Abstract
Background
Remnant Cholesterol (RC) has emerged as a significant risk factor for cardiovascular disease. However, the factors influencing RC levels remain incompletely understood. This research investigates smoking—a major modifiable risk factor—to elucidate its impact on RC levels and examine the mediating role of inflammation in this relationship.
Methods
Using NHANES data from 1999 to 2018, this study analyzed the association between serum cotinine levels (a biomarker of smoking intensity) and RC in 8,829 participants aged 20 years and older. Through complex sampling design and adjustment for multiple covariables, we examined both linear and nonlinear relationships using linear regression models, restricted cubic splines (RCS), and subgroup analyses. Additionally, mediation analyses evaluated the role of inflammatory markers—neutrophils (NEU), monocytes (MON), lymphocytes (LYM), and platelets (PLT)—in this association.
Results
The high cotinine exposure group demonstrated significantly elevated RC levels (β = 2.256, 95% CI: 1.401–3.112, p < 0.001) compared to the no/minimal exposure group. This positive association was particularly pronounced in females (p for interaction < 0.05). Restricted cubic spline analysis demonstrated a nonlinear, N-shaped relationship (p for nonlinearity < 0.05), with RC levels reaching their peak at cotinine concentrations of approximately 172 ng/mL. In the mediation analysis, inflammatory markers showed significant mediating effects: NEU (28%), LYM (14.1%), PLT (9.5%), and MON (6.9%) of the total effect.
Conclusion
A significant positive association exists between cotinine and RC levels, moderated by sex. Inflammatory markers, particularly NEU, partially mediate this association.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02372-x.
Keywords: Remnant cholesterol (RC), Cotinine; cross-sectional study, NHANES, Biomarker, Cardiovascular disease
Highlights
• Serum cotinine levels are positively associated with remnant cholesterol.
• Cotinine-remnant cholesterol association is pronounced in females.
• An n-shaped relationship is observed between cotinine and remnant cholesterol, with remnant cholesterol peaking at cotinine concentrations of approximately 172 ng/mL.
• Inflammatory markers mediate the cotinine-remnant cholesterol association; neutrophils show the strongest effect (28% of the total effect).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02372-x.
Introduction
Remnant cholesterol (RC), defined as the cholesterol content within triglyceride-rich lipoprotein (TRL) remnants, primarily originates from chylomicrons and very low-density lipoproteins (VLDL) following lipoprotein lipase hydrolysis [1, 2]. Recent evidence demonstrates that RC exhibits comparable or greater precision in cardiovascular event prediction compared to conventional markers such as LDL-C or HDL-C [3, 4]. RC shares pathogenic mechanisms with LDL-C, including the ability to penetrate the arterial endothelium and bind to proteoglycans, thereby promoting atherosclerosis through cholesterol accumulation, plaque formation, and inflammatory processes [5, 6]. Notably, elevated RC levels may increase mortality risk and recurrent event rates even among cardiovascular patients receiving lipid-lowering therapy [7, 8]. Although RC plays a pivotal role in cardiovascular disease prediction and prognosis, no clinical consensus exists regarding the management of high RC (HRC). Consequently, identifying modifiable factors that influence elevated RC levels has emerged as a critical research priority.
Among modifiable factors, smoking stands as one of the most preventable major risk factors for cardiovascular disease and mortality [9, 10]. Cigarette smoke emits numerous harmful compounds, including nicotine, polycyclic aromatic hydrocarbons, and heavy metals [11–13].
Cotinine, the principal metabolite of nicotine, serves as a highly specific and sensitive biomarker for quantifying recent tobacco exposure [12, 14]. Substantial epidemiological and experimental evidence has established that smoking disrupts lipid metabolism through multiple pathways, thereby increasing cardiovascular disease risk and associated mortality [15–17]. Specifically, smoking elevates total cholesterol (TC), triglyceride (TG), and LDL-C levels while reducing cardioprotective HDL-C concentrations [18, 19]. However, current research has predominantly examined smoking's effects on conventional lipid markers, with a limited investigation into its impact on RC. Given the crucial role of RC in cardiovascular disease pathogenesis and smoking's significance as a major modifiable risk factor, elucidating the relationship between smoking and RC could reveal novel approaches for HRC prevention.
Materials and methods
Data source and study population
This study utilized data from the National Health and Nutrition Examination Survey (NHANES) spanning 1999–2018, encompassing 8,829 participants aged 20 and above. NHANES, a comprehensive, multi-stage, probability-sampled national health survey led by the National Center for Health Statistics (NCHS), aims to assess the health and nutritional status of the non-institutionalized U.S. population. The participant selection process is illustrated in Fig. 1. Exclusion criteria comprised: 1) missing data for Cotinine and variables required to calculate RC; 2) subjects lacking data in other covariable modules; and 3) participants with zero weighting.
Fig. 1.
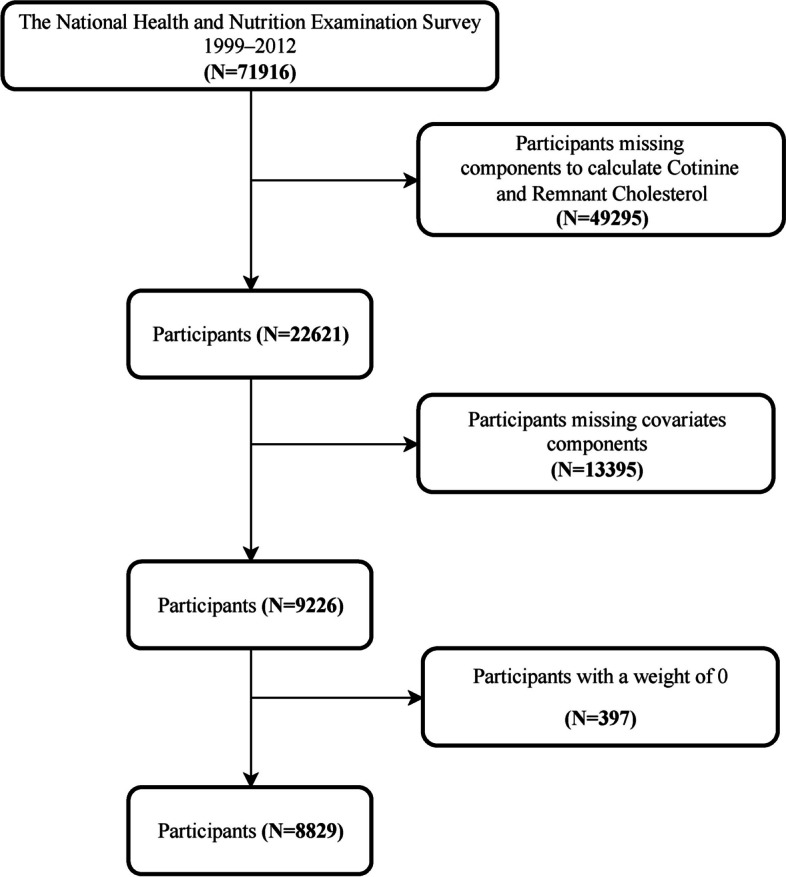
Flowchart of the participants’ selection
Measures
Primary outcome
Remnant cholesterol (RC) was the primary outcome of this study. Participants underwent an 8-h overnight fast before blood collection. TC was measured enzymatically, while HDL-C quantification involved either heparin-manganese precipitation or immunoassay techniques. The Friedewald equation was used to calculate LDL-C. A validated formula determined RC levels. All procedures adhered to the NHANES Laboratory Manual, guaranteeing standardization. This methodological approach strengthened the investigation into smoking's effects on lipid profiles.
Primary exposure
Serum cotinine, a main metabolite of nicotine, was measured as the primary exposure variable in this study, serving as a sensitive and specific biomarker to reflect smoking status. Serum cotinine was determined by isotope dilution combined with APCI-MS/MS and HPLC. Methyl-D3-cotinine internal standard was added to the alkaline serum sample for SLE extraction. After C18 HPLC separation, the extract was monitored by APCI-MS/MS, and the m/z 80 daughter ion of the m/z 177 quasi-molecular ion was used to identify cotinine [20]. Detailed processing steps can be found in the Laboratory/Medical Technology Procedure Manual on the NHANES official website. According to previous studies and literature, serum cotinine levels are divided into three groups: No/Minimal exposure (< 0. 05 ng/mL), Low exposure (0. 05–2. 99 ng/mL), High exposure (≥ 3. 00 ng/mL) [21].
Definitions of covariables
Several covariables were included in this study. These were organized into the following categories:
- Demographic characteristics
- Age (years)
- Sex (male/female)
- Race (non-Hispanic white, non-Hispanic black, other/multiracial)
- Socioeconomic factors
- Educational attainment (high school or below, college graduate or above)
- Income level (poor, not poor)
- Lifestyle factors
- Smoking status (never, former, now)
- Alcohol use (drinkers, non-drinkers)
- Physical activity (MET × min/week)
- Health Status indicators
- Body Mass Index (BMI, kg/m2)
- Cancer (yes/no)
- Heart disease (yes/no)
- Diabetes (yes/no)
The specific measurement methods for the above variables are as follows: The history of cancer, heart disease, and diabetes in participants was determined through individual interviews using questionnaires. Income level was assessed using the ratio of family income to poverty (PIR), where PIR < 1 was defined as poor and PIR ≥ 1 was not poor. Smoking status was assessed based on two questions: " Smoked at least 100 cigarettes in life?" and "Do you now smoke cigarettes? " Based on the responses, participants were categorized as never, former, and now. Alcohol use is determined by the response to "Had at least 12 alcoholic drinks/lifetime?", with "yes" classified as a drinker and "no" as a non-drinker. Physical activity levels were assessed using the World Health Organization Global Physical Activity Questionnaire (GPAQ), and weekly activity levels were converted to metabolic equivalent minutes (MET) according to WHO guidelines.
Statistical analysis
The analyses considered NHANES' complex multistage sampling design and incorporated sample weights, stratification, and clustering. Participants were stratified by serum cotinine concentrations. Continuous variables were reported as mean [95% CIs], while categorical variables as percentages [95% CIs]. Wilcoxon rank-sum tests and Rao & Scott chi-square tests assessed group differences for continuous and categorical variables, respectively. We employed weighted linear regression with four progressively adjusted models to analyze the cotinine-RC relationship. Model 1 was unadjusted; Model 2 adjusted for primary NHANES sampling factors (age, sex, and race); Model 3 further adjusted for BMI, educational attainment, physical activity, cancer, and diabetes, while Model 4 included adjustments for all covariable. The covariables for Model 3 were selected based on a greater than 10% change in the regression coefficient of cotinine levels when added to or removed from the unadjusted and fully adjusted models. Additionally, cotinine was categorized into quintiles, and trend tests evaluated the consistency of cotinine-RC associations across increasing quintiles. Restricted cubic spline analysis assessed potential nonlinear relationships between cotinine and RC after covariable adjustment, with nonlinearity evaluated by likelihood ratio tests. Subgroup analysis was conducted to investigate factors that may influence the association between cotinine and RC. Mediation analysis was conducted using a “mediation” package of R software to assess inflammatory markers as potential mediators of the effect between cotinine and RC. In all analyses, statistical significance was set at P < 0.05 (two-tailed). All analyses were conducted using R version 4.3.1.
Results
Characteristics of participants
Our study analyzed 8,829 individuals, categorized based on Cotinine exposure (Table 1). Cotinine exposure levels are strongly associated with smoking status. In the high-exposure group, 76% of the individuals were current smokers, while only 11% were never smokers. In contrast, in the no/minimal exposure group, only 0.4% of the individuals were current smokers, and 70% were never-smokers. Participants with higher Cotinine exposure exhibited distinct characteristics: they were generally younger, had lower BMI, were more likely to be male, consumed alcohol more frequently, and had lower levels of education and income. In addition, these participants have a relatively low prevalence of cancer and diabetes, but their level of physical activity is high.
Table 1.
Characteristics of participants
| Characteristic | Cotinine (N = 8829) | |||
|---|---|---|---|---|
| No/Minimal exposure, N = 4272ab | Low exposure, N = 2246ab | High exposure, N = 2311ab | P Valuec | |
| Age, years | 48 [47, 49] | 44 [43, 45] | 41 [40, 42] | < 0.001 |
| BMI (kg/m2) | 28.0 [28] | 28.8 [29] | 27.5 [27, 28] | < 0.001 |
| Sex, % | < 0.001 | |||
| Male | 44 [42, 45] | 55 [52, 57] | 63 [61, 66] | |
| Female | 56 [55, 58] | 45 [43, 48] | 37 [34, 39] | |
| Race, % | < 0.001 | |||
| Other/multiracial | 17 [15, 19] | 16 [14, 19] | 13 [11, 16] | |
| Non-Hispanic black | 6.3 [5.2, 7.5] | 13 [11, 15] | 12 [10, 14] | |
| Non-Hispanic white | 76 [74, 79] | 71 [68, 74] | 75 [72, 78] | |
| Alcohol use, % | < 0.001 | |||
| Non drinker | 13 [11, 16] | 9.3 [7.8, 11] | 2.2 [1.6, 3.0] | |
| Drinker | 87 [84, 89] | 91 [89, 92] | 98 [97, 98] | |
| Smoke status, % | < 0.001 | |||
| Never | 70 [68, 72] | 64 [61, 66] | 11 [10, 13] | |
| Former | 29 [27, 32] | 33 [31, 36] | 12 [11, 14] | |
| Now | 0.4 [0.21, 0.58] | 2.9 [2.2, 3.9] | 76 [74, 79] | |
| Education attainment, % | < 0.001 | |||
| High school or below | 26 [24, 29] | 40 [37, 43] | 55 [52, 58] | |
| College graduate or above | 74 [71, 76] | 60 [57, 63] | 45 [42, 48] | |
| Income level, % | < 0.001 | |||
| Poor | 6.7 [5.6, 8.1] | 12 [9.9, 13] | 18 [16, 20] | |
| Not poor | 93 [92, 94] | 88 [87, 90] | 82 [80, 84] | |
| Cancer, % | 9.6 [8.6, 11] | 6.3 [5.2, 7.6] | 5.3 [4.5, 6.1] | < 0.001 |
| Heart disease, % | 6.6 [5.9, 7.4] | 7.5 [6.3, 9.0] | 6.8 [5.7, 8.1] | 0.4 |
| Diabetes, % | 7.3 [6.4, 8.3] | 6.1 [5.1, 7.3] | 5.5 [4.6, 6.6] | 0.032 |
| Physical activity (MET × min/week) | 2,299 [2,067, 2,531] | 2,570 [2,288, 2,851] | 3,311 [2,920, 3,702] | 0.002 |
aMean; %
bCI Confidence Interval
cWilcoxon rank-sum test for complex survey samples; chi-squared test with Rao & Scott's second-order correction
Association between cotinine and RC
Weighted multivariable linear regression analysis revealed a significant positive association between cotinine and RC in all models (p < 0.05) (Table 2). The fully adjusted Model 4 revealed that participants in the high cotinine exposure group exhibited significantly elevated RC levels (β = 2.256, 95% CI: 1.401–3.112, p < 0.001) compared to the no/minimal exposure group. In contrast, the low exposure group showed no statistically significant difference in RC levels (β = 0.3071, 95% CI: -0.5821–1.196, p = 0.5) compared to the no/minimal exposure group.
Table 2.
Association between Cotinine and RC
| Characteristica | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CIb | p-value | Beta | 95% CIb | p-value | Beta | 95% CIb | p-value | Beta | 95% CIb | p-value | |
| Cotinine-strata | ||||||||||||
| No/Minimal exposure | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | ||||
| Low exposure | 0.3411 | -0.5853, 1.267 | 0.5 | 0.9703 | 0.0332, 1.907 | 0.043 | 0.3185 | -0.5748, 1.212 | 0.5 | 0.3071 | -0.5821, 1.196 | 0.5 |
| High exposure | 1.629 | 0.8298, 2.427 | < 0.001 | 2.425 | 1.566, 3.283 | < 0.001 | 2.291 | 1.439, 3.143 | < 0.001 | 2.256 | 1.401, 3.112 | < 0.001 |
aModels:
Model 1: Not adjusted
Model 2: Adjusted Age, Sex, Race
Model 3: Adjusted Age, Sex, BMI, Race, Education attainment, MET, Cancer, Diabetes
Model 4: Adjusted Age, Sex, BMI, Race, Income level, Education attainment, Alcohol use, MET, Cancer, Heart disease, Diabetes
bCI Confidence Interval
Trend tests of association between cotinine and RC
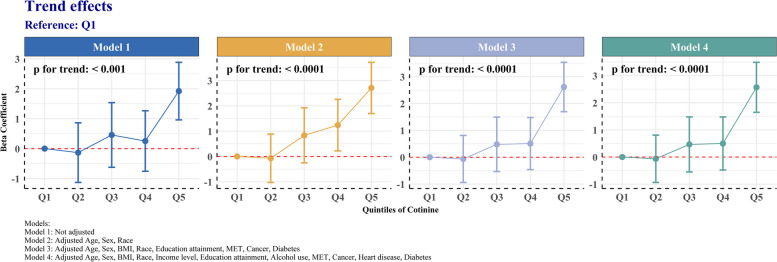
Participants were stratified into quintiles based on serum cotinine concentrations for trend analysis (Fig. 2). Although there are some fluctuations between quintiles, all four models demonstrate a clear overall upward trend in RC concentrations as cotinine levels increase across quintiles (Q1 to Q5). This positive dose–response relationship is statistically significant in all models (p for trend < 0.001).
Fig. 2.
Trend tests of association between cotinine and RC
RCS analysis of the association between cotinine and RC
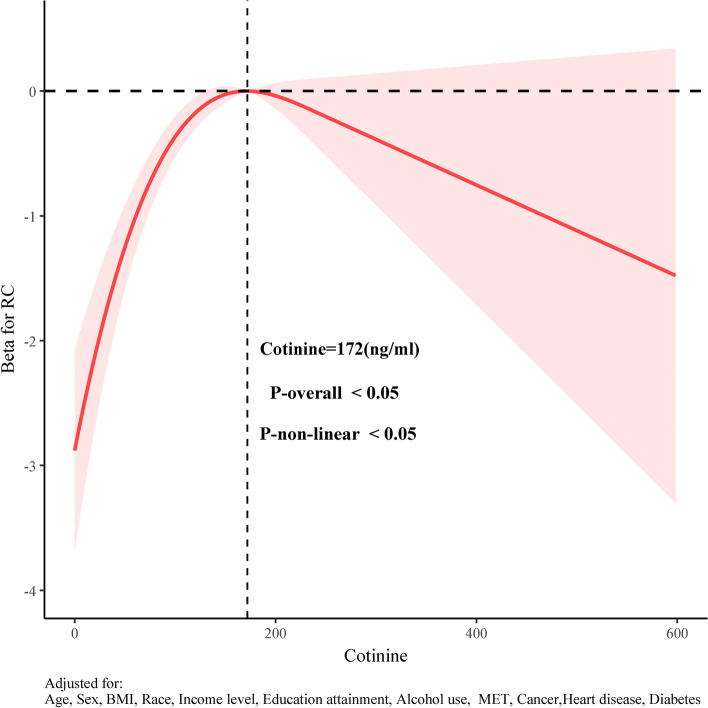
We employed weighted RCS analysis to examine the non-linear relationship between cotinine and RC, adjusting for all covariable. Figure 3 reveals a significant non-linear "n-shaped" association between cotinine and RC (P-Nonlinear < 0.05). The RC effects initially increased with rising cotinine levels, reached a peak, and then decreased. The inflection point was identified at a cotinine level of 172 ng/ml. Further stratified RCS analyses demonstrated consistent n-shaped associations between cotinine and RC across all subgroups (Supplementary file S2).
Fig. 3.
RCS analysis of the association between cotinine and RC
Subgroup analysis of the relationship between cotinine and RC
We conducted a stratified analysis of the relationship between cotinine and RC across different subgroups to investigate potential variations in this association among populations with diverse characteristics (Table 3). A statistically significant difference was observed in the subgroup of sex (p for interaction < 0.05). Specifically, in the highest quintile group of cotinine, the RC level for the female group (β = 3.22, 95% CI: 1.79–4.65) was notably higher than that of the male group (β = 2.22, 95% CI: 0.73–3.71), indicating a more pronounced association between cotinine and RC in females. This association did not show significant differences across other subgroups such as age, race, income level, alcohol use, educational attainment, cancer, heart disease, and diabetes (p for interaction > 0.05). This indicates that the association between cotinine and RC is relatively stable within these subgroups.
Table 3.
Subgroup analysis of the relationship between cotinine and RC
| Characteristicb | Q1 | Q2 | Q3 | Q4 | Q5 | p-inta |
|---|---|---|---|---|---|---|
| Age strata | 0.76 | |||||
| < 60 years | ref | -0.35(-1.41, 0.72) | 0(-1.20, 1.21) | 0.09(-1.03, 1.21) | 2.05(1.01, 3.09) | |
| ≥ 60 years | ref | 0.34(-1.03, 1.70) | 1.5(-0.52, 3.51) | 1.44(-0.55, 3.43) | 2.75(0.97, 4.53) | |
| Sex | 0.02 | |||||
| Male | ref | -0.05(-1.37, 1.28) | 0.8(-0.77, 2.38) | 1.15(-0.41, 2.71) | 2.22(0.73, 3.71) | |
| Female | ref | -0.1(-1.32, 1.12) | -0.02(-1.22, 1.18) | -0.49(-1.73, 0.75) | 3.22(1.79, 4.65) | |
| Race | 0.73 | |||||
| Other/multiracial | ref | 0.11(-1.77, 1.98) | 0.25(-1.74, 2.25) | 0.7(-1.33, 2.72) | 1.08(-1.36, 3.52) | |
| Non-Hispanic black | ref | -0.17(-2.56, 2.21) | 0.12(-2.42, 2.65) | -0.47(-2.88, 1.93) | 1.53(-0.90, 3.96) | |
| Non-Hispanic white | ref | -0.18(-1.19, 0.84) | 0.52(-0.73, 1.78) | 0.52(-0.73, 1.77) | 2.86(1.76, 3.96) | |
| Alcohol use | 0.55 | |||||
| Non drinker | ref | 0.69(-1.11, 2.50) | 0.05(-1.68, 1.78) | 0.91(-1.67, 3.49) | 4.32(0.74, 7.90) | |
| Drinker | ref | -0.23(-1.13, 0.67) | 0.5(-0.66, 1.65) | 0.37(-0.69, 1.43) | 2.39(1.39, 3.39) | |
| Income level | 0.58 | |||||
| Poor | ref | 1.01(-2.59, 4.62) | -0.88(-4.47, 2.71) | 0.98(-2.73, 4.70) | 2.78(-1.01, 6.57) | |
| Not poor | ref | -0.17(-1.03, 0.70) | 0.6(-0.51, 1.70) | 0.45(-0.63, 1.53) | 2.59(1.62, 3.55) | |
| Education attainment | 0.05 | |||||
| High school or below | ref | -0.13(-1.87, 1.62) | -0.02(-1.84, 1.80) | -1.02(-2.82, 0.79) | 1.71(-0.03, 3.46) | |
| College graduate or above | ref | -0.17(-1.14, 0.81) | 0.49(-0.64, 1.62) | 1.2(-0.13, 2.52) | 2.98(1.81, 4.16) | |
| Cancer | 0.84 | |||||
| No | ref | 0.03(-0.86, 0.92) | 0.44(-0.66, 1.54) | 0.46(-0.56, 1.49) | 2.6(1.56, 3.64) | |
| Yes | ref | -0.72(-4.19, 2.74) | 0.32(-3.41, 4.04) | 0.69(-3.18, 4.57) | 1.06(-3.24, 5.36) | |
| Heart disease | 0.25 | |||||
| No | ref | -0.1(-1.01, 0.81) | 0.61(-0.42, 1.64) | 0.62(-0.36, 1.61) | 2.49(1.58, 3.40) | |
| Yes | ref | 0.01(-3.30, 3.32) | -1.03(-4.74, 2.67) | -1.48(-5.41, 2.45) | 2.54(-1.54, 6.63) | |
| Diabetes | 0.79 | |||||
| No | ref | -0.07(-0.95, 0.82) | 0.57(-0.49, 1.63) | 0.47(-0.48, 1.42) | 2.72(1.79, 3.64) | |
| Yes | ref | -0.38(-4.71, 3.95) | -1.67(-7.90, 4.56) | 1.8(-2.53, 6.14) | 0.35(-3.64, 4.34) |
ap-int: p for interaction
bAdjusted for: Age, Sex, BMI, Race, Income level, Education attainment, Alcohol use, MET, Cancer, Heart disease, Diabetes
The mediating role of inflammatory biomarkers in the association between cotinine and RC
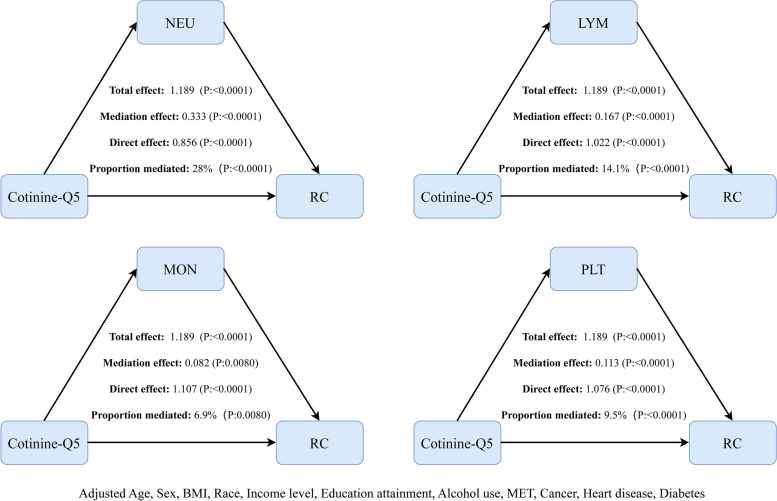
The mediation analysis results (Fig. 4 and Supplement Table S1) demonstrated a significant total effect between cotinine and RC (total effect = 1.189, p < 0.0001). All four inflammatory markers partially mediated this association (p < 0.01). Among them, NEU exhibited the strongest mediation effect, accounting for 28% of the total effect (mediation effect = 0.333, p < 0.0001), followed by LYM at 14.1% (mediation effect = 0.167, p < 0.0001). PLT and MON demonstrated smaller mediating effects, contributing 9.5% (mediation effect = 0.113, p < 0.0001) and 6.9% (mediation effect = 0.082, p = 0.0080) to the total effect, respectively.
Fig. 4.
The mediating role of inflammatory biomarkers in the association between cotinine and RC
Discussion
This study provides the first systematic investigation of the relationship between smoking intensity, measured by serum cotinine levels, and RC. Our findings reveal that blood cell inflammatory markers mediate smoking-induced elevations in RC levels. The positive association between cotinine and RC was particularly pronounced in females, demonstrating significant effect modification. Notably, we identified a nonlinear, N-shaped relationship between cotinine and RC levels, with a critical inflection point at 172 ng/mL. Furthermore, inflammatory cells—NEU, LYM, MON, and PLT—significantly mediated this association, suggesting a mechanistic link between smoking and RC metabolism.
Serum cotinine levels demonstrate a dose-dependent relationship with cardiovascular disease risk [22]. Both cross-sectional and prospective studies have established that elevated cotinine levels are associated with increased cardiovascular events and their severity [16, 23]. Smoking-induced alterations in lipid profiles accelerate atherosclerosis development [15, 24], with elevated RC particularly associated with arterial disease [25] and cardiovascular mortality [26]. Our findings identify smoking as a significant modifiable factor affecting RC levels, suggesting that smoking cessation or reduction may serve as a therapeutic target for RC management and cardiovascular risk reduction. The observed positive association between cotinine and RC levels provides additional evidence linking smoking to adverse cardiovascular outcomes. Mechanistically, cotinine—nicotine's primary metabolite—may influence RC levels through multiple pathways: enhanced oxidative stress [27], inflammatory activation [28, 29], endothelial dysfunction [30], musculoskeletal degeneration [31, 32], and dysregulation of hepatic lipid metabolism [33, 34].
Female participants exhibited heightened sensitivity to cotinine level fluctuations [35]. This enhanced sensitivity in females may be attributed to estrogen-mediated acceleration of nicotine metabolism, as reported in previous research [36]. Estrogen also directly modulates lipid metabolism by elevating HDL cholesterol while reducing LDL cholesterol, potentially explaining our observed sex-specific differences [37].
The significant positive associations between cotinine levels and inflammatory biomarkers (LYM, NEU, MON, and PLT) are consistent with findings from a Korean study of adult smokers [38]. Their research demonstrated elevated white blood cell counts in smokers compared to non-smokers, with counts increasing proportionally to cotinine concentrations [38]. Chronic tobacco smoke exposure induces low-grade systemic inflammation and promotes widespread vascular damage through multiple pathways [27]. While its cardiovascular and respiratory effects are well-reported, the impact of systemic inflammation on hepatic function warrants particular attention. Cotinine exerts hepatic effects primarily through p-38 MAPK/AP-1 and ROS/STAT-3 signaling pathway activation, triggering endothelial damage and upregulating pro-inflammatory IL-6 expression. Smokers exhibit markedly elevated IL-6 levels compared to non-smokers [28], with increased IL-6 serving as a potential harbinger of hepatocellular injury. Additionally, smoking-induced carboxyhemoglobin elevation reduces blood oxygen-carrying capacity, stimulating erythropoietin secretion. This leads to increased red blood cell mass, concurrent erythrocyte destruction, and disrupted iron metabolism, culminating in hepatocyte iron accumulation, oxidative stress, and liver damage. The resultant hepatocellular dysfunction directly impairs lipoprotein metabolism, manifesting as blood lipid abnormalities [39]. While current evidence preliminarily supports the hypothesis that cotinine influences RC levels via lipid metabolism pathway disruption, comprehensive investigation is needed to fully elucidate the relationship between residual cholesterol and cotinine levels.
These findings suggest that early intervention strategies in high-risk populations may help control RC levels [40, 41]. Our study reveals a positive association between cotinine and RC levels, providing potentially valuable insights for clinical application. Cotinine could potentially serve as a reference biomarker for RC risk assessment, contributing to the prevention and management of RC elevation. Given the heightened sensitivity to cotinine effects observed in female subjects, the implementation of sex-specific strategies in RC management warrants consideration. Furthermore, the observed relationship with inflammatory markers suggests that blood cell count monitoring could offer complementary information, especially in heavy smokers. While these findings provide promising initial evidence for developing targeted strategies in smoking-related RC management, further research would be valuable to fully understand their clinical applications.
Strengths and limitations
A primary strength of this study lies in its utilization of a large cohort of non-hospitalized participants, significantly enhancing both statistical power and the generalizability of findings. Nevertheless, several limitations warrant consideration. The cross-sectional nature of the NHANES data precludes establishing causality between smoking and RC levels, underscoring the importance of future prospective studies. While we adjusted for multiple confounding variables, the reliance on self-reported data introduces potential recall bias, and unmeasured confounders may persist. Although serum cotinine provides an objective measure of smoking status, it may not fully capture long-term smoking patterns. Furthermore, the absence of longitudinal follow-up data prevents the assessment of smoking's sustained effects on RC levels.
Conclusions
A significant positive association exists between cotinine and RC levels, moderated by sex. Inflammatory markers, particularly NEU, partially mediate this association.
Supplementary Information
Abbreviations
- RCS
Restricted Cubic Spline
- RC
Remnant cholesterol
- TC
Total cholesterol
- TG
Triglyceride
- VLDL
Very low-density lipoproteins
- LDL-C
Low-density lipoproteins-cholesterol
- HDL-C
High-density lipoproteins-cholesterol
- CIs / CI
Confidence Intervals
- PIR
Ratio of family income to poverty
- NCHS
National Center for Health Statistics
- NEU
Neutrophils
- MON
Monocytes
- LYM
Lymphocytes
- PLT
Platelets
Authors’ contributions
T.L. and Z.S. are co-first authors, jointly leading research conceptualization, statistical analysis, result interpretation, data visualization, and initial manuscript drafting. G.T., J.S., and K.H. ensured data integrity and analysis accuracy, with K.H. conducting a comprehensive study review. All authors contributed to manuscript revisions and approved the final version.
Funding
This study has no funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This research complied with the Helsinki Declaration and used NHANES data which has undergone ethical review by NCHS. As a secondary analysis of this approved dataset following STROBE guidelines, no additional ethical approval was required.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tianjie Lai and Zhihao Su contributed equally to this work.
References
- 1.Stürzebecher PE, Katzmann JL, Laufs U. What is “remnant cholesterol”? Eur Heart J. 2023;44:1446–8. 10.1093/eurheartj/ehac783. [DOI] [PubMed] [Google Scholar]
- 2.Xie YY, et al. Association between remnant cholesterol and verbal learning and memory function in the elderly in the US. Lipids Health Dis. 2022;21:120. 10.1186/s12944-022-01729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnett JR, Hooper AJ, Hegele RA. Remnant cholesterol and atherosclerotic cardiovascular disease risk. J Am Coll Cardiol. 2020;76:2736–9. 10.1016/j.jacc.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Castañer O, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol. 2020;76:2712–24. 10.1016/j.jacc.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40:537–57. 10.1210/er.2018-00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quispe R, et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J. 2021;42:4324–32. 10.1093/eurheartj/ehab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, et al. Effect of dual residual risk of cholesterol and inflammation on all-cause mortality in patients with cardiovascular disease. Cardiovasc Diabetol. 2023;22:96. 10.1186/s12933-023-01826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadström BN, Pedersen KM, Wulff AB, Nordestgaard BG. Elevated remnant cholesterol, plasma triglycerides, and cardiovascular and non-cardiovascular mortality. Eur Heart J. 2023;44:1432–45. 10.1093/eurheartj/ehac822. [DOI] [PubMed] [Google Scholar]
- 9.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43:1731–7. 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Delgado GE, Siekmeier R, März W, Kleber ME. Adiponectin and Mortality in Smokers and Non-Smokers of the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Adv Exp Med Biol. 2016;934:1–8. 10.1007/5584_2016_14. [DOI] [PubMed] [Google Scholar]
- 11.Jeong SM, et al. Smoking behavior change and risk of cardiovascular disease incidence and mortality in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2023;22:193. 10.1186/s12933-023-01930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou W, et al. Associations between smoke exposure and osteoporosis or osteopenia in a US NHANES population of elderly individuals. Front Endocrinol. 2023;14:1074574. 10.3389/fendo.2023.1074574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinucci L, et al. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H(2)O(2) and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: Implication for smokers-related osteoporosis. Free Radical Biol Med. 2018;117:6–17. 10.1016/j.freeradbiomed.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Silva AI, et al. Urinary cotinine assessment of maternal smoking and environmental tobacco smoke exposure status and its associations with perinatal outcomes: a cross-sectional birth study. Environ Res. 2022;203:111827. 10.1016/j.envres.2021.111827. [DOI] [PubMed] [Google Scholar]
- 15.Jee SH, Suh I, Kim IS, Appel LJ. Smoking and atherosclerotic cardiovascular disease in men with low levels of serum cholesterol: the Korea Medical Insurance Corporation Study. JAMA. 1999;282:2149–55. 10.1001/jama.282.22.2149. [DOI] [PubMed] [Google Scholar]
- 16.Garland C, Barrett-Connor E, Suarez L, Criqui MH, Wingard DL. Effects of passive smoking on ischemic heart disease mortality of nonsmokers. A prospective study. Am J Epidemiol. 1985;121:645–50. 10.1093/aje/121.5.645. [DOI] [PubMed] [Google Scholar]
- 17.Vazquez-Benitez G, et al. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care. 2015;38:905–12. 10.2337/dc14-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cade J, Margetts B. Cigarette smoking and serum lipid and lipoprotein concentrations. BMJ (Clinical research ed). 1989;298:1312. 10.1136/bmj.298.6683.1312-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen SS, Hatsukami D, Gorsline J. Cholesterol changes in smoking cessation using the transdermal nicotine system Transdermal Nicotine Study Group. Prev Med. 1994;23:190–6. 10.1006/pmed.1994.1026. [DOI] [PubMed] [Google Scholar]
- 20.She D, Jiang S & Yuan S. Association between serum cotinine and hepatic steatosis and liver fibrosis in adolescent: a population-based study in the United States. Scientific Reports. 2024;14. 10.1038/s41598-024-61771-3 [DOI] [PMC free article] [PubMed]
- 21.Zhu N, Zhu J, Lin S, Yu H & Cao C. Correlation analysis between smoke exposure and serum neurofilament light chain in adults: a cross-sectional study. BMC Public Health. 2024;24, 10.1186/s12889-024-17811-8 [DOI] [PMC free article] [PubMed]
- 22.Nikam KS, Wingkar KC, Joshi RK, Kallur RK. Correlation between cotinine urinary levels & cardiovascular autonomic function tests among smokeless tobacco chewers: A cross-sectional study. Indian J Med Res. 2020;152:633–7. 10.4103/ijmr.IJMR_1815_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukhram SD, et al. Serum cotinine as a predictor of lipid-related indices in Turkish immigrants with type 2 diabetes: a clinic-based cross-sectional study. Front Med. 2023;10:1011045. 10.3389/fmed.2023.1011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadström BN, Pedersen KM, Wulff AB, Nordestgaard BG. Elevated remnant cholesterol and atherosclerotic cardiovascular disease in diabetes: a population-based prospective cohort study. Diabetologia. 2023;66:2238–49. 10.1007/s00125-023-06016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doi T, Langsted A, Nordestgaard BG. Elevated remnant cholesterol reclassifies risk of ischemic heart disease and myocardial infarction. J Am Coll Cardiol. 2022;79:2383–97. 10.1016/j.jacc.2022.03.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian Y, et al. Associations of remnant cholesterol with cardiovascular and cancer mortality in a nationwide cohort. Science bulletin. 2024;69:526–34. 10.1016/j.scib.2023.12.035. [DOI] [PubMed] [Google Scholar]
- 27.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res. 2006;7:132. 10.1186/1465-9921-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumboyono K, et al. Detection of vascular inflammation and oxidative stress by cotinine in smokers: measured through interleukin-6 and superoxide dismutase. Int J Gen Med. 2022;15:7319–28. 10.2147/ijgm.S367125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henein MY, Vancheri S, Longo G & Vancheri F. The Role of Inflammation in Cardiovascular Disease. Int J Mol Sci. 2022;23. 10.3390/ijms232112906 [DOI] [PMC free article] [PubMed]
- 30.Liu C, et al. Oral nicotine aggravates endothelial dysfunction and vascular inflammation in diet-induced obese rats: Role of macrophage TNFα. PLoS One. 2017;12:e0188439. 10.1371/journal.pone.0188439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rom O, Reznick AZ, Keidar Z, Karkabi K, Aizenbud D. Smoking cessation-related weight gain–beneficial effects on muscle mass, strength and bone health. Addiction (Abingdon, England). 2015;110:326–35. 10.1111/add.12761. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Li H, Song C, Sun J, Liu W. Association between serum cotinine and muscle mass: results from NHANES 2011–2018. BMC Public Health. 2024;24:2093. 10.1186/s12889-024-19623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heeren J, Scheja L. Metabolic-associated fatty liver disease and lipoprotein metabolism. Mol Metab. 2021;50:101238. 10.1016/j.molmet.2021.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muraba Y, et al. The role of plasma lipoprotein lipase, hepatic lipase and GPIHBP1 in the metabolism of remnant lipoproteins and small dense LDL in patients with coronary artery disease. Clin Chim Acta Int J Clin Chem. 2018;476:146–53. 10.1016/j.cca.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Hwang SW, et al. The effect of hidden female smoking on the relationship between smoking and cardiovascular disease. Cardiol J. 2021;28:716–27. 10.5603/CJ.a2020.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P 3rd. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79:480–8. 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Tuck CH, Holleran S, Berglund L. Hormonal Regulation of Lipoprotein(a) Levels: effects of estrogen replacement therapy on lipoprotein(a) and acute phase reactants in postmenopausal women. Arterioscler Thromb Vasc Biol. 1997;17:1822–9. 10.1161/01.ATV.17.9.1822. [DOI] [PubMed] [Google Scholar]
- 38.Choi WJ, Lee JW, Cho AR & Lee YJ. Dose-dependent toxic effect of cotinine-verified tobacco smoking on systemic inflammation in apparently healthy men and women: a nationwide population-based study. Int J Environ Res Public Health. 2019;16. 10.3390/ijerph16030503 [DOI] [PMC free article] [PubMed]
- 39.Marti-Aguado D, Clemente-Sanchez A, Bataller R. Cigarette smoking and liver diseases. J Hepatol. 2022;77:191–205. 10.1016/j.jhep.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Hoogeveen RC, Ballantyne CM. Residual Cardiovascular Risk at Low LDL: Remnants, Lipoprotein(a), and Inflammation. Clin Chem. 2021;67:143–53. 10.1093/clinchem/hvaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krauss RM, King SM. Remnant lipoprotein particles and cardiovascular disease risk. Best Pract Res Clin Endocrinol Metab. 2023;37:101682. 10.1016/j.beem.2022.101682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.