Abstract
Background
Risk factors for local recurrence in patients with metastatic spinal cord compression (MSCC) has not been clearly investigated. So, the purpose of this study was to identify risk factors causing local recurrence following surgeries in patients with MSCC.
Methods
We conducted a retrospective comparative study on 304 patients who underwent surgery for MSCC between March 2014 and February 2020. Local recurrence rate (LRR) was analyzed according to demographic variables, radiological variables such as level of spinal metastasis, number of non-spinal bone metastases, degree of spinal cord compression, spinal instability, and pathological fracture, and treatment-related variables such as origin of tumor, surgical treatment methods, and pre- and post- operative radiation therapy. Univariate and multivariate logistic regression analyses were performed to reveal the risk factors for local recurrence.
Results
Among 304 patients with MSCC, 50 patients (16.4%) experienced local recurrence after surgery. Of the surgical methods, decompression alone (26/50, 52.0%) showed higher LRR compared to decompression with fixation (9/177, 5.1%) or corpectomy (11/89, 12.4%), (P = 0.002 and P = 0.018, respectively). Patients with renal cell carcinoma revealed higher LRR compared to other types (P = 0.014). It was found that the 3 or more level of spinal metastasis (P = 0.001), the 3 or more of extraspinal bone metastases (P = 0.028), and pathologic fracture (P = 0.003) were related with higher LRR. Smoking is also an independent risk factor for local recurrence in patients who underwent fixation (P = 0.026).
Conclusions
Symptomatic local recurrence may be influenced by several factors, including the extent of spinal and extraspinal bone metastasis, pathologic fractures, surgical approach, and tumor origin (RCC). These factors should be carefully considered by surgeons when evaluating the risk of symptomatic local recurrence after surgery.
Keywords: Spinal tumor, Metastasis, Cord compression, Local recurrence, Risk factor
Background
In general, metastasis occurs in the spine in approximately 5% of patients with primary cancer, and metastatic spinal tumors are one of the most common cancers in the spine [1, 2]. Approximately 6% of patients with metastatic spinal tumors develop metastatic spinal cord compression (MSCC) [2, 3]. Because MSCC can cause progressive neurologic deficit, it is important to perform timely surgical treatment if patients started to show the progression of paralysis [3–5].
There have been several surgical strategies provided: decompression alone, posterior decompression and stabilization, corpectomy with stabilization, or en-bloc excision [6–8]. Regardless of any surgical techniques, the one of important objective in the treatment of MSCC is to minimize local recurrence. If patients experienced local recurrence, there is limited chance of local control because of difficulties of surgical treatment as well as lack of further treatment options. Because the prognosis is poor when local recurrence occurs in patients with MSCC, the understanding of the related factors for local recurrence in patients with MSCC is important [9–14].
Although several studies revealed the risk of local recurrence following decompressive surgery in patients with MSCC, there have been suggested conflicting evidences because of heterogenous population and small number of patients. In addition, related factors for higher local recurrence rate (LRR) have not been clearly investigated. So, the purpose of this study was to reveal the incidence of symptomatic local recurrence in various conditions, and to identify risk factors for higher LRR following surgeries in patients with MSCC [2–5, 9–25].
There were about two studies on risk factors that occurred after surgery in MSCC patients. One was a paper on risk factors that occurred after en bloc spondylectomy. In an analysis of 91 patients, local recurrence occurred in 10 patients, and radiation history was found to be the only risk factor [10]. Another paper is about the risk factor that occurred after surgery for spinal metastasis. In an analysis of 99 patients, local recurrence occurred in 32 patients, and melanoma was found to be the only risk factor among various primary cancers [11]. Despite these studies, the number of patient groups was relatively small and studies on various risk factors were not analyzed, so this study was planned and started.
Methods
Patients and operative methods
This study was approved by the Institutional Review Board of our institution (IRB number: 2022 − 0684). Informed consent was waived for this study because of the retrospective nature of the study. This study is a retrospective comparative study including 304 patients who underwent surgical treatment because of MSCC between March 2014 and February 2020. It was designed and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for the cohort.
Surgery was performed on patients who were diagnosed with MSCC with severe pain or neurological deficits. Sometimes, surgical treatment was indicated if the degree of cord compression was severe (Bilsky grade 3) in patients with not severe symptom to prevent neurologic deterioration. The decision of treatment strategy including whether to perform surgery was discussed among medical oncologists, radiation oncologists and spine surgeons. However, in emergency situations such as progressive limb weakness, surgery was performed relying on the experience of spine surgeon. Decision of operative methods depend on surgeons’ various considerations such as life expectancy, other treatment options, general performances, etc. The decision on operative methods primarily depends on the Tomita scoring system, which leads to a range of surgical options with varying degrees of invasiveness [7]. When longer survival is anticipated, there is an increased risk of local recurrence, necessitating a more aggressive approach. If radiological findings indicate instability, fixation is the preferred method. However, the actual decision largely relies on the surgeon’s experience, considering factors such as life expectancy, other treatment options including chemotherapy or radiotherapy, general performance, and so on. In some cases, only decompression without fixation was performed if the patient’s condition was not optimal, even though progressive weakness was observed, which constituted an emergent situation.
If surgical treatment is not indicated, radiotherapy is initially administered. If surgical treatment is performed, postoperative radiotherapy is typically conducted 2 to 3 weeks later, provided there are no contraindications such as wound complications or the risk of spinal cord infarction due to previous radiation doses. Most patients received conventional external beam radiotherapy (EBRT). Data on exact dosages and frequencies were not available because many patients underwent radiotherapy at various hospitals.
If surgery was planned, preoperative embolization was usually performed especially in hyper vascular tumor [15].
Study variables
Epidemiologic variables included sex, age, height, weight and BMI. Surgical method, primary cancer type, level of spinal metastasis, number of bone metastases other than spine, degree of compression of spinal cord, pre- and postoperative radiation therapy, pathologic fracture, degree of spinal instability, and pre- and postoperative neurological changes were investigated by the review of medical chart and PACS (picture archiving and communication system). Radiographs, CT and MRI were used to evaluate various radiological parameters [25].
Surgical treatment methods for patients undergoing MSCC surgery were divided into four types: Decompression alone, fixation alone, decompression with fixation, and corpectomy with fixation. The extent of spinal metastasis was classified into 1 level, 2 level, and 3 or more levels based on the number of vertebral bodies metastasized from the primary cancer, regardless of cervical, thoracic, lumbar, or sacral vertebra. The number of bone metastases other than the spine was classified based on the number of metastasized bones, excluding carpal bone and tarsal bone, and was classified into 1, 2, and 3 or more.
The degree of spinal cord compression was classified using the Bilsky grades [26], and the degree of spinal instability was classified using the spinal instability neoplastic score (SINS) [27, 28]. The presence of a pathologic fracture was determined based on the acute pathologic fracture confirmed on MRI before the first surgery, and pathologic fractures that occurred after the first surgery were excluded. Smoking history was defined as both current smokers and individuals who have a history of smoking for more than one year.
Motor function was described using the six point scale motor scoring (0–5 grade), and was described based on the muscle showing the highest grade among the major muscles. Pre- and post- motor function changes were expressed based on differences in motor grade of the major muscles described.
Gait analysis used the Functional Mobility Scale (FMS scale), the pre-operative gait grade indicated the state immediately before surgery, and the post-operative gait grade analyzed the state immediately before discharge after the first surgery.
“Symptomatic local recurrence” was defined as follows [29]. At first, the symptom should be clearly resolved following index surgery. For example, pain diminished or the motor function improved. After that, aggravation was confirmed in two ways: when clinical symptoms worsen (increased pain or worsening neurological symptoms) and when aggravation of tumor (recurred cord compression) in the level of index surgery was confirmed on CT or MRI. Among patients who underwent surgery, analysis was conducted by dividing them into two groups: those in which local recurrence occurred [LR(+)] and those in which it did not [LR(-)].
Statistical analyses
Various demographic, clinical and radiological parameters were compared according to the existence of symptomatic local recurrence, using the Student’s t-test for continuous variables and the chi-square test for categorical variables. Only the variables with a P value less than 0.05 were entered into the multivariate analysis to maintain model parsimony and reduce the risk of overfitting. Forward conditional method was used for logistic regression analysis. To reveal the time effect of operation method and origin of tumor on local recurrence, log-rank test was used. Statistical analysis was performed using SPSS 21, and a P value of < 0.05 was considered statistically significant.
Results
Comparison of demographic parameters between groups
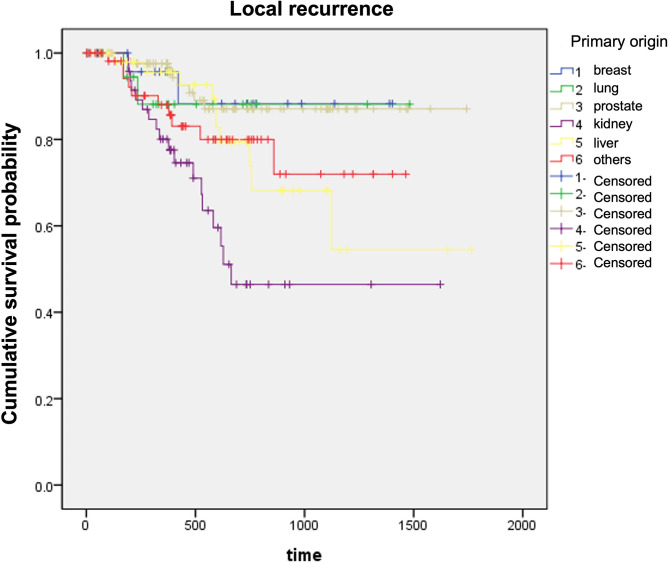
The study included 304 patients with MSCC who underwent surgery, of which 254 (83.5%) did not develop local recurrence and 50 (16.5%) patients developed local recurrence. The average age did not differ between LR(+) and LR(-) groups (56.7 and 59.2 yrs, P = 0.264). The most frequent origin of tumors was lung cancer (91/304,29.9%), followed by HCC (54/304,17.8%), and RCC (52/304,17.1%). Local recurrence was most frequent in renal cell carcinoma (RCC) (18/52, 34.6%) compared to other origins (P = 0.042). Average time to local recurrence was 405.8 ± 213.9 days. In the log rank test, RCC was found to be one of the risk factors statistically significantly involved in local recurrence (Fig. 1).
Fig. 1.
Log-rank test for local recurrence according to origin of tumor. Higher local recurrence was observed in kidney cancer, compared to breast (P = 0.012), lung (P = 0.083), prostate (P < 0.001), liver (P = 0.015), and others (P = 0.036)
Smoking was also related with local recurrence (P = 0.040). The other demographic and clinical variables did not reveal any differences between groups (Table 1).
Table 1.
Comparison of demographic factors among groups
| Total (N = 304) | |||
|---|---|---|---|
| LR [-] (N = 254) | LR [+] (N = 50) | P-value | |
| Age (years) | 59.2 ± 12.7 | 56.7 ± 12.1 | 0.264 |
| Sex (M: F) | 160 : 94 | 33 : 17 | 0.507 |
| Height (cm) | 164.8 ± 7.2 | 166.5 ± 7.7 | 0.266 |
| Weight (kg) | 60.8 ± 10.8 | 63.2 ± 8.3 | 0.302 |
| BMI (kg/m2) | 22.3 ± 3.1 | 22.8 ± 2.4 | 0.517 |
| Origin of primary tumor, N (%) | |||
| Breast cancer | 23 (92.0) | 2 (8.0) | 0.042 |
| Lung cancer | 83 (91.2) | 8 (8.8) | |
| Prostate cancer | 22 (91.7) | 2 (8.3) | |
| RCC | 34 (65.4) | 18 (34.6) | |
| HCC | 44 (81.5) | 10 (18.5) | |
| Other | 48 (82.8) | 10 (17.2) | |
| Past medical history, N (%) | |||
| HTN | 78 (30.7) | 18 (36.0) | 0.382 |
| DM | 50 (19.7) | 12 (24.0%) | 0.294 |
| Liver disease | 40 (15.7%) | 9 (18.0%) | 0.917 |
| Pulmonary disease | 30 (11.8%) | 4 (8.0%) | 0.356 |
| Smoking | 41 (16.1%) | 15 (30.0%) | 0.040 |
Data are expressed as the mean value and standard deviation for continuous variables and numbers for categorical variables
LR, local recurrence; N, number; BMI, body mass index; RCC, Renal cell cancer, HCC, Hepatocellular cancer; HTN, Hypertension; DM, diabetes mellitus
Comparison of tumor-related factors between groups
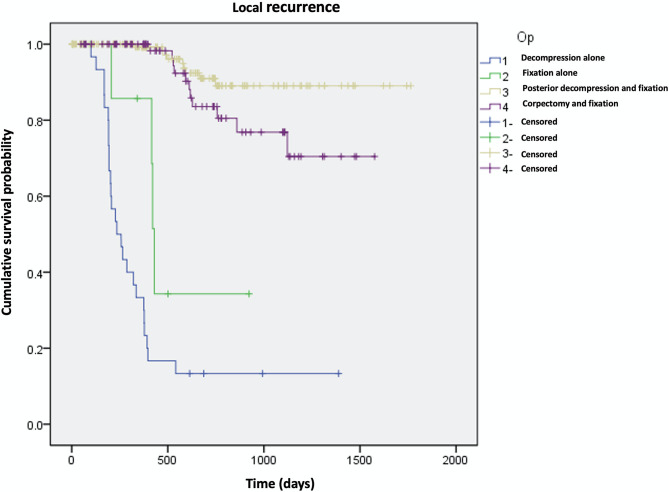
When only decompression was performed as a surgical method, 26 of 30 patients (86%) recurred. However, less local recurrence was expected in patients who underwent decompression/fixation or corpectomy (9/117, 5% and 11/89, 12%, respectively, P < 0.001). In the log rank test, similar results were found (Fig. 2).
Fig. 2.
Log-rank test for local recurrence according to surgical methods. Higher local recurrence was observed in patients who underwent decompression alone compared to whom underwent fixation (P < 0.001). No differences were found between decompression with fixation group and corpectomy with fixation group (P = 0.082)
For extent of spinal lesion, 3 or more level of spinal metastasis was related with higher LRR (39/60, 65%) compared to 1 (4/87, 4.6%) or 2 level cases (7/157, 4.5%) (P < 0.001). For additional bone metastasis other than spine, in patients with 3 or more extraspinal bone metastases showed higher LRR (40/55, 72.7%) compared to 2 or less extraspinal bone lesions (10/249, 4.0%) (P < 0.001). Pathologic fracture was also related with higher LRR (P < 0.001). In the case without pathological fracture, only 5 out of 162 patients (3.1%) had recurrence, and in the case with pathological fracture, 45 out of 142 patients (31.7%) had recurrence. Patients who underwent radiotherapy postoperatively showed less LRR compared to whom did not (23/196, 11.7% vs. 27/108, 25%, P = 0.006). Greater SINS related with higher LRR (P < 0.001). However, preoperative radiotherapy was not related with LRR (Table 2).
Table 2.
Comparison of tumor related factors among groups
| Total (N = 304) | |||
|---|---|---|---|
| LR [-] (N = 254) | LR [+] (N = 50) | P-value | |
| Surgical method, N (%) | |||
| Decompression | 4 (13.3) | 26 (86.7) | < 0.001 |
| Fixation | 4 (50.0) | 4 (50.0) | |
| Decompression with fixation | 168 (94.9) | 9 (5.1) | |
| Corpectomy with fixation | 78 (87.6) | 11 (12.4) | |
| Number of spinal metastases, N (%) | |||
| 1 | 83 (95.4) | 4 (4.6) | < 0.001 |
| 2 | 150 (95.5) | 7 (4.5) | |
| 3 or more | 21 (35.0) | 39 (65.0) | |
| Number of non-spinal bone metastases, N (%) | |||
| None | 41 (93.2) | 3 (6.8) | < 0.001 |
| 1 | 118 (95.9) | 5 (4.1) | |
| 2 | 80 (97.6) | 2 (2.4) | |
| 3 or more | 15 (27.3) | 40 (72.7) | |
| Degree of spinal cord compression (Bilsky grade), N (%) | |||
| 0 | 7 (70.0) | 3 (30.0) | 0.664 |
| 1 | 28 (80.0) | 7 (20.0) | |
| 2 | 83 (83.8) | 16 (16.2) | |
| 3 | 136 (85.0) | 24 (15.0) | |
| Radiation therapy, N (%) | |||
| Preoperative RTx | 146 (57.5) | 22 (44.0) | 0.104 |
| Postoperative RTx | 173 (68.1) | 23 (46.0) | 0.006 |
| Pathologic fracture, N (%) | |||
| Yes | 97 (38.2) | 45 (90.0) | < 0.001 |
| SINS | 7.71 ± 1.95 | 11.42 ± 2.43 | < 0.001 |
Data are expressed as the mean value and standard deviation for continuous variables and numbers for categorical variables
LR, local recurrence; N, number; RTx, radiation therapy; SINS: spinal instability neoplastic score
Risk factors for local recurrence
In multivariate logistic regression analysis, surgical method, the number of spinal metastasis, the number of extraspinal bone metastasis, pathologic fracture, and origin of tumor (RCC or not) were independent risk factors for higher LRR (Table 3). Furthermore, smoking had a trend of increasing local recurrence (P = 0.057).
Table 3.
Multivariate logistic regression analyses
| β ± SE | Exp (β) | 95% CI | P-value | ||
|---|---|---|---|---|---|
| Surgical method (compared to decompression only) | |||||
| Decompression with fixation | -7.920 ± 2.572 | 0.000 | 0.000-0.056 | 0.002 | |
| Corpectomy with fixation | -4.918 ± 2.072 | 0.007 | 0.000-0.425 | 0.018 | |
| Level of spinal metastasis (compared to 1 level) | |||||
| 2 | -0.104 ± 1.321 | 0.901 | 0.068–11.996 | 0.937 | |
| 3 or more | 6.382 ± 1.952 | 591.084 | 12.875-27137.053 | 0.001 | |
| Number of non-spinal bone metastases (compared to no bone metastases except spine) | |||||
| 1 | -0.634 ± 2.151 | 0.530 | 0.008–35.943 | 0.768 | |
| 2 | -3.498 ± 2.680 | 0.030 | 0.000-5.781 | 0.192 | |
| 3 or more | 5.509 ± 2.502 | 246.994 | 1.832-33300.574 | 0.028 | |
| Pathologic fracture | 5.987 ± 1.990 | 398.303 | 8.061-19680.314 | 0.003 | |
| RCC | 3.843 ± 1.557 | 46.689 | 2.206-987.992 | 0.014 | |
| Smoking | 1.419 ± 0.745 | 4.133 | 0.960-17.798 | 0.057 | |
| Postoperative RTx | 0.876 | ||||
| SINS | 0.194 | ||||
Data are expressed as the mean value and standard deviation
SE, standard error; RCC: renal cell carcinoma; RTx, Radiation therapy; SINS: spinal instability neoplastic score
Postoperative neurologic status
Leg motor function was improved postoperatively in 44.5% (113/254) in LR[-] group, whereas only 12.0% (6/50) in LR[+] group (P < 0.001). Overall neurologic status (including sensory function) was improved postoperatively in 66.9% (170/254) in LR[-] group, whereas only 38.0% (19/50) in LR[+] group (P < 0.001).
Subgroup analysis
Subgroup analysis for patients with decompression/corpectomy with fixation is shown in Table 4. Among 266 patients who underwent decompression or corpectomy with fixation, symptomatic local recurrence developed in 20 patients (7.5%). In multivariate logistic regression analysis, specific origin of tumor (RCC), smoking, pathologic fracture, number of spinal metastases, and number of extraspinal bone metastases were independent risk factors for local recurrence.
Table 4.
Subgroup analysis in patients who underwent fixation (N = 266)
| LR [-] (N = 246) | LR [+] (N = 20) | Univariate P-value |
Multivariate P-value |
|
|---|---|---|---|---|
| RCC | 33 (13.4%) | 8 (40.0%) | 0.002 | 0.019 |
| HTN | 76 (30.9%) | 7 (35.0%) | 0.703 | NE |
| DM | 49 (19.9%) | 6 (30.0%) | 0.284 | NE |
| Liver disease | 37 (15.0%) | 7 (35.0%) | 0.021 | 0.188 |
| Smoking | 40 (16.3%) | 9 (45.0%) | 0.001 | 0.026 |
| Surgical method (decompression/corpectomy) | 168/78 | 9/11 | 0.034 | 0.081 |
| Pathologic fracture | 96 (39.0%) | 18 (90.0%) | < 0.001 | 0.034 |
| Number of spinal metastases (1/2/3 or more) | 80/146/20 | 2/2/16 | < 0.001 | 0.001 |
| Number of extraspinal bone metastases (0/1/2/3 or more) | 40/114/77/15 | 1/1/1/17 | < 0.001 | 0.003 |
| Preoperative RTx | 142 (57.7%) | 8 (40.0%) | 0.124 | NE |
| Postoperative RTx | 170 (69.1%) | 8 (40.0%) | 0.008 | 0.130 |
| SINS | 7.69 ± 1.95 | 10.55 ± 2.16 | < 0.001 | 0.230 |
Data are expressed as the mean value and standard deviation for continuous variables and numbers for categorical variables
LR, local recurrence; N, number; RCC, renal cell carcinoma; HTN, hypertension; DM, diabetes mellitus; RTx, Radiation therapy; SINS: spinal instability neoplastic score
Discussion
Local recurrence is the important problem among the many problems that can occur after surgery for MSCC. When local recurrence occurs in MSSC patients, not only does the patient’s survival rate decrease, but the quality of life significantly deteriorates [9–11, 17, 18, 30]. Therefore, research on local recurrence in MSCC patients can have a huge impact in predicting treatment results or prognosis.
In two previously published papers, 10 out of 91 patients relapsed, showing a recurrence rate of 11% [10], and in another paper, 32 out of 99 patients relapsed, resulting in a recurrence rate of 32.3% [11]. In our study, local recurrence occurred in approximately 16.5% of patients who received surgical treatment for MSCC. These figures were found to be almost similar to prior research results.
Among the surgical treatment methods, the local recurrence rate was found to be the highest in the group where only decompression was performed. This is because when only decompression was performed, procedures such as pediculectomy or wide resection involving the facet joint were not performed to maintain spinal stability although we aimed to remove as much tumor tissue as possible. Preservation of the pedicle or facet joint may lead to faster compression of the dural sac or nerve roots.
Furthermore, the relatively low incidence of local recurrence in cases where decompression and posterior fixation were performed together can be attributed to the surgeons’ approach. They removed as much surrounding tissue, including the pedicle and posterior longitudinal ligament, along with the visible tumor mass, with the fixation being performed afterward. This comprehensive resection likely contributed to a reduced local recurrence rate. Another reason why LRR was high in patients who only underwent decompression is the possibility of worsening the collapse of the pathologic fracture. If decompression is performed in a situation where there is a pathologic fracture, the vertebral body weakened by tumor invasion will eventually collapse over time, which is thought to progress to local recurrence.
In this study, local recurrence was found to be more frequent when pathologic fracture was present in patients who only underwent decompression. The results of this study show that pathologic fracture is a significant risk factor for local recurrence regardless of fixation. Probably pathologic fracture itself means advanced status of cancer, which lead to more possible local recurrence. However, fixation must be performed if pathologic fracture is present because the surgical outcomes of decompression without fixation was very poor.
In a prior paper, it was reported that melanoma was the only cancer independent of local recurrence, but this was not the case [11]. Various types of cancer were analyzed, and the following results were obtained. We found RCC is independent risk factor of symptomatic local recurrence (34.6%). Although the reason of higher LRR, the possible explanation is as follows. Because RCC is a hypervascular tumor, there is a possibility that the tumor removal was insufficient due to poor visibility due to bleeding. Due to the nature of the tumor, it forms a lot of blood vessels. Since RCC induce more blood vessel formation, the possibility of local recurrence is thought to be high. In addition, if the type of primary cancer is lung cancer, the patient’s survival rate and prognosis are very poor, so there is a possibility that the patient expired before local recurrence. In the case of RCC, the survival rate is relatively high compared to other cancers, and the progression rate is relatively faster than prostate or breast cancer, so the rate of local recurrence may be high.
The level of spinal metastasis and number of non-spinal bone metastasis are used as prognostic factors in the Tomita score and Tokuhashi score [7, 31]. High scores on these factors indicate rapid tumor progression and rapid worsening of clinical symptoms. And high level of spinal metastasis and a large number of extra-spinal bone metastasis ultimately mean that the tumor has progressed to a high degree, and since the degree of cancer metastasis has already progressed to a significant extent, there are inevitably fewer options for additional medical treatment. And for patients with high levels and large number compared to low levels, complete removal through surgery is not easy, and extensive surgery causes a lot of bleeding, which appears to result in a high local recurrence rate.
The correlation between preoperative radiation therapy and local recurrence rate was not significant, which may be due to the nature of MSCC patients, who often require surgical treatment as an emergency, so they often undergo surgery without preoperative radiation therapy. In the case of postoperative radiation therapy, there is evidence in the literature that adjuvant therapy reduces local recur and separation surgery with postoperative radiotherapy is known to be helpful [12, 17, 27, 30]. But in this study post-operative radiation therapy and LRR were not found to be related. Possible reasons are the insufficient therapeutic dose and the inappropriate timing of radiation therapy. Due to the risk of cord infarction, there is a high possibility that additional postoperative radiation therapy could not be performed or that minimal dose of radiation therapy was used. It is generally known that the appropriate time for post radiation therapy is to be performed within 2 weeks after surgery. However, it is believed that for the patients who participated in this study, there were many cases in which the procedure could not be performed within 2 weeks due to the patients’ condition and the schedule of collaborative treatment with other departments.
Although smoking was not identified as a risk factor in the overall cohort (Table 3, P = 0.057), it was significantly associated with local recurrence in patients who underwent fixation surgery (Table 4, P = 0.026). This suggests that while other factors, such as progressive collapse of the vertebral body, may be more influential in the general cohort, smoking becomes a clearer risk factor in patients who underwent fixation. However, there were no papers that studied smoking in local recurrence studies in MSCC patients although many studies have shown that smoking increases the recurrence rate in various primary cancers. It has been reported that recurrence increases in various cancers such as breast cancer, prostate cancer, etc. in smokers [30, 32–34]. Smoking is thought to affect the immune system, increasing the recurrence of the tumor itself, and further increasing local recurrence. Furthermore, recovery process following mass reduction may be delayed in smokers, leading to delayed union if fusion is attempted, which could subsequently affect clinical outcomes. Smoking has also been shown to impair healing and tissue regeneration, potentially influencing both the dynamics of recurrence and overall recovery [35].
Regarding surgical methods, no significant difference in local recurrence was observed between decompression with fixation and corpectomy (P = 0.081). This suggests that, at least in our cohort, more invasive procedures may not necessarily confer a substantial advantage in terms of reducing local recurrence. Further studies are ongoing to investigate whether regional factors (such as cervical, thoracic, or lumbar metastasis) or other factors may influence recurrence rates differently depending on the surgical method.
This study has several limitations. The surgery for cord compression is often performed under emergent conditions, and the heterogeneity of primary tumor types causing cord compression contributed to the variability in the dataset. This heterogeneity is an inherent issue in studies of metastatic spinal tumors. In addition, there is another inherent problem caused by asymmetry of patient cohorts. The asymmetric distribution between the LR [+] and LR [-] groups may have reduced the statistical power of our analysis, particularly given that only 2 patients in each of the breast and prostate cancer cohorts were classified in the LR [+] group. We selected surgical options primarily based on patient survival, as guided by the Tomita scoring system. However, recent studies have reported that several scoring systems, including the Tomita score, the revised Tokuhashi score, the classic Skeletal Oncology Research Group (SORG) algorithm, and the New England Spinal Metastasis Score (NESMS), are not effective in predicting patient survival. Consequently, it is inherently challenging to define surgical options for metastatic spinal cord compression [36]. To address this limitation, we included a wide range of parameters and a large study population. However, we could not obtain data on medical treatment options or the effects of previous treatments, which is challenging to acquire and analyze. Additionally, we could not fully obtain details on specific radiation therapy protocols. However, most patients underwent conventional radiotherapy rather than the more recently popularized stereotactic radiotherapy, which is now commonly used in combination with separation surgery [12, 17]. We were also unable to obtain complete past medical histories, which prevented us from accurately assessing patient comorbidities using the Charlson Comorbidity Index. However, we did not find any significant differences between the groups regarding hypertension, diabetes mellitus, liver disease, and pulmonary disease.
However, this study includes a significantly larger number of patients than previous studies, and contains information on patients with symptomatic local recurrence, which is clinically very important in relation to the survival rate of tumor patients. In addition, it faithfully contains information on the risk factors of symptomatic local recurrence patients, so it will be a paper that will play a very important role in making quick decisions about patient survival and determining prognosis. And although it is a heterogenous population, I believe it will be directly helpful in actual situations by most faithfully reflecting actual clinical situations.
Indeed, the results of this study on the risk factors for symptomatic local recurrence can be utilized in various clinical settings. First, in preoperative assessments: the identification of risk factors such as the extent of spinal metastasis, the presence of pathologic fractures, and specific tumor origins (e.g., renal cell carcinoma) can help stratify patients based on their risk of local recurrence, enabling more tailored discussions regarding surgical options and expected outcomes. Second, in postoperative monitoring: understanding the risks associated with factors such as smoking and the number of extraspinal bone metastases can inform postoperative surveillance protocols. Patients identified as high-risk could benefit from closer monitoring for signs of recurrence, allowing for timely intervention if necessary. Third, in the development of individualized treatment plans: our findings suggest that surgical strategies may need to be adjusted based on individual risk profiles. For instance, patients with a higher likelihood of recurrence following surgical treatment might be considered for more aggressive surgical approaches or adjunct therapies, such as postoperative radiation.
Conclusions
In conclusion, symptomatic local recurrence may be influenced by several factors, including the extent of spinal and extraspinal bone metastasis, pathologic fractures, surgical approach, and tumor origin (RCC). These factors should be carefully considered by surgeons when evaluating the risk of symptomatic local recurrence after surgery.
Acknowledgements
Not applicable.
Author contributions
JHC designed this study. JBK, SJB, and THK acquired data. JBK and JHC performed analysis and interpretation of data, JBK initially drafted the manuscript. SP, CJH and DHL supervised this study and underwent the critical revision. JHC obtained funding. All authors read and approved final manuscript.
Funding
This study was funded by Asan Medical Life Research Institute (No. 2022IF0026). The submitted manuscript does not contain information about any medical device(s)/drug(s).
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by our facility’s Institutional Review Board of our institution (IRB number: 2022 − 0684). Informed consent was waived due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bilsky MH, Lis E, Raizer J, Lee H, Boland P. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459–69. [PubMed] [Google Scholar]
- 2.Sutcliffe P, Connock M, Shyangdan D, Court R, Kandala NB, Clarke A. A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol Assess. 2013;17:1–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawton AJ, Lee KA, Cheville AL, Ferrone ML, Rades D, Balboni TA, Abrahm JL. Assessment and Management of patients with metastatic spinal cord Compression: a multidisciplinary review. J Clin Oncol. 2019;37:61–71. [DOI] [PubMed] [Google Scholar]
- 4.Shiue K, Sahgal A, Chow E, Lutz ST, Chang EL, Mayr NA, Wang JZ, Cavaliere R, Mendel E, Lo SS. Management of metastatic spinal cord compression. Expert Rev Anticancer Ther. 2010;10:697–708. [DOI] [PubMed] [Google Scholar]
- 5.Chaichana KL, Woodworth GF, Sciubba DM, McGirt MJ, Witham TJ, Bydon A, Wolinsky JP, Gokaslan Z. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62:683–92. discussion 683–692. [DOI] [PubMed] [Google Scholar]
- 6.Strickland BA, McCutcheon IE, Chakrabarti I, Rhines LD, Weinberg JS. The surgical treatment of metastatic spine tumors within the intramedullary compartment. J Neurosurgery: Spine. 2017;28:79–87. [DOI] [PubMed] [Google Scholar]
- 7.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001;26:298–306. [DOI] [PubMed] [Google Scholar]
- 8.Steinmetz MP, Mekhail A, Benzel EC. Management of metastatic tumors of the spine: strategies and operative indications. Neurosurg Focus. 2001;11:e2. [DOI] [PubMed] [Google Scholar]
- 9.Knöll P, Lenschow M, Lenz M, Neuschmelting V, von Spreckelsen N, Telentschak S, Olbrück S, Weber M, Rosenbrock J, Eysel P, Walter SG. Local recurrence and development of spinal cord syndrome during Follow-Up after Surgical Treatment of metastatic spine disease. Cancers (Basel) 2023, 15. [DOI] [PMC free article] [PubMed]
- 10.Igarashi T, Murakami H, Demura S, Kato S, Yoshioka K, Yokogawa N, Tsuchiya H. Risk factors for local recurrence after total en bloc spondylectomy for metastatic spinal tumors: a retrospective study. J Orthop Sci. 2018;23:459–63. [DOI] [PubMed] [Google Scholar]
- 11.Lau D, Than KD, La Marca F, Park P. Independent predictors for local recurrence following surgery for spinal metastasis. Acta Neurochir (Wien). 2014;156:277–82. [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Chang BS, Kim H, Hong SH, Chang SY. Separation surgery followed by stereotactic ablative radiotherapy for metastatic epidural spinal cord compression: a systematic review and meta-analysis for local progression rate. J Bone Oncol. 2022;36:100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaveri GR, Jain R, Mehta N, Garg B. An overview of decision making in the management of metastatic spinal tumors. Indian J Orthop. 2021;55:799–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong SH, Chang BS, Kim H, Kang DH, Chang SY. An updated review on the Treatment Strategy for Spinal Metastasis from the spine surgeon’s perspective. Asian Spine J. 2022;16:799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park S, Park JW, Park JH, Lee CS, Lee DH, Hwang CJ, Yang JJ, Cho JH. Factors affecting the prognosis of recovery of motor power and ambulatory function after surgery for metastatic epidural spinal cord compression. Neurosurg Focus. 2022;53:E11. [DOI] [PubMed] [Google Scholar]
- 16.Cho W, Chang UK. Neurological and survival outcomes after surgical management of subaxial cervical spine metastases. Spine (Phila Pa 1976). 2012;37:E969–977. [DOI] [PubMed] [Google Scholar]
- 17.Bishop AJ, Tao R, Rebueno NC, Christensen EN, Allen PK, Wang XA, Amini B, Tannir NM, Tatsui CE, Rhines LD, et al. Outcomes for Spine Stereotactic Body Radiation Therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys. 2015;92:1016–26. [DOI] [PubMed] [Google Scholar]
- 18.Bendfeldt GA, Chanbour H, Chen JW, Gangavarapu LS, LaBarge ME, Ahmed M, Jonzzon S, Roth SG, Chotai S, Luo LY et al. Does low-Grade Versus High-Grade Bilsky score influence local recurrence and overall survival in metastatic spine tumor surgery? Neurosurgery 2023, 93:1319–30. [DOI] [PubMed]
- 19.Chaichana KL, Pendleton C, Sciubba DM, Wolinsky JP, Gokaslan ZL. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. Clinical article. J Neurosurg Spine. 2009;11:56–63. [DOI] [PubMed] [Google Scholar]
- 20.Lau D, Leach MR, La Marca F, Park P. Independent predictors of survival and the impact of repeat surgery in patients undergoing surgical treatment of spinal metastasis. J Neurosurg Spine. 2012;17:565–76. [DOI] [PubMed] [Google Scholar]
- 21.Chong S, Shin SH, Yoo H, Lee SH, Kim KJ, Jahng TA, Gwak HS. Single-stage posterior decompression and stabilization for metastasis of the thoracic spine: prognostic factors for functional outcome and patients’ survival. Spine J. 2012;12:1083–92. [DOI] [PubMed] [Google Scholar]
- 22.Missenard G, Lapresle P, Cote D. Local control after surgical treatment of spinal metastatic disease. Eur Spine J. 1996;5:45–50. [DOI] [PubMed] [Google Scholar]
- 23.Hibberd CS, Quan GMY. Risk factors for pathological fracture and metastatic epidural spinal cord Compression in patients with spinal metastases. Orthopedics. 2018;41:e38–45. [DOI] [PubMed] [Google Scholar]
- 24.Chaichana KL, Pendleton C, Wolinsky JP, Gokaslan ZL, Sciubba DM. Vertebral compression fractures in patients presenting with metastatic epidural spinal cord compression. Neurosurgery. 2009;65:267–74. discussion 274 – 265. [DOI] [PubMed] [Google Scholar]
- 25.Kim GU, Park WT, Chang MC, Lee GW. Diagnostic Technology for Spine Pathology. Asian Spine J. 2022;16:764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilsky MH, Laufer I, Fourney DR, Groff M, Schmidt MH, Varga PP, Vrionis FD, Yamada Y, Gerszten PC, Kuklo TR. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13:324–8. [DOI] [PubMed] [Google Scholar]
- 27.Fisher CG, Schouten R, Versteeg AL, Boriani S, Varga PP, Rhines LD, Kawahara N, Fourney D, Weir L, Reynolds JJ, et al. Reliability of the spinal instability neoplastic score (SINS) among radiation oncologists: an assessment of instability secondary to spinal metastases. Radiat Oncol. 2014;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fourney DR, Frangou EM, Ryken TC, Dipaola CP, Shaffrey CI, Berven SH, Bilsky MH, Harrop JS, Fehlings MG, Boriani S, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the spine oncology study group. J Clin Oncol. 2011;29:3072–7. [DOI] [PubMed] [Google Scholar]
- 29.Park SJ, Park JS, Kang DH, Lee CS, Chang BS, Kim H, Chang SY, Lee J. Posterior aggressive debulking versus minimal decompression surgery in patients with metastatic spinal cord compression: propensity-score–matching analysis from a multicenter study cohort. J Neurosurg Spine. 2024;18:1–10. [DOI] [PubMed] [Google Scholar]
- 30.Foerster B, Pozo C, Abufaraj M, Mari A, Kimura S, D’Andrea D, John H, Shariat SF. Association of Smoking Status with recurrence, metastasis, and Mortality among patients with localized prostate Cancer undergoing prostatectomy or Radiotherapy: a systematic review and Meta-analysis. JAMA Oncol. 2018;4:953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tokuhashi Y, Uei H, Oshima M. Classification and scoring systems for metastatic spine tumors: a literature review. Spine Surg Relat Res. 2017;1:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon IJ, Lee DY, Suh MW, Han DH, Kim ST, Min YG, Lee CH, Rhee CS. Cigarette smoking increases risk of recurrence for sinonasal inverted papilloma. Am J Rhinol Allergy. 2010;24:325–9. [DOI] [PubMed] [Google Scholar]
- 33.Mantziari S, Allemann P, Winiker M, Demartines N, Schäfer M. Locoregional Tumor Extension and Preoperative Smoking are significant risk factors for early recurrence after Esophagectomy for Cancer. World J Surg. 2018;42:2209–17. [DOI] [PubMed] [Google Scholar]
- 34.Bishop JD, Killelea BK, Chagpar AB, Horowitz NR, Lannin DR. Smoking and breast cancer recurrence after breast conservation therapy. Int J Breast Cancer. 2014;2014:327081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shariel Sayardoust O, Omar O, Norderyd P, Thomsen. Implant-associated gene expression in the jaw bone of smokers and nonsmokers: a human study using quantitative qPCR. Clin Oral Implants Res. 2018;29:937–53. [DOI] [PubMed] [Google Scholar]
- 36.Truong VT, Al-Shakfa F, Roberge D, Masucci GL, Tran TPY, Dib R, Yuh SJ, Wang Z. Assessing the performance of prognostic scores in patients with spinal metastases from Lung Cancer undergoing non-surgical treatment. Asian Spine J. 2023;17:739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.