Abstract
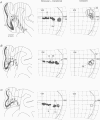
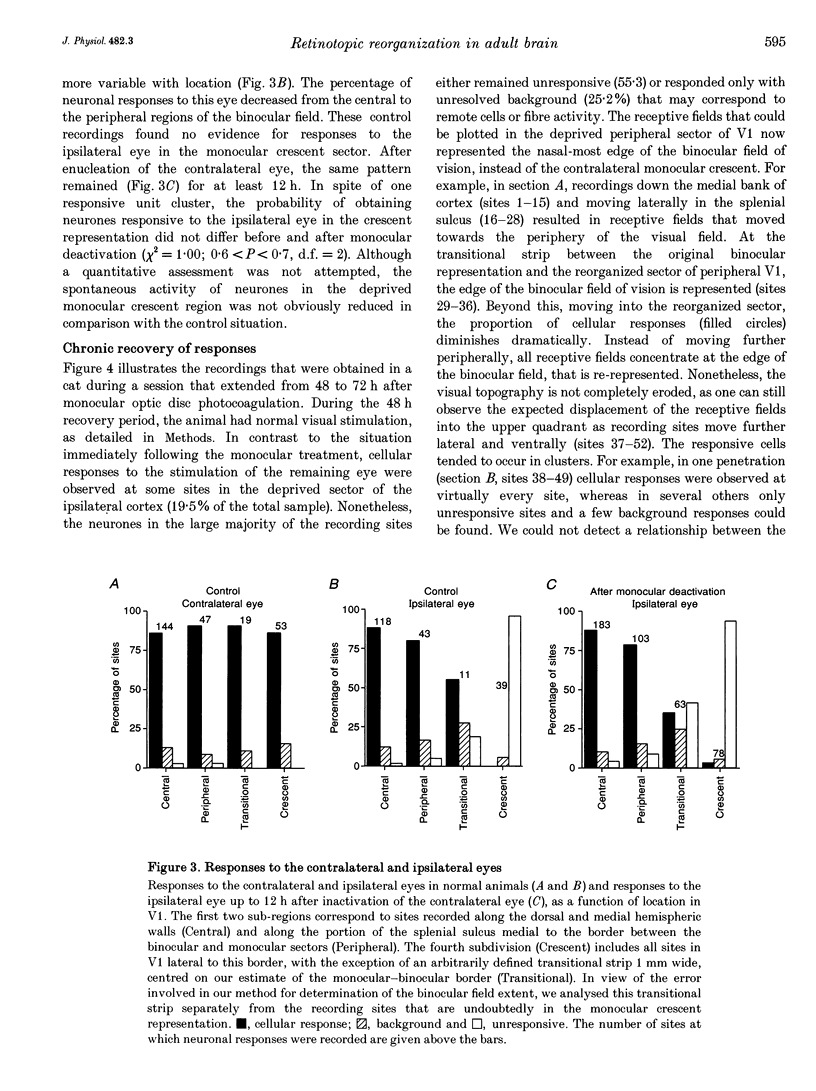
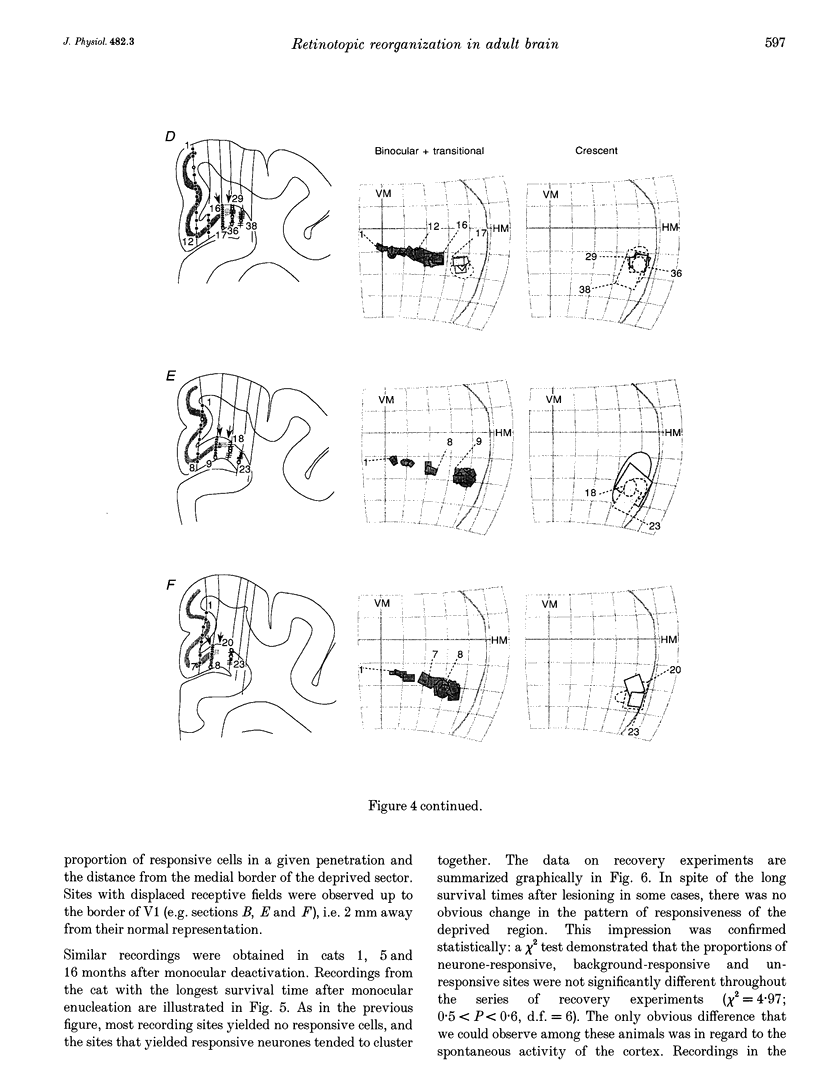
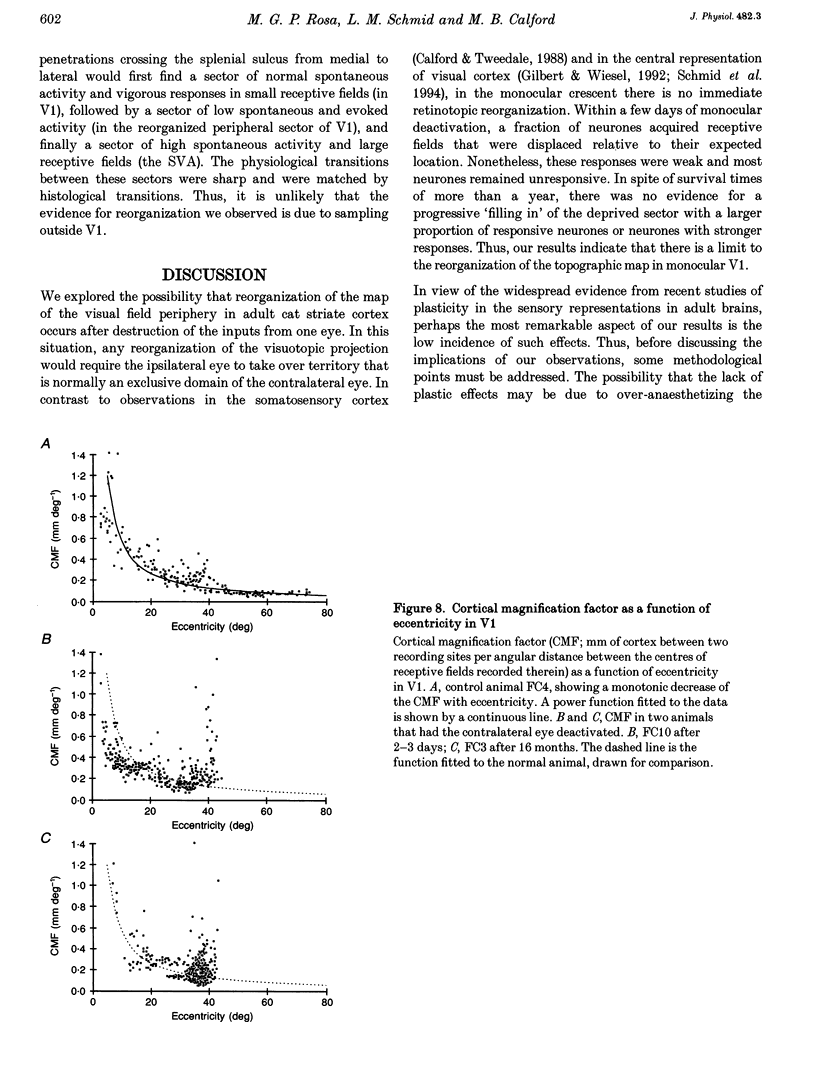
1. Recordings were made from neurones in the splenial sulcus of normal adult cats and adult cats which had one eye inactivated by enucleation or photocoagulation of the optic disc. Two visually responsive regions were observed, corresponding to the peripheral representation of visual area 1 (V1) and the splenial visual area. In normal animals, responses to the ipsilateral eye in V1 were restricted to the medial half of the splenial sulcus, up to 45-50 deg eccentricity. Thus, by inactivating the eye contralateral to the experimental hemisphere, we created a region in V1, 1-2 mm wide, that lacked normal inputs. 2. In contrast to results from previous experiments where lesions were placed in the central retina, neurones in the deprived peripheral representation remained unresponsive to light stimuli for up to 12 h after deactivation of the contralateral eye. 3. In animals that were allowed to recover from the monocular deactivation for periods of 2 days to 16 months, there was rearrangement of the retinotopic maps. Receptive fields in regions of cortex that normally represented the monocular crescent were displaced to the temporal border of the binocular field of vision. However, most neurones in the deprived peripheral representation remained unresponsive to visual stimuli even more than 1 year after treatment. This is also in marked contrast with the extensive reorganization that is observed in the central representation of V1 after restricted retinal lesions. Analysis of the cortical magnification factor demonstrates that the change in visual topography is local, and does not involve an overall centro-peripheral shift of the retinotopic map. 4. Among the neurones that did show displaced receptive fields, the response properties were clearly abnormal. They showed a notable lack of spontaneous activity, low firing rates and rapid habituation to repeated stimulation. 5. The low potential for reorganization of the monocular sector of V1 demonstrates that the capacity for plasticity of mature sensory representations varies with location in cortex. Even relatively small pieces of cortex, such as the monocular crescent representations, may not reorganize completely if certain conditions are not met. These results suggest the existence of natural boundaries that may limit the process of reorganization of sensory representations.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford M. B., Tweedale R. Acute changes in cutaneous receptive fields in primary somatosensory cortex after digit denervation in adult flying fox. J Neurophysiol. 1991 Feb;65(2):178–187. doi: 10.1152/jn.1991.65.2.178. [DOI] [PubMed] [Google Scholar]
- Calford M. B., Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988 Mar 31;332(6163):446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Clarke R. J., Datskovsky B. W., Grigonis A. M., Murphy E. H. The effects of monocular enucleation on visual topography in area 17 in the rabbit. Exp Brain Res. 1992;91(2):303–310. doi: 10.1007/BF00231663. [DOI] [PubMed] [Google Scholar]
- DANIEL P. M., WHITTERIDGE D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961 Dec;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysel U. T., Gonzalez-Aguilar F., Mayer U. A functional sign of reorganization in the visual system of adult cats: lateral geniculate neurons with displaced receptive fields after lesions of the nasal retina. Brain Res. 1980 Jan 13;181(2):285–300. doi: 10.1016/0006-8993(80)90613-7. [DOI] [PubMed] [Google Scholar]
- Eysel U. T., Gonzalez-Aguilar F., Mayer U. Time-dependent decrease in the extent of visual deafferentation in the lateral geniculate nucleus of adult cats with small retinal lesions. Exp Brain Res. 1981;41(3-4):256–263. doi: 10.1007/BF00238882. [DOI] [PubMed] [Google Scholar]
- Eysel U. T. Maintained activity, excitation and inhibition of lateral geniculate neurons after monocular deafferentation in the adult cat. Brain Res. 1979 Apr 27;166(2):259–271. doi: 10.1016/0006-8993(79)90212-9. [DOI] [PubMed] [Google Scholar]
- Fiorani Júnior M., Rosa M. G., Gattass R., Rocha-Miranda C. E. Dynamic surrounds of receptive fields in primate striate cortex: a physiological basis for perceptual completion? Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8547–8551. doi: 10.1073/pnas.89.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Clustered intrinsic connections in cat visual cortex. J Neurosci. 1983 May;3(5):1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Receptive field dynamics in adult primary visual cortex. Nature. 1992 Mar 12;356(6365):150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Heinen S. J., Skavenski A. A. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp Brain Res. 1991;83(3):670–674. doi: 10.1007/BF00229845. [DOI] [PubMed] [Google Scholar]
- Hughes A. A supplement to the cat schematic eye. Vision Res. 1976;16(2):149–154. doi: 10.1016/0042-6989(76)90091-2. [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Krubitzer L. A., Chino Y. M., Langston A. L., Polley E. H., Blair N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990 Apr 13;248(4952):229–231. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- Kalia M., Whitteridge D. The visual areas in the splenial sulcus of the cat. J Physiol. 1973 Jul;232(2):275–283. doi: 10.1113/jphysiol.1973.sp010269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwel S., Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science. 1992 Jan 10;255(5041):209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- Löwel S., Singer W. The pattern of ocular dominance columns in flat-mounts of the cat visual cortex. Exp Brain Res. 1987;68(3):661–666. doi: 10.1007/BF00249809. [DOI] [PubMed] [Google Scholar]
- McConnell S. K., LeVay S. Anatomical organization of the visual system of the mink, Mustela vison. J Comp Neurol. 1986 Aug 1;250(1):109–132. doi: 10.1002/cne.902500110. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J. T., Sur M., Nelson R. J., Felleman D. J. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983 Nov;10(3):639–665. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Nelson R. J., Stryker M. P., Cynader M. S., Schoppmann A., Zook J. M. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984 Apr 20;224(4):591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Montero V. M., Bravo H., Fernández V. Striate-peristriate cortico-cortical connections in the albino and gray rat. Brain Res. 1973 Apr 13;53(1):202–207. doi: 10.1016/0006-8993(73)90781-6. [DOI] [PubMed] [Google Scholar]
- Nikara T., Bishop P. O., Pettigrew J. D. Analysis of retinal correspondence by studying receptive fields of binocular single units in cat striate cortex. Exp Brain Res. 1968;6(4):353–372. doi: 10.1007/BF00233184. [DOI] [PubMed] [Google Scholar]
- Pettet M. W., Gilbert C. D. Dynamic changes in receptive-field size in cat primary visual cortex. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8366–8370. doi: 10.1073/pnas.89.17.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J. D., Cooper M. L., Blasdel G. G. Improved use of tapetal reflection for eye-position monitoring. Invest Ophthalmol Vis Sci. 1979 May;18(5):490–495. [PubMed] [Google Scholar]
- Pons T. P., Garraghty P. E., Ommaya A. K., Kaas J. H., Taub E., Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991 Jun 28;252(5014):1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Rajan R., Irvine D. R., Wise L. Z., Heil P. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol. 1993 Dec 1;338(1):17–49. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- Rasmusson D. D. Reorganization of raccoon somatosensory cortex following removal of the fifth digit. J Comp Neurol. 1982 Mar 10;205(4):313–326. doi: 10.1002/cne.902050402. [DOI] [PubMed] [Google Scholar]
- Recanzone G. H., Merzenich M. M., Jenkins W. M., Grajski K. A., Dinse H. R. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992 May;67(5):1031–1056. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Rosa M. G., Gattass R., Fiorani M., Jr, Soares J. G. Laminar, columnar and topographic aspects of ocular dominance in the primary visual cortex of Cebus monkeys. Exp Brain Res. 1992;88(2):249–264. doi: 10.1007/BF02259100. [DOI] [PubMed] [Google Scholar]
- Tusa R. J., Palmer L. A., Rosenquist A. C. The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol. 1978 Jan 15;177(2):213–235. doi: 10.1002/cne.901770204. [DOI] [PubMed] [Google Scholar]
- Weinberger N. M., Hopkins W., Diamond D. M. Physiological plasticity of single neurons in auditory cortex of the cat during acquisition of the pupillary conditioned response: I. Primary field (AI). Behav Neurosci. 1984 Apr;98(2):171–188. doi: 10.1037//0735-7044.98.2.171. [DOI] [PubMed] [Google Scholar]