interest in participating in interviews at a later date. The purpose of the qualitative portion of the study was to obtain additional context and insights about the survey and chart review findings.
Statistical analyses
We performed descriptive statistical analyses (means, frequencies) of the aggregated data using Q Research Software for Windows (A Division of Displayr, Inc., New South Wales, Australia). Categorical data are expressed as frequencies and proportions. Continuous and count variables are presented using mean, standard deviation (SD), median, and interquartile range (IQR). Missing data and outliers were removed from calculations and were not imputed. Responses to open-ended questions were coded, categorized thematically, and quantified. PAH risk status was calculated using COMPERA 2.0, REVEAL 2.0, and REVEAL Lite 2 for the patient records for which the risk calculator parameters were available. Qualitative data was analyzed thematically; the analysis consisted of coding the transcripts to identify emerging themes and constructs.
Dosing data was collected as an exploratory endpoint for the chart review. However, upon review of the data, it was determined that there were a substantial number of “outliers” that were largely inconsistent with clinical plausibility, which may have been due to inconsistent reporting/documentation. Thus, the dosing data is not reported in this analysis, but information from the qualitative interviews which sought to understand how PAH treatments are typically dosed and documented in the electronic health record (EHR) is included.
Tests of differences (t-tests for means and z-tests for proportions) for subgroup analyses by practice setting (community-based and accredited PH centers) were performed using Q Research Software tables for the HCP survey results. Statistical significance was set at p < 0.05, using 2-tailed tests. Hierarchical Bayes modeling was used to generate the mean relative importance score for each attribute assessed in the maximum difference scaling exercise in the HCP survey. Attribute scores are positive values ranging from 0 to 100, that sum to 100. The scores have the valuable property of ratio-scaling, meaning for example, that an attribute with a rating of 20 is twice as important as an attribute with a rating of 10.
Results
Healthcare professional sample
A total of 1,289 HCP respondents entered the survey, of which 139 qualified for, and participated in this study. The remaining respondents did not complete the survey, did not qualify, or were over the target quota. The sample was comprised primarily of HCPs specializing in cardiology or pulmonology and was similarly split between those affiliated with PH centers and those who were community-based. Sample characteristics of healthcare providers are shown in Table 1.
Table 1.
Characteristics of healthcare professional participants
| Characteristics | Healthcare professionals (N = 139) |
|---|---|
| Age, mean ± SD | 49.4 ± 8.4 |
| Years in practice, mean ± SD | 16.2 ± 6.3 |
| Role, n (%) | |
| Physician | 134 (96%) |
| NP/PA/APRN | 5 (4%) |
| Specialty, n (%) | |
| Cardiology | 72 (52%) |
| Pulmonology | 50 (36%) |
| Rheumatology | 17 (12%) |
| Affiliation, n (%) | |
| Community-based | 73 (53%) |
| PH center | 66 (47%) |
| Hospital affiliation type, n (%) | |
| Major medical center, university/research hospital, or tertiary referral center | 57 (41%) |
| Community teaching hospital | 39 (28%) |
| Community non-teaching hospital | 39 (28%) |
| None of the above / practice is not affiliated with a hospital | 4 (3%) |
| Region, n (%) | |
| Northeast | 34 (24%) |
| Midwest | 26 (19%) |
| South | 44 (32%) |
| West | 35 (25%) |
| Practice setting | |
| Urban | 76 (55%) |
| Suburban | 59 (42%) |
| Rural | 4 (3%) |
| Number of PAH patients personally initiated treatment for in past year, median (IQR) | 50 (30.0, 90.0) |
Abbreviations: APRN, advanced practice registered nurse; NP, nurse practitioner; PA, physician assistant; PAH, pulmonary arterial hypertension; PH, accredited pulmonary hypertension center; SD, standard deviation; IQR, interquartile range
The qualitative phase of this research included 15 HCPs agreeing to participate in the 45-minute telephone interviews. All of the HCPs participating in the qualitative interviews were physicians; five were cardiologists; five were pulmonologists, and five were rheumatologists. Affiliation was split between community-based practices (n = 8) and PH centers (n = 7). Additionally, representation was similar across regions (n = 3, Northeast; n = 5 Midwest; n = 3 South; n = 4 West).
Healthcare professional survey
PAH patient population
The survey asked HCPs about their overall PAH patient population. HCPs surveyed reported that of their patients with PAH (Group 1 PH) whom they currently manage, 29% would be classified as low risk, 42% as intermediate risk, and 29% as high risk based on their clinical judgment; HCP-reported risk stratification was similar among those practicing in PH centers and those who were community-based. HCPs indicated that of their intermediate-risk patients whom they currently manage, they would classify most as unchanged (36%) or improving (38%), with one-quarter (25%) declining. When asked about the WHO/NYHA FC of their intermediate-risk patients, HCPs reported that most were FC II or III at diagnosis (28% and 32%, respectively); 21% were FC I, 17% were FC IV, and 2% were unknown.
Regarding their overall PAH patient population, HCPs reported that of their patients being treated with a prostacyclin (any route of administration), 22% were low risk, 41% were intermediate-risk, and 37% were high risk prior to starting prostacyclin therapy. For patients on a parenteral prostacyclin, 18% were low risk, 35% were intermediate risk, and 47% were high risk prior to starting this therapy. As reported by HCPs, on average, 56% of their patients were initiated on non-parenteral prostacyclin therapy prior to escalating to a parenteral prostacyclin, with an average of 13 months between initiation of the two treatment types. HCPs reported that of their patients at intermediate risk, 27% were currently being treated with monotherapy, 40% with a dual combination therapy, and 27% with at least three PAH-specific drugs; 6% were not treated with any PAH-specific therapy.
PAH risk assessment and monitoring
On average, the HCPs surveyed reported performing a formal risk assessment via a risk stratification method in 58% of their PAH patient visits. Among those conducting formal risk assessments, HCPs reported using ESC/ERS guidelines 37% of the time; REVEAL 2.0 and REVEAL Lite 2 were used less frequently (17% and 12% of the time, respectively). COMPERA 2.0 was also infrequently used (6% of the time among all HCPs) but use was significantly higher among HCPs practicing in PH centers than those in community-based practices (10% vs. 3%, p < 0.05). HCPs reported using “gestalt/clinical impression” 19% of the time, driven primarily by those in community-based practices (25% vs. 11% among those in PH centers, p < 0.05). The main reasons cited (unaided) by HCPs for not conducting formal risk assessments at every visit with their PAH patients include patient stability (20%), assessments conducted informally (17%), and formal assessments being time-consuming (15%).
When inquiring about awareness of clinical guidelines, only three of the 15 HCPs interviewed qualitatively reported being aware of the changes to the 2022 ESC/ERS guidelines recommending that parenteral prostacyclin therapy be considered in patients who present at intermediate-high or high risk. However, some HCPs (n = 6) noted the new recommendations would not change their prescribing patterns because it is in line with their current practice; others stated that patient willingness and symptom assessment will continue to drive their treatment decisions.
HCPs surveyed reported monitoring their patients’ response to PAH-specific drug therapy using various methods. Vital signs were used most frequently, with nearly all (93%) reporting using this method at least every six months, followed by WHO/NYHA FC (85%), 6MWD (71%) and BNP/NT-proBNP (70%). Although most HCPs (80%) reported using echocardiograms every six or 12 months, differences were seen in the frequent use of echocardiograms (every three months) between HCPs practicing in PH centers (17%) and community-based practices (3%).
During the qualitative interviews, participants were asked how long they typically wait to assess the treatment progress of their patients not on a parenteral prostacyclin before escalating treatment. The period varied between every six weeks to six months, with several participants (n = 7) indicating the assessments occur between two to three months on current therapy. However, some HCPs (n = 6) also mentioned that they re-evaluate patients sooner in case of active worsening.
Attitudes and approaches towards PAH treatment
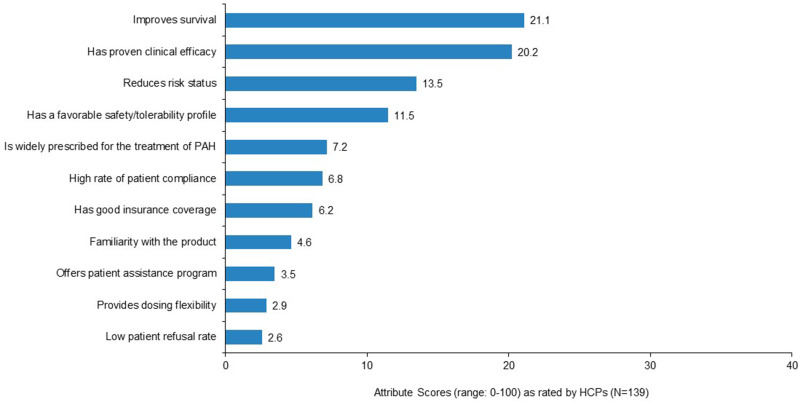
As assessed by the maximum difference scaling exercise, the most important attributes to HCPs when prescribing a therapy to patients with PAH at intermediate risk were “improves survival” and “has proven clinical efficacy.” Low patient refusal rate and dosing flexibility were least important (Fig. 1).
Fig. 1.
Importance of attributes influencing healthcare professionals’ PAH treatment decisions for patients at intermediate risk. Note: Responses to the survey question “We would now like you to consider several attributes that might influence your decision when prescribing a therapy for the treatment of PAH to your intermediate-risk patients. Considering only the attributes listed on the screen, please select the attribute that is most important in your therapy selection and then select the least important attribute.” Maximum difference scaling output consists of a rank ordering of attributes along with standard scores that represent the strength (magnitude) of the associated importance. Attribute scores are positive values ranging from 0 to 100, that sum to 100. The scores have the valuable property of ratio-scaling, meaning for example, that an attribute with a rating of 20 is twice as important as an attribute with a rating of 10. Abbreviations: HCP, healthcare provider; PAH, pulmonary arterial hypertension
When asked about the extent to which several clinical characteristics influence them to initiate a patient on parenteral prostacyclin therapy (at any risk level), HCPs indicated that a WHO/NYHA FC of IV or classification of high risk had the most influence (58% and 49% rated as “great deal of influence,” respectively). Patient demographics and characteristics such as cost concerns, health insurance coverage, age, education level, geographic distance from office/clinic, having a good support system, patient concern about site pain, and having a history of drug use were less influential overall, only having a great deal of influence for at most 25% of the HCPs surveyed.
According to HCPs, the main reasons for escalating therapy in patients at intermediate-high risk to parenteral prostacyclin therapy were “patient is declining on their current therapy at the 3-month (or 6-month) follow-up visit” (59% and 58%, respectively), “patient is not improving on their current therapy” (53%), and “patient has right heart hemodynamics consistent with higher risk” (47%). HCPs cited “patients refuse treatment” (51%) and “patient is improving on current therapy” (49%) as the primary reasons for not escalating patients at intermediate-high risk to parenteral prostacyclin therapy. Reasons for escalating/not escalating patients at intermediate-low risk to parenteral prostacyclin therapy were similar.
Upon exploration of patient receptivity to parenteral prostacyclin therapy, most HCPs participating in the qualitative interviews (n = 8) reported that their patients are receptive to this treatment, but some (n = 4) indicated the opposite. However, HCPs with less-receptive patients primarily saw low-risk or intermediate-risk patients. Most HCPs interviewed (n = 11) believed patients start parenteral prostacyclin therapy because their symptoms are progressing quickly and their condition is worsening, essentially leaving patients with “no choice” but to start therapy. HCPs believed that inconvenience of and unfamiliarity with intravenous (IV)/subcutaneous (SC) administration were the main reasons patients resist initiation of parenteral prostacyclin therapy. However, HCPs also indicated that very few patients refuse parenteral prostacyclin therapy once their symptoms progress to a point that they severely impact their quality of life.
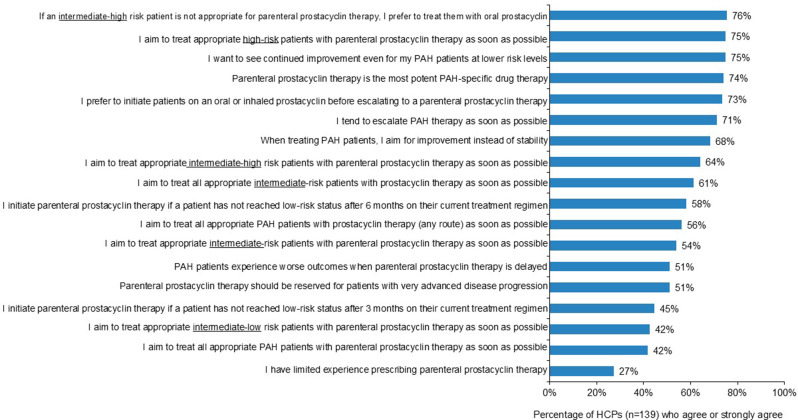
Although most HCPs surveyed “prefer to initiate patients on an oral or inhaled prostacyclin before escalating to a parenteral therapy,” 75% of HCPs reported they “aim to treat appropriate high-risk patients with parenteral prostacyclin therapy as soon as possible” (Fig. 2). Three-quarters of HCPs indicated that they “want to see continued improvement even for [their] PAH patients at lower risk levels” (Fig. 2).
Fig. 2.
Healthcare professionals’ attitudes towards parenteral prostacyclin therapy. Note: Rated using a 5-point scale where one indicates “strongly disagree” and five indicates “strongly agree.” Abbreviations: HCP, healthcare provider; PAH, pulmonary arterial hypertension
Parenteral prostacyclin therapy dosing, documentation, and visit timing
Due to inconsistencies in the dosing information entered in the patient chart forms, we explored how parenteral prostacyclin therapy was typically dosed, titrated, and recorded in EHRs, as well as frequency of follow-up visits. HCPs participating in the qualitative interviews reported that on average, 40% of their patients are initiated on a parenteral prostacyclin therapy in an inpatient setting, and 60% in an outpatient setting. Many HCPs (n = 10) reported initiating patients in both inpatient and outpatient settings. HCPs indicated that the target doses they set for their patients depend on a variety of factors including worsening of symptoms/condition, tolerability/side effects, and disease severity. According to the HCPs interviewed, titration approaches are largely based on mode of parenteral administration (IV vs. SC) and setting (inpatient/outpatient). Over half of HCPs (n = 8) reported that they typically see their patients for the initial follow-up visit within two weeks of starting parenteral prostacyclin therapy. Most (n = 12) see patients for their comprehensive follow-up visit within three months of parenteral prostacyclin therapy initiation.
When asked how PAH treatment is documented in patients’ medical records, HCPs interviewed reported that EHR information is entered by all members of the care team, including physicians and nurses managing titration (inpatient and outpatient). Nearly all HCPs (n = 14) indicated that dosing changes are recorded in the EHR, but the location of that information varies based on the type of EHR system the hospital uses. Some HCPs stated that they record dosing and titration information in the notes section, while others record this information in the medication reconciliation section.
PAH chart review
Patient characteristics
A total of 350 patient records were provided by participating HCPs. The average age was 54 years, and the sample was almost evenly split between males and females. Patient record characteristics are shown in Table 2. The most common etiology was idiopathic PAH (50%), followed by PAH associated with connective tissue disease (23%). At diagnosis, most patients were WHO/NYHA FC II or III. Across the study population, the median time from diagnosis to the chart data extraction was 3.8 years.
Table 2.
Characteristics of intermediate-risk PAH patient records
| Characteristics | Patient records (N = 350) |
|---|---|
| Age, mean ± SD | 54.1 ± 15.3 |
| Sex, n (%) | |
| Female | 182 (52%) |
| Male | 166 (47%) |
| Other | 2 (1%) |
| Race/ethnicitya, n (%) | |
| Caucasian or White | 202 (58%) |
| African American or Black | 85 (24%) |
| Hispanic/Latino | 26 (7%) |
| Asian or South Asian | 25 (7%) |
| Other | 14 (4%) |
| Affiliationb, n (%) | |
| PH center | 165 (47%) |
| Community-based | 185 (53%) |
| Specialtyb, n (%) | |
| Cardiology | 194 (55%) |
| Pulmonology | 114 (33%) |
| Rheumatology | 42 (12%) |
| Number of years since PAH diagnosis, mean ± SD | 4.3 ± 2.0 |
| PAH etiologyc, n (%) | |
| Idiopathic | 175 (50%) |
| PAH associated with connective tissue disease | 79 (23%) |
| PAH associated with congenital heart disease | 25 (7%) |
| Heritable | 23 (7%) |
| PAH associated with HIV infection | 16 (5%) |
| Drug- and toxin-induced | 14 (4%) |
| PAH associated with other conditions | 11 (3%) |
| PAH associated with portal hypertension | 7 (2%) |
| Comorbiditiesa | |
| Hypertension | 117 (33%) |
| Obesity | 68 (19%) |
| Autoimmune disorders | 66 (19%) |
| Hyperlipidemia | 63 (18%) |
| Depression | 62 (18%) |
| Asthma | 58 (17%) |
| Anemia | 55 (16%) |
| Coronary artery disease | 55 (16%) |
| Chronic obstructive pulmonary disease | 53 (15%) |
| Sleep apnea | 50 (14%) |
| Type 2 diabetes | 46 (13%) |
| Thyroid disease | 27 (8%) |
| Interstitial lung disease | 23 (7%) |
| Other condition | 80 (23%) |
| None | 46 (13%) |
| WHO/NYHA FC at diagnosis, n (%) | |
| I | 26 (7%) |
| II | 136 (39%) |
| III | 176 (50%) |
| IV | 8 (2%) |
| Unknown/not recorded | 4 (1%) |
a Multiple responses allowed
b Of the healthcare professionals providing the patient record data
c Indicates numbers may not add to 100 due to rounding
Note: PAH etiology response option of “PAH associated with other conditions” and comorbidities option of “other conditions” not specified or defined in the survey
Abbreviations: FC, functional class; HIV, human immunodeficiency virus; PAH, pulmonary arterial hypertension; PH, pulmonary hypertension; SD, standard deviation; NYHA, New York Heart Association; WHO, World Health Organization
Visit timing and parenteral prostacyclin therapy use
The median time between the index visit (intermediate risk classification) and parenteral prostacyclin initiation (n = 323 patient records with available data) was 4.0 months (IQR: 1.0, 14.0). Between parenteral prostacyclin initiation and the follow-up visit (n = 313), the median duration was 3.0 months (IQR: 2.0, 7.0). Following their intermediate risk classification, 53% of patients were initiated on SC treprostinil, 25% on IV treprostinil, and 21% on IV epoprostenol. Over half (55%, n = 192) of patients on parenteral prostacyclin therapy did not transition from another prostacyclin; among those who did, 35% (n = 55) were previously taking oral treprostinil, 28% (n = 44) inhaled treprostinil, 26% (n = 41) oral selexipag, and 12% (n = 19) inhaled iloprost. HCPs indicated that the main reasons (unaided) for initiating patients on parenteral prostacyclin therapy were clinical worsening/progression (26%), symptoms (17%), efficacy (15%), lack of improvement on prior therapy (11%), risk status (9%), and functional class (8%).
Change in risk status and clinical characteristics
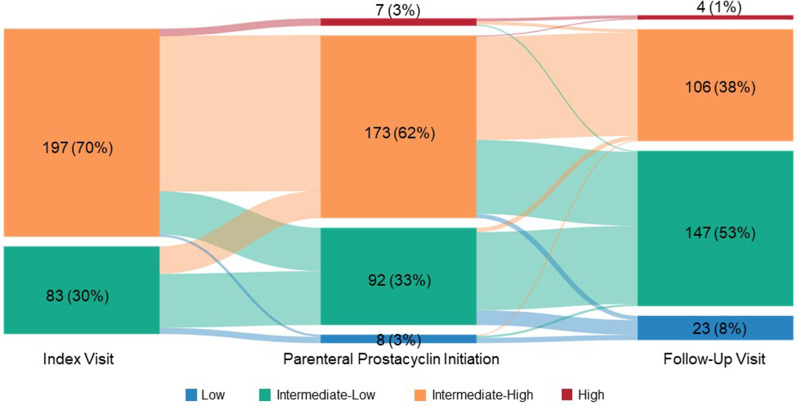
At the index visit, the majority of patients (70%) were classified as intermediate-high risk per COMPERA 2.0 risk assessment (Fig. 3). Risk status at index was also calculated using the REVEAL Lite 2 and REVEAL 2.0 risk calculators using available data from the patient records. Among the patient records for which the required parameters were provided in the chart review, 11% were considered low risk, 36% intermediate risk, and 53% high risk, per REVEAL Lite (n = 333); 11% were considered low risk, 27% intermediate risk, and 62% high risk, per the REVEAL 2.0 risk calculator (n = 338).
Fig. 3.
COMPERA 2.0 risk status at index, parenteral prostacyclin initiation, and follow-up (patient record data). Note: PAH Patient Records with all COMPERA 2.0 parameters available at index visit, parenteral prostacyclin initiation, and follow-up visit (n = 280). The boxes represent the proportion of patients at each timepoint, and the arcs represent the change between each timepoint; their width is proportional to the number of patients shifting between risk status categories. Index visit indicates timing of intermediate-risk assessment. Abbreviations: COMPERA, Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; PAH, pulmonary arterial hypertension
Risk status improvements were seen across all three risk calculators. Using REVEAL Lite 2 and REVEAL 2.0 risk assessments at the follow-up visit, 29%/32%, 40%/29%, and 31%/39% were considered low risk, intermediate risk, and high risk, respectively. Per COMPERA 2.0, risk status improved after initiating a parenteral prostacyclin therapy, with 61% of patients at intermediate-low or low risk at their follow-up visit (Fig. 3). Improvements in the individual COMPERA 2.0 risk calculator parameters were seen between parenteral prostacyclin therapy initiation and follow-up, as shown in Table 3. Table 4 presents the clinical characteristics of the patient records at index and follow-up visits. Improvements were seen in disease progression, heart rate, mean right atrial pressure, stroke volume, pericardial effusion, right ventricular function, systolic pulmonary arterial pressure, tricuspid regurgitation severity, and evidence of systolic interventricular septal flattening.
Table 3.
COMPERA 2.0 parameters at index, parenteral prostacyclin initiation, and follow-up (patient record data)
| Parameters | At index visit | At parenteral prostacyclin initiation | At follow-up |
|---|---|---|---|
| WHO/NYHA FC, n (%) | n = 350 | n = 348 | n = 350 |
| I | 25 (7%) | 24 (7%) | 29 (8%) |
| II | 133 (38%) | 95 (27%) | 188 (54%) |
| III | 189 (54%) | 207 (59%) | 121 (35%) |
| IV | 3 (1%) | 22 (6%) | 12 (3%) |
| 6MWD, median (m) (IQR) | n = 350 | n = 289 | n = 346 |
|
250.0 (125.0, 332.0) |
225.0 (120.0, 300.0) |
300.0 (160.0, 380.0) |
|
| BNP or NT-proBNP, median (ng/L) (IQR) | |||
| n = 269 | n = 222 | n = 268 | |
| BNP |
215.0 (90.0, 450.0) |
209.5 (95.0, 420.0) |
152.3 (71.3, 280.0) |
| n = 161 | n = 57 | n = 101 | |
| NT-proBNP |
500.0 (200.0, 735.0) |
680.0 (432.0, 855.0) |
500.0 (200.0, 690.0) |
Abbreviations: 6MWD, 6-minute walk distance; BNP, brain natriuretic peptide; FC, functional class; IQR, interquartile range; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; WHO, World Health Organization
Table 4.
Clinical characteristics at index and follow-up (patient record data)
| Characteristics (index / follow-up)a | At index visit (patient records) |
At follow-up (patient records) |
|---|---|---|
| Disease progression, n (%) | n = 332 | n = 340 |
| Unchanged | 96 (29%) | 60 (18%) |
| Improving | 85 (26%) | 245 (72%) |
| Declining | 151 (45%) | 35 (10%) |
| Heart rate | n = 327 | n = 325 |
| BPM > 96 | 158 (48%) | 77 (24%) |
| BPM ≤ 96 | 169 (52%) | 248 (76%) |
| Systolic blood pressure, | n = 322 | n = 321 |
| ≥ 110 mmHg | 203 (63%) | 209 (65%) |
| < 110 mmHg | 119 (37%) | 112 (35%) |
| eGFR < 60mL/min/1.73m2 or renal insufficiency, n (%) | n = 290 | n = 283 |
| 50 (17%) | 59 (21%) | |
| Predicted DLCO | n = 251 | n = 222 |
| < 40 | 83 (33%) | 66 (30%) |
| ≥ 40 | 168 (67%) | 156 (70%) |
| Hemodynamic parameters | ||
| PVR (Woods units), median (IQR) | n = 123 | n = 28 |
| 5.0 (4.0, 7.0) | 4.0 (3.0, 6.8) | |
| PVR (dynes/sec/cm− 5), median (IQR) | n = 39 | n = 9 |
| 400.0 (85.0, 480.0) | 100.0 (51.0, 300.0) | |
| mPAP (mmHg), median (IQR) | n = 200 | n = 43 |
| 45.5 (36.0, 56.0) | 43.0 (35.0, 56.0) | |
| mRAP (mmHg) | n = 205 | n = 53 |
| > 20 mmHg | 126 (61%) | 26 (49%) |
| ≤ 20 mmHg | 79 (39%) | 27 (51%) |
| Cardiac index (L/min/m2), median (IQR) | n = 131 | n = 30 |
| 2.5 (2.0, 3.0) | 2.7 (2.3, 4.0) | |
| Cardiac output (thermodilution measurement L/min), median (IQR) | n = 71 | n = 13 |
| 4.1 (3.9, 5.0) | 5.4 (4.2, 7.5) | |
| Cardiac output (Fick measurement L/min), median (IQR) | n = 42 | n = 9 |
| 4.5 (4.0, 4.7) | 4.1 (4.0, 4.6) | |
| Stroke volume (mL), median (IQR) | n = 51 | n = 9 |
| 50.0 (40.0, 65.0) | 58.0 (50.0, 80.0) | |
| Echocardiogram parameters | ||
| Pericardial effusion, n % | n = 281 | n = 152 |
| None | 152 (54%) | 95 (63%) |
| Minimal | 103 (37%) | 47 (31%) |
| Moderate or large | 26 (9%) | 10 (7%) |
| RV function, n % | n = 283 | n = 154 |
| Normal | 29 (10%) | 16 (10%) |
| Mildly reduced | 113 (40%) | 80 (52%) |
| Moderately reduced | 119 (42%) | 51 (33%) |
| Severely reduced | 22 (8%) | 7 (5%) |
| RA size, n % | n = 283 | n = 153 |
| Normal | 23 (8%) | 16 (10%) |
| Mildly enlarged | 118 (42%) | 64 (42%) |
| Moderately enlarged | 119 (42%) | 67 (44%) |
| Severely enlarged | 23 (8%) | 6 (4%) |
| RV size, n % | n = 281 | n = 153 |
| Normal | 25 (9%) | 13 (8%) |
| Mildly enlarged | 110 (39%) | 73 (48%) |
| Moderately enlarged | 118 (42%) | 57 (37%) |
| Severely enlarged | 28 (10%) | 10 (7%) |
| TAPSE (mm), median (IQR) | n = 69 | n = 34 |
| 11.0 (2.0, 16.0) | 15.0 (3.0, 17.0) | |
| sPAP (mmHg), median (IQR) | n = 184 | n = 104 |
| 57.5 (50.0, 66.5) | 40 (48.0, 56.0) | |
| TR severity | n = 256 | n = 142 |
| None | 7 (3%) | 7 (5%) |
| Mild | 73 (29%) | 59 (42%) |
| Moderate | 155 (61%) | 68 (48%) |
| Severe | 21 (8%) | 8 (6%) |
| Did the echocardiogram show systolic interventricular septal flattening? | n = 246 | n = 143 |
| Yes | 108 (44%) | 39 (27%) |
| No | 138 (56%) | 104 (73%) |
a Sample sizes reflect the number of patient records where information was known/provided
Abbreviations: BPM, beats per minute; DLCO, diffusing capacity of the lungs for carbon monoxide; eGFR, estimated glomerular filtration rate; IQR, interquartile range; mPAP, mean pulmonary artery pressure; mRAP, mean right atrial pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV, right ventricular; SD, standard deviation; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation
Discussion
This study aimed to gain a better understanding of parenteral prostacyclin therapy use in the intermediate-risk PAH population in a real-world setting. In our patient chart review study, 50% of patients had idiopathic PAH, similar to that seen in REVEAL and in a global physician survey and chart review study [21, 26]. Slightly more than half of patients were female, less than that seen in other studies of patients with PAH [11, 27–29]. Patients were primarily WHO/NYHA FC II or III at diagnosis, similar to that observed in other research [11, 21, 28, 29]. The affiliation of the treating HCP was almost evenly split between PH centers and community-based institutions.
We found that a median of 4 months passed between the index visit of intermediate-risk classification and initiation of parenteral prostacyclin therapy, with this route of administration being the first prostacyclin therapy for the majority of patients. Worsening of PAH symptoms was the primary reason HCPs cited for initiating patients on parenteral prostacyclin therapy. Patients in our analysis showed improvement in risk status according to the COMPERA 2.0 risk assessment between parenteral prostacyclin therapy initiation and the follow-up visit, which occurred six months later, on average. Approximately two-thirds of patients (for whom all COMPERA 2.0 parameters available were available at index visit, parenteral prostacyclin initiation, and follow-up visit) were at intermediate-high risk at treatment initiation, decreasing to 38% at follow-up. Our findings are similar to improvements seen in the French PH Registry between diagnosis and first re-evaluation (median of 4.4 months) among patients enrolled between 2006 and 2016 taking monotherapy or combination PAH targeted therapy [30] and a database cohort analysis of patients diagnosed with idiopathic PAH between 1999 and 2009 at three- and 12-month follow-up [31].
Parenteral prostacyclin therapy has been shown to have positive effects on invasive hemodynamic measures, which we observed across several parameters in our real-world research. A single-site study conducted between 2007 and 2016 among patients with PAH initiating IV epoprostenol or IV or SC treprostinil therapy showed that early initiation of parenteral prostacyclin therapy was associated with decreases in hemodynamic measures including mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance [32]. In a retrospective pilot study of patients newly diagnosed with PAH, mPAP was reduced after four months of treatment with upfront triple combination therapy with IV epoprostenol [33]. In high-risk patients with PAH, upfront triple therapy with SC treprostinil was associated with improvements in clinical and hemodynamic parameters and right heart reverse remodeling [34]. Boucly et al. found that triple combination therapy with parenteral prostacyclin was associated with a significantly lower risk of death in intermediate-risk patients as compared with dual combination therapy or monotherapy [35].
We found that the dosing information collected in the patient chart records were varied and inconsistent. One of our assumptions was that dosing may be dependent on the location of parenteral prostacyclin therapy initiation or route of administration (e.g., patients taking SC treprostinil may have been switched from IV administration after hospital discharge); however, information about where patients received their first dose of parenteral prostacyclin therapy (i.e., inpatient or outpatient) was not collected in our study. In the qualitative interviews, we found that approaches to parenteral prostacyclin therapy initiation and titration varied by patient and by treating HCP. It is also possible that dosing changes may not be reflected in real-time in some EHRs and instead may be reflected elsewhere in the patient’s chart, which was supported by the qualitative interviews. Additionally, as parenteral prostacyclin dosing is calculated based on patient weight to provide a flow rate specific to a pump, there are several pieces of information that may be documented. Lastly, the drug order in the EHR may not be accurate/adjusted and may be documented in the notes under “medication reconciliation.” Although the survey and chart review form were pilot tested, it is possible that HCPs were uncertain as to how to record the dosing information.
The demographics of our HCP survey were similar to that of another recent survey of clinicians evaluating the use of risk assessment tools in PAH management; [36] however, our study had a greater representation of cardiologists and those practicing at a PH center. HCPs self-reported performing risk assessments in less than 60% of their PAH patient visits, with the COMPERA 2.0 risk status method rarely used. HCPs reported relying on gestalt/their clinical impression 19% of the time, particularly those in community-based practices. Despite ESC/ERS Guidelines since 2015 recommending the use of risk stratification methods to manage PAH [23] and shown superiority over physician gestalt [37], low use of risk assessment tools has been reported in the literature [36, 38]. In our study, reasons for not conducting formal risk assessments at every PAH visit were patient stability, performing informal assessments, and the time-intensiveness of formal assessments.
The “paradox” of delaying or underutilizing parenteral prostacyclin therapy despite the known survival benefits of parenteral prostacyclin therapy discussed in Schilz et al. were also observed in our survey results [39]. Improvement in survival and clinical efficacy were selected by HCPs as the most important attributes when prescribing therapy to patients with PAH at intermediate risk. On the contrary, FC IV or high-risk status most influenced HCPs to initiate patients on parenteral prostacyclin therapy. Respondents escalated patients at intermediate-high risk to parenteral prostacyclin therapy due to various factors including lack of improvement or progression and right heart hemodynamics. Most HCPs (75%) reported aiming to treat appropriate high-risk patients with parenteral prostacyclin therapy as soon as possible, but fewer agreed regarding their patients at intermediate-high (64%), intermediate (54%), or intermediate-low risk (42%).
Only half of HCPs agreed that patients experienced worse outcomes when parenteral therapy is delayed. However, treatment delays can negatively impact survival [40]. Earlier initiation may positively affect prognosis as seen in two small studies [33, 34]. Additionally, an analysis of patients enrolled in the French PH Registry initiated on PAH targeted medications within three months of PAH diagnosis demonstrated that initial triple combination therapy including a parenteral prostacyclin was associated with a higher rate of survival [35]. Potential barriers to prescribing parenteral prostacyclin therapy may include patient age, comorbidities, patient discomfort with pump therapy, geographic distance from the office/clinic/PH center, concerns about overall medication compliance, lack of an appropriate support system, and history of drug use [41–43]; however, we found that patient demographics and characteristics were least influential in initiating parenteral prostacyclin therapy according to the HCPs surveyed.
In our survey, HCPs cited patient refusal as one of the key reasons for not escalating patients at intermediate-high or intermediate-low risk to parenteral prostacyclin therapy. Patients may refuse this therapy option for a variety of reasons including fear of negative impact on quality of life, administering the medication, challenges with having to wear an external pump, and site pain/infections [44]. There may also be healthcare and provider barriers such as limitations of risk assessment tools (e.g., lack of right ventricular assessment variables, for which most other components are surrogates), access to care, lack of infrastructure or nursing staff to support parenteral prostacyclin therapy in the community care setting, suboptimal communication when recommending the treatment option to patients, infrequently performing risk assessments, and lack of knowledge or experience with parenteral prostacyclin therapy.
Although prescribing PAH targeted treatment at initial diagnosis is relatively straightforward following current clinical guidelines, HCPs face challenges in deciding when and how to escalate treatment for patients on therapy. Overcoming barriers to earlier initiation of parenteral prostacyclin therapy will likely take a multifaceted approach including encouraging earlier referral to PH centers [45], increasing education about echocardiogram and other right heart parameters that can inform the need for parenteral prostacyclin therapy, education on how to evaluate echocardiogram parameters over time, and using patient/nurse videos to educate patients and instill confidence in their ability to manage parenteral prostacyclin therapy. Additional research could provide evidence for setting higher expectations for specific treatment goals in the first three to six months. Prospective randomized studies are also needed to determine extended survival as seen in recent research.
Limitations
Assessing HCP perceptions of PAH management and its treatment with a chart review allows us to contextualize attitudes and behaviors with practice patterns, including those of HCPs who are community-based. We followed best practices for chart audit studies discussed in Vassar et al. [46], including creating well-defined, clearly articulated research questions, considering sampling issues a priori, using standardized data collection forms, explicitly specifying inclusion and exclusion criteria, and conducting pilot testing; however, this study has limitations. These include the retrospective nature of the study, potential selection bias of patient chart records, recall bias, and the accuracy of the patient records. As a retrospective chart review, the study is limited to describing the associations between treatment and outcomes rather than causality. Potential confounding variables could affect patient outcomes, including concomitant therapies or variations in clinical practice. The majority of our survey respondents were cardiologists who have a baseline standard training in echocardiography and right heart catheterization; thus, practice patterns described in our study may not be generalizable to other HCPs treating patients with PAH. Additionally, the demographic characteristics of the HCPs who responded to the survey and provided patient records may differ from those who did not respond to and complete the survey. The duration of follow-up captured in the chart review was brief (median of 3.0 months), which limits our ability to understand long-term outcomes and durability of treatment effects. Further, the study was sponsored by a pharmaceutical company that develops treatments for PAH; however, the study sponsor was blinded to the study respondents; clinical experts were involved in the design of the study, survey, and chart review form; and a third party conducted the data collection and analyzed the results. The inability to analyze the parenteral prostacyclin dosing for the patient records is also a limitation and prevents us from making any potential associations regarding dosing and outcomes. However, even with a short follow-up period, improvements were observed in patients’ risk status and clinical parameters between treatment initiation and first comprehensive follow-up. Further research is needed to confirm these findings and refine treatment strategies for patients with PAH.
Conclusions
The HCPs surveyed aim to improve the outcomes of their patients with PAH at lower risk levels and agree that parenteral prostacyclin therapy is effective. According to the chart review, patients’ risk status and clinical and hemodynamic parameters improved between parenteral prostacyclin therapy at follow-up, supporting recent guidelines suggesting earlier use of this treatment in intermediate-risk patients. Additional education and resources are needed to increase awareness of the advantages of using formal risk assessments and the benefits of parenteral prostacyclin therapy. A graphical abstract summarizing this research can be found in Supplementary Material 2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Isabella Barbagallo, Andrea Stoltz, and Nick Henderson of KJT Group, Inc. (Rochester, NY, USA) for their support in the conduct of and statistical analysis of this study, and Claire Thrasher, PharmD, of United Therapeutics Corporation for her review and contributions to the manuscript drafts. The authors also wish to thank Rebecca Hahn, MPH and Ladan Panahi, PharmD, of KJT Group, Inc. for providing medical writing assistance and support, which was funded by United Therapeutics Corporation (Research Triangle Park, NC, USA) in accordance with Good Publication Practice (GPP 2022) guidelines.
Abbreviations
- PAH
Pulmonary arterial hypertension
- COMPERA
Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension
- HCPs
Healthcare professionals
- WHO
World Health Organization
- FC
Functional Class
- 6MWD
6-minute walk distance
- BNP
B-type natriuretic peptide
- NT-proBNP
N-terminal pro B-type natriuretic peptide
- REVEAL
Registry to Evaluate Early and Long-Term PAH Disease Management
- ESC
European Society of Cardiology
- ERS
European Respiratory Society
- NYHA
New York Heart Association
- SD
Standard Deviation
- IQR
Interquartile range
- EHR
Electronic Health Record
- IV
Intravenous
- SC
Subcutaneous
- mPAP
Mean pulmonary artery pressure
Author contributions
O.A.S, A.V., M.R.S, and M.B. participated in the design of the study and development of the study materials. M.R.S and M.B. oversaw the data collection. O.A.S, A.V., M.R.S, and M.B. participated in the analysis and interpretation of the data, preparation of and critical review of the manuscript, and approval of the final manuscript version for submission.
Funding
The study was funded by United Therapeutics Corporation. United Therapeutics Corporation participated in the design of the study, data analyses, interpretation, and preparation of the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All participants provided informed consent prior to completing the survey and could withdraw at any time, and participants who completed the entire survey received a modest monetary incentive. The study protocol was submitted to the WCG Institutional Review Board for ethical approval and was determined to qualify for exemption status because sufficient protections were in place to protect the privacy of subjects and to maintain the confidentiality of data. The study and data accumulation were in conformity with all country, federal, or state laws, and the study adhered to the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
O.A.S. is on the Speakers’ Bureau for United Therapeutics Corporation and is a consultant for Altavant Sciences Inc, Acceleron Pharma Inc, and Merck; she is also on an advisory board for Janssen Pharmaceuticals and received support from Ferrer for travel to meetings. A.V. is a consultant for United Therapeutics Corporation, Merck, and Janssen Pharmaceuticals; she is also on the Speakers’ Bureau for United Therapeutics Corporation, Janssen Pharmaceuticals, and Bayer. M.R.S. and M.B. are employees and shareholders of United Therapeutics Corporation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021;11(1):2045894020977300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–731. [DOI] [PubMed] [Google Scholar]
- 3.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–56. [DOI] [PubMed] [Google Scholar]
- 4.Benza RL, Farber HW, Selej M, Gomberg-Maitland M. Assessing risk in pulmonary arterial hypertension: what we know, what we don’t. Eur Respir J. 2017;50(2). [DOI] [PubMed]
- 5.Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141(2):354–62. [DOI] [PubMed] [Google Scholar]
- 6.Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156(2):323–37. [DOI] [PubMed] [Google Scholar]
- 7.Benza RL, Kanwar MK, Raina A, Scott JV, Zhao CL, Selej M, et al. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest. 2021;159(1):337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kylhammar D, Kjellström B, Hjalmarsson C, Jansson K, Nisell M, Söderberg S, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39(47):4175–81. [DOI] [PubMed] [Google Scholar]
- 9.Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2). [DOI] [PubMed]
- 10.Boucly A, Weatherald J, Savale L, de Groote P, Cottin V, Prévot G et al. External validation of a refined four-stratum risk assessment score from the French pulmonary hypertension registry. Eur Respir J. 2022;59(6). [DOI] [PMC free article] [PubMed]
- 11.Hoeper MM, Pausch C, Olsson KM, Huscher D, Pittrow D, Grünig E et al. COMPERA 2.0: a refined four-stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J. 2022;60(1). [DOI] [PMC free article] [PubMed]
- 12.Sitbon O, Vonk Noordegraaf A. Epoprostenol and pulmonary arterial hypertension: 20 years of clinical experience. Eur Respir Rev. 2017;26(143). [DOI] [PMC free article] [PubMed]
- 13.Irene ML, Sean PG. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. Eur Respiratory Rev. 2015;24(138):630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25, Supplement):D60–72. [DOI] [PubMed]
- 15.Manthena P, Ghosh S, Shah T, Chin K. Effects of parenteral prostacyclin therapy on echocardiographic variables. J Heart Lung Transplantation. 2021;40(4):S465–6. [Google Scholar]
- 16.Stubbe B, Opitz CF, Halank M, Habedank D, Ewert R. Intravenous prostacyclin-analogue therapy in pulmonary arterial hypertension - A review of the past, present and future. Respir Med. 2021;179:106336. [DOI] [PubMed] [Google Scholar]
- 17.Barnes H, Yeoh HL, Fothergill T, Burns A, Humbert M, Williams T. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2019;5(5):Cd012785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farber HW, Miller DP, Meltzer LA, McGoon MD. Treatment of patients with pulmonary arterial hypertension at the time of death or deterioration to functional class IV: insights from the REVEAL Registry. J Heart Lung Transpl. 2013;32(11):1114–22. [DOI] [PubMed] [Google Scholar]
- 19.Burger CD, Pruett JA, Lickert CA, Berger A, Murphy B, Drake W 3. Prostacyclin use among patients with pulmonary arterial hypertension in the United States: a retrospective analysis of a large health care claims database. J Manag Care Spec Pharm. 2018;24(3):291–302. [DOI] [PMC free article] [PubMed]
- 20.Farber H, Miller D, Beery F, McGoon M. Use of parenteral prostanoids at time of death in patients with pulmonary arterial hypertension enrolled in REVEAL. Chest. 2011;140(4):903A. [Google Scholar]
- 21.Preston IR, Hinzmann B, Heinz S, Gall H, Jenkins D, Kim NH, et al. An international physician survey of pulmonary arterial hypertension management. Pulmonary Circulation. 2016;6(3):338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathai S, Morland K, Classi P, Chen H, Forys A, Nelsen A. Low utilization of prostacyclin therapy prior to death among Medicare patients with pulmonary arterial hypertension. Chest. 2020;158(4):A2164–5. [Google Scholar]
- 23.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. [DOI] [PubMed] [Google Scholar]
- 24.Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest. 2019;155(3):565–86. [DOI] [PubMed] [Google Scholar]
- 25.Cascino TM, McLaughlin VV. Upfront combination therapy for pulmonary arterial hypertension: time to be more ambitious than AMBITION. Am J Respir Crit Care Med. 2021;204(7):756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary arterial hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164–72. [DOI] [PubMed] [Google Scholar]
- 27.Souza R, Channick RN, Delcroix M, Galiè N, Ghofrani HA, Jansa P, et al. Association between six-minute walk distance and long-term outcomes in patients with pulmonary arterial hypertension: data from the randomized SERAPHIN trial. PLoS ONE. 2018;13(3):e0193226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White RJ, Jerjes-Sanchez C, Bohns Meyer GM, Pulido T, Sepulveda P, Wang KY, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind placebo-controlled clinical trial. Am J Respir Crit Care Med. 2020;201(6):707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwicke DL, Restrepo-Jaramillo R, Alnuaimat H, Gordon K, Broderick M, Edwards LD, et al. A multicenter retrospective study of patients with pulmonary hypertension transitioned from inhaled to oral treprostinil. Pulmonary Circulation. 2021;11(1):2045894021998203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boucly A, Weatherald J, Savale L, Jaïs X, Cottin V, Prevot G et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2). [DOI] [PubMed]
- 31.Nickel N, Golpon H, Greer M, Knudsen L, Olsson K, Westerkamp V, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2012;39(3):589. [DOI] [PubMed] [Google Scholar]
- 32.Bartolome SD, Sood N, Shah TG, Styrvoky K, Torres F, Chin KM. Mortality in patients with pulmonary arterial hypertension treated with continuous prostanoids. Chest. 2018;154(3):532–40. [DOI] [PubMed] [Google Scholar]
- 33.Sitbon O, Jaïs X, Savale L, Cottin V, Bergot E, Macari EA, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J. 2014;43(6):1691–7. [DOI] [PubMed] [Google Scholar]
- 34.D’Alto M, Badagliacca R, Argiento P, Romeo E, Farro A, Papa S, et al. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest. 2020;157(2):376–83. [DOI] [PubMed] [Google Scholar]
- 35.Boucly A, Savale L, Jaïs X, Bauer F, Bergot E, Bertoletti L, et al. Association between initial treatment strategy and long-term survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2021;204(7):842–54. [DOI] [PubMed] [Google Scholar]
- 36.Sahay S, Balasubramanian V, Memon H, Poms A, Bossone E, Highland K, et al. Utilization of risk assessment tools in management of PAH: a PAH provider survey. Pulmonary Circulation. 2022;12(2):e12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS ONE. 2020;15(11):e0241504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. 2020;10(3):2045894020950186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schilz R, Park M. Selection of infusion prostacyclin therapy in pulmonary arterial hypertension: not just a last resort. Adv Pulmonary Hypertens. 2017;16(1):14–9. [Google Scholar]
- 40.Badagliacca R, Pezzuto B, Poscia R, Mancone M, Papa S, Marcon S, et al. Prognostic factors in severe pulmonary hypertension patients who need parenteral prostanoid therapy: the impact of late referral. J Heart Lung Transpl. 2012;31(4):364–72. [DOI] [PubMed] [Google Scholar]
- 41.Narechania S, Torbic H, Tonelli AR. Treatment discontinuation or interruption in pulmonary arterial hypertension. J Cardiovasc Pharmacol Therap. 2019;25(2):131–41. [DOI] [PubMed] [Google Scholar]
- 42.Tonelli AR, Dweik RA. Why patients who die of worsening pulmonary arterial hypertension are not on parenteral prostacyclin analog treatment? J Heart Lung Transpl. 2014;33(2):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huston JH, Hemnes AR. Parenteral prostacylin use in pulmonary arterial hypertension. In: Ford HJ, Heresi GA, Risbano MG, editors: Pulmonary hypertension: controversial and emerging topics. Cham: Humana; 2020.
- 44.Morland K, Raina A, Nails A, Classi P, Etschmaier M, Frantz RP. Reasons for refusing parenteral therapy: a qualitative study of patients with pulmonary arterial hypertension. Pulmonary Circulation. 2021;11(4):20458940211046761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandras SA, Ventura HO, Corris PA. Breaking down the barriers: why the delay in referral for pulmonary arterial hypertension? Ochsner J. 2016;16(3):257–62. [PMC free article] [PubMed] [Google Scholar]
- 46.Vassar M, Holzmann M. The retrospective chart review: important methodological considerations. J Educ Eval Health Prof. 2013;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.