Abstract
Background
Open pancreaticoduodenectomy (OPD) is an essential surgical procedure for expert hepato-biliary-pancreatic (HBP) surgeons. However, there is no standard for how many surgeries must be performed by a surgeon in training before they are considered to have enough experience to ensure surgical safety.
Methods
Cumulative Sum (CUSUM) analysis was performed using the surgical data of OPDs performed during the training period of board-certified expert surgeons of the Japanese Society of Hepato-Biliary-Pancreatic Surgery.
Results
Fourteen HBP surgeons participated in this study and performed 334 OPDs during their training period. The median (interquartile range) values for operative time, blood loss, and length of hospital stay were 455 (397–519) minutes, 450 (234–-716) ml, and 28 (21–38) days, respectively. CUSUM analysis showed inflection points at 20 surgeries performed for operative time. After 20 procedures, operative time was significantly shorter (461 min vs. 425 min, p = 0.021) and blood loss was significantly lower (470 ml vs. 340 ml, p = 0.038). No significant differences between within 20 and after 21 procedures were found in the complication rate (53% vs. 48%, p = 0.424) and rate of in-hospital deaths (1.5% vs.1.4%. p = 0.945). Up to 20 surgeries, PDAC and another malignant tumor had longer operative time than benign/low malignant diseases (486 min vs. 472 min vs. 429 min, p < 0.001), and higher blood loss (500 ml vs. 502 ml vs. 355 ml, p < 0.001). Mortality rate was higher at PDAC cases (5% vs. 0% vs. 0%, p = 0.01). After the 21 procedures, these outcomes were improved and no differences in by primary disease were observed. Multivariable analysis showed that within 20 surgeries were independent risk factors of longer operative time (HR2.6, p = 0.013) and higher blood loss (HR2.0, p = 0.049).
Conclusions
To stabilize the surgical outcome of OPD for malignant disease, at least 20 surgeries should be performed at a certified institution during surgeon training.
Trial registration
Clinical trial number: Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12893-024-02677-9.
Keywords: Education, High-volume hospital, Learning curve, Pancreaticoduodenectomy
Background
Pancreaticoduodenectomy (PD) is one of the challenging procedures characterized by high complication and mortality rates that requires advanced skills and specialized anatomical knowledge of hepato-biliary-pancreatic (HBP) surgeons.
While it is crucial that HBP surgeons perform open PD (OPD) safely and certainly, the number of surgeries that should be performed during the surgeon training period remains uncertain.
The required number of surgeries experienced needed to achieve a stable procedure varies depending on the background of the surgeon or the institution. Previous analyses of the learning curve for OPD have been limited to a single surgeon [1–4] or a single center [5–9], and a considerable number of studies used an arbitrary split-group approach (e.g., postgraduate year, 50 cases), rather than a statistical calculation (e.g., Cumulative Sum analysis).
This multicenter, cohort study aimed to investigate the learning curve of OPDs performed by board-certified HBP surgeons during their training periods at board-certified institutions of the board certification system of the Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS).
Methods
Study participants
The study included total of 334 adult patients who underwent OPD by 14 surgeons between January 2008 and December 2022 from five medical institutions (Okayama University Hospital, Okayama Saiseikai General Hospital, National cancer center hospital east, Japanese Red Cross Society Himeji Hospital, and Tottori Municipal Hospital). All institutions were board-certified training institutions for the JHPBS during the training period [10, 11]. Of five hospitals, four were “Training Institution A” that performs more than 50 cases of highly advanced surgery for Hepatobiliary and Pancreatic field in a calendar year, and one was “Training Institution B” that performs more than 30 such surgeries in a calendar year.
Postoperative management
The patients were mobilized the day after surgery. A liquid diet on day 3 and a solid diet on day 4 were offered if tolerated. Drain fluid amylase level and drain fluid culture were measured on postoperative day 1 and 3; and drain was removed within day 5 according to the patient’s clinical conditions, in absence of sinister appearance of the effluent. Somatostatin analogs were not routinely used.
The following demographic and clinical data were reviewed through medical records: sex, age, preoperative diagnosis, surgical procedure, operative time, blood loss, and length of postoperative hospital stay (LOS). In this study, discharge was Included transfers to other hospital.
Postoperative complications in 90 days after the intervention were defined as those of Clavien-Dindo class IIIa or higher [12]. Postoperative pancreatic fistulas (POPFs) were assessed according to the International Study Group of Pancreatic Surgery (ISGPS) [13].
In this study, the primary endpoint was operative time, and the secondary endpoints were, blood loss, hospital stay, mortality rate, and surgical complications.
Statistical analysis
Clinical variables were compared using the Mann-Whitney U test for continuous data and Pearson’s correlation coefficient for categorical data. Continuous variables are presented as medians and interquartile range (IQR). Values of p < 0.05 were considered significant.
CUSUM analysis was performed to assess the change by number of experiences for operative time and to identify the number of procedures necessary to reach optimal performance [14]. A chronological arrangement of all cases from the first to the last by the trainee was performed. CUSUM values were calculated according to the following formula: CUSUM = Σ (xi − µ), where xi was the operative time of the individual case and µ was the median operative time of the trainee. Finally, the CUSUM values were plotted on the vertical axis according to their case number on the horizontal axis. Flection point could be determined by visual interpretation of the chart.
Risk factors for operative time and blood loss of top 25% for each trainee identified by univariable and multivariable analyses. Variables found to be associated with operative time and blood loss on univariable analysis (p < 0.05) were entered into a stepwise logistic regression model for multivariable analysis of risk factors.
All statistical analyses were performed using JMP version 14 (SAS Institute Inc., Cary, NC, USA).
Results
The background characteristics of the 14 HBP surgeons are summarized in Table 1. All participants were male, board-certified surgeons in gastroenterology with around 12 years of postgraduate experience at the beginning of the training. Three surgeons (21%) had received HBP training at two institutions during their training period.
Table 1.
Summary of HBPs trainees
| All (N=14) | |
|---|---|
| Years of post-graduate experience at HBPs training start, y, median (IQR) | 12 (10-13) |
| Sex | |
| Female, n (%) | 0 (0) |
| Male, n (%) | 14 (100) |
| Training period, y, median (IQR) | 5 (4-5) |
| Number of training institutions trainees belonged to | |
| One, n (%) | 11 (79) |
| Two, n (%) | 3 (21) |
| Number of OPD procedures performed during the training period, n, median (IQR) | 25.5 (19-28) |
| Board certified training institution | |
| institution A ( >50 high level HBP surgeries per year), n (%) | 13 (93) |
| institution B ( >30 high level HBP surgeries per year), n (%) | 1 (7) |
HBPs: hepatobiliary pancreatic surgery, IQR; Interquartile Range, OPD; open pancreaticoduodenectomies
Of the five hospitals in this study, “Institution A” performed about 100 to 200 cases of highly advanced HBP surgery with two board-certified instructors, and “Institution B” performed about 30 to 40 surgeries with one board-certified instructor. About the number of OPDs, Institution A had about 40 to 80 cases and Institution B had about 10 OPDs per year. Trainees performed highly advanced HBP surgeries including about 26 OPDs during their training periods (median 5 years) under the guidance of instructors at training institutions.
Finally, all trainees submitted videos of OPD or highly advanced hepatectomy for assessment of the applicant’s surgical skills for the JHPBS and passed this evaluation.
The enrolled patients’ characteristics and the surgical outcomes of the OPD cases are summarized in Table 2. Patients were categorized into three groups based on number of surgeries performed at each surgeons (group 1 (1–10), group 2 (11–20), group 3 (21≦). The most frequent primary disease was pancreatic ductal adenocarcinoma (n = 113, 34%), followed by ampulla or duodenal tumor (n = 70, 21%), IPMN (n = 67, 20%), bile duct cancer (n = 50, 15%), low-malignant pancreatic tumor (n = 18, 5%), and others (n = 16, 5%).
Table 2.
Summary of clinical characteristics and surgical outcomes of 334 OPD cases: total cohort and groups based on case number of each surgeon
| Total | Group 1 (1-10) | Group 2 (11-20) | Group 3 (>20) | p value | |
|---|---|---|---|---|---|
| Number of pancreaticoduodenectomies | 334 | 140 | 123 | 71 | |
| Training institution | |||||
| institution A, n (%) | 303 (91) | 130 (93) | 113 (92) | 60 (85) | 0.122 |
| institution B, n (%) | 31 (9) | 10(7) | 10 (8) | 11 (15) | |
| Age y, median (IQR) | 70 (63-76) | 69 (62-75) | 70 (63-77) | 71 (63-76) | 0.393 |
| Sex | |||||
| Female, n (%) | 137 (41) | 58 (41) | 42 (34) | 37 (52) | 0.049 |
| Male, n (%) | 197 (59) | 82 (59) | 81 (66) | 34 (48) | |
| BMI kg/m2 (IQR) | 22 (20-25) | 22 (21-25) | 22 (20-25) | 22 (20-24) | 0.866 |
| Disease | |||||
| PDAC, n (%) | 113 (34) | 37 (26) | 44 (36) | 32 (45) | 0.204 |
| Ampulla of Vater/duodenal tumor, n (%) | 70 (21) | 33 (24) | 27 (22) | 10 (14) | |
| IPMN, n (%) | 67 (20) | 35 (25) | 20 (16) | 12 (17) | |
| Bile duct cancer, n (%) | 50 (15) | 20 (14) | 21 (17) | 9 (13) | |
| Pancreatic NEN/SPN/SCN | 18 (5) | 10 (7) | 4 (3) | 4 (6) | |
| Others, n (%) | 16 (5) | 5 (4) | 7 (6) | 4 (6) | |
| Preoperative biliary drainage yes, n (%) | 144 (43) | 49 (35) | 59 (48) | 36 (51) | 0.037 |
| Combined vascular resection yes, n (%) | 54 (16) | 16 (11) | 24 (20) | 14 (20) | 0.136 |
| Operative time min, median (IQR) | 455 (397-519) | 465 (394-519) | 459 (405-532) | 425 (382-491) | 0.056 |
| Blood loss ml, median (IQR) | 450 (234-716) | 500 (263-743) | 450 (215-815) | 340 (200-560) | 0.090 |
| Blood transfusion n (%) | 32 (10) | 14 (10) | 16 (13) | 2 (3) | 0.066 |
| Hospital deaths n (%) | 5 (1.5) | 2 (1.4) | 2 (1.6) | 1 (1.4) | 0.989 |
| Surgical complications of Clavien-Dindo ≥IIIa | |||||
| All | 166 (50) | 66 (47) | 66 (54) | 34 (48) | 0.541 |
| POPF ≥ grade B, n (%) | 96 (29) | 42 (30) | 38 (31) | 16 (23) | 0.423 |
| DGE, n (%) | 27 (8) | 10 (7) | 9 (7) | 8 (11) | 0.540 |
| SSI, n (%) | 19 (6) | 10 (7) | 6 (5) | 3 (4) | 0.611 |
| Bile leak, n (%) | 13 (4) | 5 (4) | 6 (5) | 2 (3) | 0.749 |
| Abdominal bleeding, n (%) | 6 (2) | 1 (1) | 5 (4) | 0 (0) | 0.055 |
| LOS days, median (IQR) | 28 (21-38) | 28 (19-38) | 28 (22-39) | 24 (19-39) | 0.229 |
PDAC: Pancreatic Ductal Adenocarcinoma, IPMN: Intraductal Papillary Mucinous Neoplasm, NEN: Neuroendocrine Neoplasm, Neoplasm
SPN: Solid Pseudopapillary Neoplasm, SCN: Serous Cyst
POPF: Postoperative Pancreatic Fistula, DGE: Delayed Gastric Emptying, SSI: Surgical Site Infection
LOS: length of postoperative hospital stay
In the total cohort, the median operative time was 455 (IQR: 397–519) minutes, blood loss was 450 (IQR: 234–716) ml, and the postoperative hospital stay was 28 (IQR: 21–38) days.
There were 6 (1%) in-hospital deaths. Clinical POPFs were seen in 96 cases (29%), delayed gastric emptying was seen in 29 (9%), and bile leakage was found in 13 (4%).
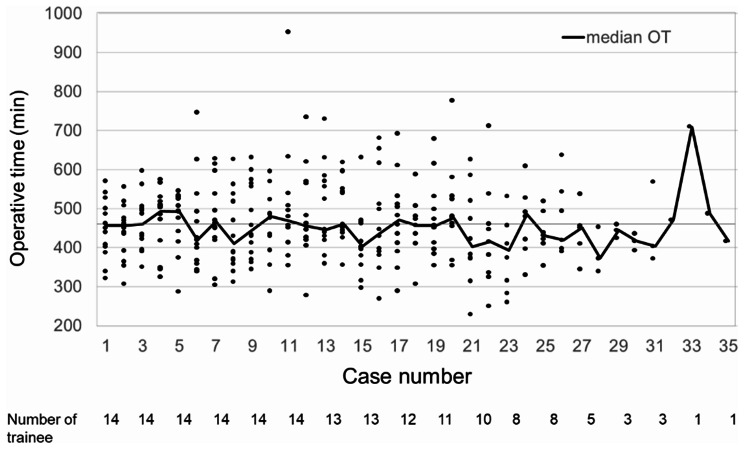
The operative time ordered by the number of cases was shown in Fig. 1. The number of trainees decreased after 11 cases because trainees who have passed the board-certified examination were removed from this study.
Fig. 1.
Operative time of the OPD procedure. Horizontal axis shows case number of OPD procedure for trainee
In CUSUM analysis of operation time including all trainees, the flection point was found after the 20 procedures (Fig. 2).
Fig. 2.
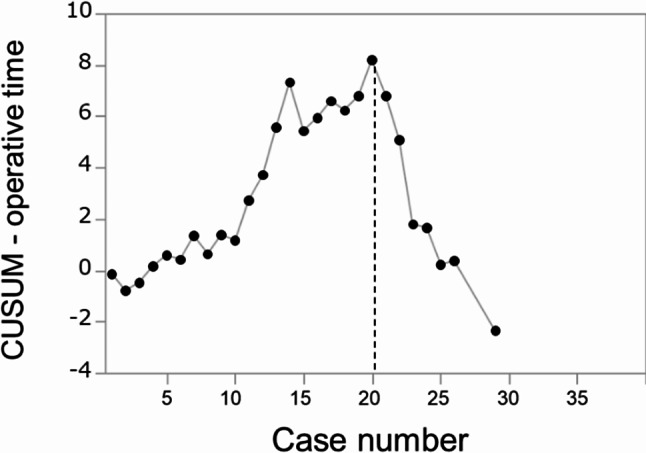
CUSUM analysis of the operative time. The flection point was found after the 20nd procedure
Cases and outcomes were compared before and after the 20 cases (Table 3). After 20 procedures, the PDAC rate was significantly higher (31% vs. 45%, p = 0.024), operative time was significantly shorter (461 min vs. 425 min, p = 0.021), and blood loss was significantly lower (470 ml vs. 340 ml, p = 0.038). On the other hand, no differences in these outcomes by before and after 10 procedures were observed. (supplemental Table e1). No significant differences between within 20 and after 21 procedures were found in the complication rate (53% vs. 48%, p = 0.424) or rate of in-hospital deaths (1.5% vs. 1.4%. p = 0.945). This trend was similar to subgroup analysis on Institution B only (supplemental Table e2).
Table 3.
Comparison of clinical characteristics and surgical outcomes before/after 20 cases
| Within 20 cases (1-20) |
After 21 cases ( ≥21 ) |
p value | |
|---|---|---|---|
| Number of pancreaticoduodenectomies | 263 | 71 | |
| Age y, median (IQR) | 69 (62-76) | 71 (63-76) | 0.547 |
| Sex | |||
| Female, n (%) | 100 (38) | 37 (52) | 0.032 |
| Male, n (%) | 163 (62) | 34 (48) | |
| BMI kg/m2 (IQR) | 22 (20-25) | 22 (20-24) | 0.612 |
| Disease | |||
| PDAC, n (%) | 81 (31) | 32 (45) | 0.024 |
| Others, n (%) | 182 (69) | 39 (55) | |
| Preoperative biliary drainage yes, n (%) | 108 42) | 36 (50) | 0.175 |
| Combined vascular resection yes, n (%) | 40 (15) | 14 (20) | 0.388 |
| Operative time min, median (IQR) | 461 (402-523) | 425 (382-491) | 0.021 |
| Blood loss ml, median (IQR) | 470 (250-779) | 340 (200-560) | 0.038 |
| Blood transfusion n (%) | 30 (11) | 2 (3) | 0.029 |
| Hospital deaths n (%) | 4 (1.5) | 1 (1.4) | 0.945 |
| Surgical complications of Clavien-Dindo ≥IIIa | |||
| All | 140 (53) | 34 (48) | 0.424 |
| POPF ≥ grade B, n (%) | 80 (30) | 16 (23) | 0.193 |
| DGE, n (%) | 21 (8) | 8 (11) | 0.383 |
| SSI, n (%) | 20 (8) | 3 (4) | 0.318 |
| Bile leak, n (%) | 11 (4) | 2 (3) | 0.598 |
| Abdominal bleeding, n (%) | 6 (2) | 0 (0) | 0.199 |
| LOS days, median (IQR) | 28 (22-38) | 24 (19-39) | 0.141 |
PDAC: Pancreatic Ductal Adenocarcinoma, IPMN: Intraductal Papillary Mucinous Neoplasm, NEN: Neuroendocrine Neoplasm, SPN: Solid Pseudopapillary Neoplasm, SCN: Serous Cyst Neoplasm
POPF: Postoperative Pancreatic Fistula, DGE: Delayed Gastric Emptying, SSI: Surgical Site Infection
LOS: length of postoperative hospital stay
The subgroup analysis depend on primary diseases is shown in Table 4. OPD cases were classified three groups: PDAC, another malignant tumor (bile duct cancer, ampullary cancer, gastric cancer), and benign/low malignant diseases (intraductal papillary mucinous neoplasm, neuroendocrine neoplasm, solid pseudopapillary neoplasm, gastrointestinal stromal tumor, chronic pancreatitis) Up to 20 surgeries, PDAC and another malignant tumor had longer operative time than benign/low malignant diseases (486 min vs. 472 min vs. 429 min, p < 0.001), and higher blood loss (500 ml vs. 502 ml vs. 355 ml, p < 0.001). Mortality rate was higher at PDAC cases (5% vs. 0% vs. 0%, p = 0.01) and all complication rates were higher at another malignant tumor (35% vs. 62% vs. 52%, p = 0.04). After the 21 procedures, operative time and blood loss were improved and no differences in by primary disease were observed: (430 min vs. 445 min vs. 410 min, p = 0.328), (345 ml vs. 400 ml vs. 315 ml, p = 0.642). Mortality and complication rate were similar between three groups.
Table 4.
Comparison of surgical outcomes and primary diseases before/after 20 cases for PDAC, other malignant tumors, and benign or low-malignant diseases
| Within 20 cases | After 21 cases | |||||||
|---|---|---|---|---|---|---|---|---|
| PDAC | Other malignant tumors | Benign or low-malignant diseases | p value | PDAC | Other malignant tumors | Benign or low-malignant diseases | p value | |
| Number of pancreaticoduodenectomies | 81 | 100 | 82 | 32 | 19 | 20 | ||
| Age y, median (IQR) | 70 (65-76) | 71 (62-77) | 66 (59-72) | 0.003 | 72 (65-76) | 74 (65-79) | 66 (59-72) | 0.022 |
| Sex | ||||||||
| Female, n (%) | 33 (41) | 39 (39) | 28 (34) | 0.665 | 19 (59) | 11 (58) | 7 (35) | 0.194 |
| Male, n (%) | 48 (59) | 61 (61) | 54 (66) | 13 (41) | 8 (42) | 13 (65) | ||
| BMI kg/m2 (IQR) | 22 (20-25) | 22 (21-24) | 22 (21-25) | 0.752 | 21 (19-23) | 22 (21-25) | 23 (22-24) | 0.038 |
| Preoperative biliary drainage yes, n (%) | 42 (52) | 64 (64) | 2 (3) | <0.001 | 21 (66) | 14 (74) | 1 (5) | <0.001 |
| Combined vascular resection yes, n (%) | 31 (38) | 7 (7) | 2 (3) | <0.001 | 14 (44) | 0 (0) | 0 (0) | <0.001 |
| Operative time min, median (IQR) | 486 (430-544) | 472 (410-532) | 429 (378-477) | <0.001 | 430 (397-494) | 445 (375-471) | 410 (337-458) | 0.328 |
| Blood loss ml, median (IQR) | 500 (303-840) | 502 (280-884) | 355 (140-578) | <0.001 | 345 (205-568) | 400 (215-550) | 315 (81-618) | 0.642 |
| Blood transfusion n (%) | 9 (11) | 18 (18) | 3 (4) | 0.010 | 1 (3) | 1 (5) | 0 (0) | 0.089 |
| Hospital deaths n (%) | 4 (5) | 0 (0) | 0 (0) | 0.010 | 0 (0) | 0 (0) | 1 (5) | 0.274 |
| Surgical complications of Clavien-Dindo ≥ IIIa | ||||||||
| All | 35 (43) | 62 (62) | 43 (52) | 0.041 | 13 (41) | 9 (47) | 12 (60) | 0.396 |
| POPF ≥ grade B, n (%) | 14 (17) | 38 (38) | 28 (4) | 0.007 | 4 (3) | 6 (32) | 6 (30) | 0.185 |
| DGE, n (%) | 8 (10) | 7 (7) | 6 (7) | 0.750 | 4 (13) | 2 (11) | 2 (10) | 0.955 |
| SSI, n (%) | 3 (4) | 15 (15) | 2 (2) | 0.002 | 2 (6) | 0 (0) | 1 (5) | 0.551 |
| Bile leak, n (%) | 2 (2) | 4 (4) | 5 (6) | 0.509 | 1 (3) | 0 (0) | 1 (5) | 0.634 |
| Abdominal bleeding, n (%) | 0 (0) | 4 (4) | 2 (2) | 0.199 | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| LOS days, median (IQR) | 28 (18-389) | 30 (24-41) | 26 (20-35) | 0.062 | 23 (17-32) | 33 (6-41) | 25 (18-33) | 0.043 |
PDAC: Pancreatic Ductal Adenocarcinoma,
POPF: Postoperative Pancreatic Fistula, DGE: Delayed Gastric Emptying, SSI: Surgical Site Infection
LOS: Length Of Postoperative Hospital Stay
In the sub analysis of PDAC, the percentage of PDAC was significantly increased in every 5 OPDs, and frequency of preoperative biliary drainage was also increased. (Supplemental Table e3)
However, operative time and blood loss of OPDs for PDAC were significantly improved after 21 procedures. (Supplemental Table e4)
The factors predicting top 25% of operative time and intraoperative bleeding for each trainee’s surgeries were analyzed. The results are summarized in Table 5. Multivariable analysis showed that male, BMI ≧ 25, vascular resection, and within 20 surgeries were independent risk factors of longer operative time (p = 0.011, = 0.010, < 0.001, = 0.013 respectively). And, multivariable analysis also showed that male, BMI ≧ 25, and within 20 surgeries were independent predictors of a higher blood loss (p = 0.012, < 0.001, = 0.049 respectively). In the PDACs cases, logistic regression analysis showed that younger than 65 years, male, BMI ≧ 25, vascular resection, and within 20 surgeries were independent risk factors of longer operative time, and only within 20 surgeries was the independent predictors of higher blood loss (supplemental Table 5).
Table 5.
Logistic regression analysis to examine risk factors for top 25% of operative time and intraoperative bleeding for each trainee’s surgeries
| Top 25% of operative time | Top 25% of blood loss | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| HR | P value | HR | P value | HR | P value | HR | P value | ||
| Age | ≥65 y | 0.083 | 0.703 | ||||||
| Sex | male | 2.0 | 0.011 | 2.2 | 0.008 | 2.1 | 0.006 | 2.0 | 0.012 |
| BMI | ≥25 kg/m2 | 2.2 | 0.010 | 2.5 | 0.005 | 2.7 | <0.001 | 2.8 | <0.001 |
| Disease | PDAC | 2.0 | 0.009 | 0.225 | 0.730 | ||||
| Preoperative biliary drainage | yes | 0.290 | 0.196 | ||||||
| Combined vascular resection | yes | 3.8 | <0.001 | 4.2 | <0.001 | 0.267 | |||
| Number of procedures | within 20 cases | 2.3 | 0.013 | 2.6 | 0.019 | 2.2 | 0.026 | 2.0 | 0.049 |
BMI: Body Mass Index, PDAC: Pancratic Ductal Adenocarcinoma
Discussion
This is the first multicenter study to analyze the OPD learning curve during the training period of gastroenterological surgeons who later became board-certified HBP expert surgeons. In the field of laparoscopic or robotic PD, multicenter analyses and clinical trials have been reported previously [15–20]. On the other hand, there has been no standard for the number of OPDs performed before stabilization of safety-related outcomes for HBP experts.
The results of learning curve analyses varied by training institution or outcome (such as operative time, bleeding, and surgical complications) [21]. The JSHBPS has established a certification system to certify expert HBP surgeons since 2008 [11], and its usefulness and safety has been reported [10, 22–25]. The quality of the present study was assured by these JSHBPS-certified institutions and by accepting as participants only expert HBP surgeons who had been certified through a video evaluation process with a pass rate of around 40%. Because the aim of the present study was to determine the cut-off for number of surgeries performed needed to adequately learn a safe surgical procedure, blood loss was set as the primary outcome [26].
The postoperative complication and mortality rates were comparable to the national average in Japan, even though this study’s patient population consisted of those treated during the HBP surgeons’ training period [25, 27].
The CUSUM analysis showed that, despite the high individual or institutional variability of surgical outcomes for OPD, the operating time decreased after 20 procedures performed in many HBP trainees. The inflection point at 20 surgeries performed was lower than that in previous studies, which reported 30 (range: 20–50) surgeries performed during training as being necessary for ensuring safe OPD procedures [9, 28, 29]. This might be attributable to the fact that participants in the present study were limited to those who were experts in gastrointestinal surgery at board-certified institutions with standardized OPD procedures.
In addition to the skill of each surgeon, the institutional environment such as volume of surgery at single institutions, number of tutors, and the frequency of other surgical experience over the period also has a significant impact on learning curve. In this study, while institution B had a low number of HBP surgery and OPD compared for Institution A, the experienced number of OPDs at trainee did not change during institutions. The reason for this might be that the larger hospital has more trainees and the number of OPD cases was more dispersed.
The problems in evaluating surgical outcomes of OPD are that the technique for pancreatic cancer is significantly different from that for benign/low-malignant tumor, resulting in variability in surgical difficulty. Furthermore, as the percentage of PDAC patients increases with the number of experienced cases, the improvement of surgical outcome by learning may be counteracted by the difficulty of cases.
In this study, the proportion of advanced PDAC which required preoperative biliary drainage increased along with the training period progressed, as trainee performed more difficult OPD. However, operative time and blood loss of OPDs for PDAC were significantly improved after 21 procedures.
In the subgroup analysis of PDAC case, logistic regression analysis showed that not only patients factor such as younger than 65 years, male, and BMI ≧ 25 but also vascular resection and number of cases were independent risk factors of longer operative time.
We investigated comparison between the primary disease and found that, during the learning curve, PDAC cases had longer operative time, higher blood loss and higher risk of hospital death than case of benign/low-malignant tumor. However, after 21 cases, the dramatic improvement was shown in operative time and blood loss for PDAC cases, and safety was ensured regardless of the disease. Finaly, we found that not only patient’s factor such as male, obesity, and vascular resection, but also the experience of 20 surgeries obviously affects surgical outcome of OPD during HBP training period. These results mean that in HBP training, difficulty cases should be performed later in the training period to ensure patient safety.
The limitations of this study are the few procedures per surgeon and individual differences in experience doing OPD at the start of training. There was no female trainee during study period in our institutions. This may be because female surgeons in Japan have few opportunities to receive surgical training especially HBPs surgery. [30]
Conclusions
The hepato-biliary-pancreatic surgery training at certified institutions was done safely. To stabilize the surgical outcome of OPD, at least 20 surgeries should be performed at certified institutions during surgeon training. In addition, difficult cases should be experienced step by step.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank their colleagues who contributed to data collection for this study: Daisuke Sato, Ken Koujima, Kenji Mizuno, Kenta Sui, Masaki Tokumo, Masashi Utsumi, Nobuyuki Watanabe, Susumu Shinoura, Takashi Kuise, Toru Kojima, Kazuteru Monden.
Author contributions
T. F: Analysis and interpretation of data, drafting of manuscript. Y.U: study conception and design. T. M: analysis. K.T, M.H, R.Y, Y.E, K.Y, K.M, D.N and T.F: Acquisition of data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due owing to data privacy policy at our facility but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study conformed to the Declaration of Helsinki on Human Research Ethics standards and was approved by the Okayama University Hospital Institutional Ethics Board (2112-040). The need for written informed consent was waived by the Okayama University Hospital Institutional Ethics Board because of the retrospective design of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10–5. 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardacre JM. (2010) Is there a learning curve for pancreaticoduodenectomy after fellowship training? HPB Surg 2010:230287. 10.1155/2010/230287 [DOI] [PMC free article] [PubMed]
- 3.Noda H, Kamiyama H, Kato T, Watanabe F, Toyama N, Konishi F. Risk factor for pancreatic fistula after pancreaticoduodenectomy performed by a surgeon during a learning curve: analysis of a single surgeon’s experiences of 100 consecutive patients. Hepatogastroenterology. 2012;59(118):1990–3. 10.5754/hge11821. [DOI] [PubMed] [Google Scholar]
- 4.Fisher WE, Hodges SE, Wu MF, Hilsenbeck SG, Brunicardi FC. Assessment of the learning curve for pancreaticoduodenectomy. Am J Surg. 2012;203(6):684–90. 10.1016/j.amjsurg.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Tseng JF, Pisters PW, Lee JE, Wang H, Gomez HF, Sun CC, Evans DB. The learning curve in pancreatic surgery. Surgery. 2007;141(5):694–701. 10.1016/j.surg.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Park HM, Han SS, Park SJ, Kim SW. Learning curve for pancreatoduodenectomy: can it be generalized? ANZ J Surg. 2020;90(7–8):1414–21. 10.1111/ans.15874. [DOI] [PubMed] [Google Scholar]
- 7.Relles DM, Burkhart RA, Pucci MJ, Sendecki J, Tholey R, Drueding R, Sauter PK, Kennedy EP, Winter JM, Lavu H, Yeo CJ. Does resident experience affect outcomes in complex abdominal surgery? Pancreaticoduodenectomy as an example. J Gastrointest Surg. 2014;18(2):279–85. 10.1007/s11605-013-2372-5. discussion 285. [DOI] [PubMed] [Google Scholar]
- 8.Shirai Y, Shiba H, Horiuchi T, Saito N, Furukawa K, Sakamoto T, Gocho T, Ishida Y, Yanaga K. Assessment of Outcome after Pancreaticoduodenectomy by Junior surgeons. Anticancer Res. 2016;36(7):3505–10. [PubMed] [Google Scholar]
- 9.Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, Nakeeb A, Howard TJ, Pitt HA, Lillemoe KD. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010;145(7):634–40. 10.1001/archsurg.2010.118. [DOI] [PubMed] [Google Scholar]
- 10.Miura F, Yamamoto M, Gotoh M, Konno H, Fujimoto J, Yanaga K, Kokudo N, Yamaue H, Wakabayashi G, Seto Y, Unno M, Miyata H, Hirahara N, Miyazaki M. Validation of the board certification system for expert surgeons (hepato-biliary-pancreatic field) using the data of the National Clinical Database of Japan: part 2 - pancreatoduodenectomy. J Hepatobiliary Pancreat Sci. 2016;23(6):353–63. 10.1002/jhbp.348. [DOI] [PubMed] [Google Scholar]
- 11.Miura F, Yamamoto M, Gotoh M, Konno H, Fujimoto J, Yanaga K, Kokudo N, Yamaue H, Wakabayashi G, Seto Y, Unno M, Miyata H, Hirahara N, Miyazaki M. Validation of the board certification system for expert surgeons (hepato-biliary-pancreatic field) using the data of the National Clinical Database of Japan: part 1 - hepatectomy of more than one segment. J Hepatobiliary Pancreat Sci. 2016;23(6):313–23. 10.1002/jhbp.344. [DOI] [PubMed] [Google Scholar]
- 12.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 13.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M, International Study Group on Pancreatic S. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584–91. 10.1016/j.surg.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Egberts JH, Welsch T, Merboth F, Korn S, Praetorius C, Stange DE, Distler M, Biebl M, Pratschke J, Nickel F, Muller-Stich B, Perez D, Izbicki JR, Becker T, Weitz J. Robotic-assisted minimally invasive Ivor Lewis esophagectomy within the prospective multicenter German Da Vinci Xi registry trial. Langenbecks Arch Surg. 2022;407(4):1–11. 10.1007/s00423-022-02520-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim JS, Jackson T, Kurtz J, Cho EE, Vedantam S, Nagatomo K, Osman H, Jeyarajah DR. Overcoming the arduous transition for robotic Hepatopancreatobiliary cases: a multi-procedure learning curve study utilizing CUSUM Analysis. World J Surg. 2021;45(3):865–72. 10.1007/s00268-020-05861-z. [DOI] [PubMed] [Google Scholar]
- 16.Lyman WB, Passeri MJ, Murphy K, Siddiqui IA, Khan AS, Iannitti DA, Martinie JB, Baker EH, Vrochides D. An objective approach to evaluate novice robotic surgeons using a combination of kinematics and stepwise cumulative sum (CUSUM) analyses. Surg Endosc. 2021;35(6):2765–72. 10.1007/s00464-020-07708-z. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt CR, Harris BR, Musgrove KA, Rao P, Marsh JW, Thomay AA, Hogg ME, Zeh HJ, Zureikat AH, Boone BA. Formal robotic training diminishes the learning curve for robotic pancreatoduodenectomy: implications for new programs in complex robotic surgery. J Surg Oncol. 2021;123(2):375–80. 10.1002/jso.26284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takagi K, Umeda Y, Yoshida R, Yagi T, Fujiwara T, Zureikat AH, Hogg ME, Koerkamp BG. Surgical training model and safe implementation of robotic pancreatoduodenectomy in Japan: a technical note. World J Surg Oncol. 2021;19(1):55. 10.1186/s12957-021-02167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyutyunnik P, Klompmaker S, Lombardo C, Lapshyn H, Menonna F, Napoli N, Wellner U, Izrailov R, Baychorov M, Besselink MG, Abu Hilal M, Fingerhut A, Boggi U, Keck T, Khatkov I, European Consortium on Minimally Invasive Pancreatic S. Learning curve of three European centers in laparoscopic, hybrid laparoscopic, and robotic pancreatoduodenectomy. Surg Endosc. 2022;36(2):1515–26. 10.1007/s00464-021-08439-5. [DOI] [PubMed] [Google Scholar]
- 20.Zwart MJW, Nota CLM, de Rooij T, van Hilst J, Te Riele WW, van Santvoort HC, Hagendoorn J, Rinkes I, van Dam JL, Latenstein AEJ, Takagi K, Tran TCK, Schreinemakers J, van der Schelling G, Wijsman JH, Festen S, Daams F, Luyer MD, de Hingh I, Mieog JSD, Bonsing BA, Lips DJ, Hilal MA, Busch OR, Saint-Marc O, 3rd Zeh HJ, Zureikat AH, Hogg ME, Molenaar IQ, Besselink MG, Koerkamp BG, Dutch Pancreatic Cancer G. Outcomes of a Multicenter Training Program in robotic pancreatoduodenectomy (LAELAPS-3). Ann Surg. 2021. 10.1097/SLA.0000000000004783. [DOI] [PubMed] [Google Scholar]
- 21.Kassite I, Bejan-Angoulvant T, Lardy H, Binet A. A systematic review of the learning curve in robotic surgery: range and heterogeneity. Surg Endosc. 2019;33(2):353–65. 10.1007/s00464-018-6473-9. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto D, Okawa T, Matsumura F. Training in the Japanese Society of Hepato-Biliary-Pancreatic Surgery board certification system for expert surgeons during 225 consecutive pancreaticoduodenectomies. Ann Hepatobiliary Pancreat Surg. 2019;23(2):145–54. 10.14701/ahbps.2019.23.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsubo T, Kobayashi S, Sano K, Misawa T, Ota T, Katagiri S, Yanaga K, Yamaue H, Kokudo N, Unno M, Fujimoto J, Miura F, Miyazaki M, Yamamoto M. Safety-related outcomes of the Japanese Society of Hepato-Biliary-Pancreatic Surgery board certification system for expert surgeons. J Hepatobiliary Pancreat Sci. 2017;24(5):252–61. 10.1002/jhbp.444. [DOI] [PubMed] [Google Scholar]
- 24.Otsubo T, Kobayashi S, Sano K, Misawa T, Katagiri S, Nakayama H, Suzuki S, Watanabe M, Ariizumi S, Unno M, Tanabe M, Nagano H, Kokudo N, Hirano S, Nakamura M, Shirabe K, Suzuki Y, Yoshida M, Takada Y, Nakagohri T, Horiguchi A, Ohdan H, Eguchi S, Ohtsuka M, Sho M, Rikiyama T, Hatano E, Taketomi A, Fujii T, Yamaue H, Miyazaki M, Yamamoto M, Takada T, Endo I. A nationwide certification system to increase the safety of highly advanced hepatobiliary-pancreatic surgery. J Hepatobiliary Pancreat Sci. 2023;30(1):60–71. 10.1002/jhbp.1186. [DOI] [PubMed] [Google Scholar]
- 25.Mise Y, Hirakawa S, Tachimori H, Kakeji Y, Kitagawa Y, Komatsu S, Nanashima A, Nakamura M, Endo I, Saiura A. Volume- and quality-controlled certification system promotes centralization of complex hepato-pancreatic-biliary surgery. J Hepatobiliary Pancreat Sci. 2023;30(7):851–62. 10.1002/jhbp.1307. [DOI] [PubMed] [Google Scholar]
- 26.Aramaki O, Takayama T, Higaki T, Nakayama H, Ohkubo T, Midorikawa Y, Moriguchi M, Matsuyama Y. Decreased blood loss reduces postoperative complications in resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2014;21(8):585–91. 10.1002/jhbp.101. [DOI] [PubMed] [Google Scholar]
- 27.Marubashi S, Takahashi A, Kakeji Y, Hasegawa H, Ueno H, Eguchi S, Endo I, Goi T, Saiura A, Sasaki A, Takiguchi S, Takeuchi H, Tanaka C, Hashimoto M, Hiki N, Horiguchi A, Masaki T, Yoshida K, Gotoh M, Konno H, Yamamoto H, Miyata H, Seto Y, Kitagawa Y, National Clinical D. Surgical outcomes in gastroenterological surgery in Japan: report of the National Clinical Database 2011–2019. Ann Gastroenterol Surg. 2021;5(5):639–58. 10.1002/ags3.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMillan MT, Malleo G, Bassi C, Sprys MH, Vollmer CM Jr. Defining the practice of pancreatoduodenectomy around the world. HPB (Oxford). 2015;17(12):1145–54. 10.1111/hpb.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy GT, McMillan MT, Maggino L, Sprys MH, Vollmer CM Jr. Surgical experience and the practice of pancreatoduodenectomy. Surgery. 2017;162(4):812–22. 10.1016/j.surg.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Emiko Kono MD, Urara Isozumi MS, Sachiyo Nomura MD, KaeOkoshi PD,MD, PhD;Hiroyuki Yamamoto MD, Hiroaki MPHPD, Miyata PD, Itaru Yasufuku MD, Hiromichi Maeda MD, PhD;Junichi Sakamoto MD, Kazuhisa Uchiyama PD, Yoshihiro Kakeji MDPD. Surgical Experience Disparity between Male and Female surgeons in Japan. JAMA Surg. 2017;157(9):e222938. 10.1001/jamasurg.2022.2938. ,MD, PhD; KazuhiroYoshida,MD, PhD; Yuko Kitagawa,MD, PhD. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due owing to data privacy policy at our facility but are available from the corresponding author on reasonable request.