Abstract
Purpose
This study aimed to develop postsurgical progression/hyperprogression recurrence (type III-IV recurrence) prediction models for hepatocellular carcinoma (HCC) patients with macroscopic vascular invasion (MaVI) and to guide treatment strategies in the accurate healthcare era.
Patients and methods
393 HCC patients with MaVI from two central hospitals made up the entire study population. In developmental (290 patients) and validation (103 patients) cohorts, all patients were randomized into one or the other. Two prediction models for type III-IV recurrence were developed, based on the findings of univariate and multivariate analysis in the development cohort, and multidimensional verification was carried out in both cohorts.
Results
The postoperative recurrence rate of type III-IV in 393 HCC patients with MaVI was 70.9%. Young age, large tumor size (≥ 10 cm), node number, incomplete tumor capsule, postoperative complications, and high Ki67 index were the independent risk factors for relapse of type III-IV. In the development cohort, two nomograms (pre- and postoperative) had the Area Under the ROC curve (AUC) of 0.827 and 0.891, respectively. The two nomograms performed well, according to multidimensional verification methods such as clinical impact curves, decision curve analysis (DCA), and calibration curves. The validation cohort saw similar encouraging results. Both nomograms could separate patients into two distinct prognosis subgroups with ideal cutoff values of 170.3 presurgery and 175.0 postsurgery (both P < 0.05).
Conclusion
We constructed two novel and potentially clinically valuable models for predicting type III-IV recurrence. These two models can develop strategies for treating those suffering from HCC with MaVI owing to their strong prediction performance and availability.
Keywords: Hepatocellular carcinoma (HCC), Macroscopic vascular invasion, Nomogram, Progression/hyperprogression recurrence (type III-IV recurrence)
Introduction
The sixth most frequent neoplasm worldwide is hepatocellular carcinoma (HCC), which is also one of the main reasons why people die from cancer [1–3]. Patients with HCC who have macroscopic vascular invasion (MaVI) are classified as having advanced-stage HCC (BCLC stage C) and are advised to receive systemic or targeted therapy [4]. However, multiple studies have found that surgery is also a potential option for HCC patients with MaVI, and some patients are still benefiting from this course of treatment [5–7]. But as surgical adaptability grows, so does the likelihood of postoperative recurrence [8–12].
HCC patients with MaVI experience varying surgical results. Some patients benefit from curative hepatic resection (CHR), while others have a very poor prognosis [6, 8–10, 12–17]. With the integration of recurrence traits, survival, effects on liver and system-wide function, and prospective therapies following recurrence, we previously proposed an original “four-types recurrent HCC classification” with significant potential usefulness in predicting postsurgical survival. Of the four recurrence patterns, a particularly bad prognosis was noted by progression/hyperprogression recurrence (type III-IV recurrence) [18]. In our opinion, the significant postsurgical type III-IV recurrence rate contributes to the very poor prognosis of some HCC patients with MaVI. Therefore, it is essential to develop preventive strategies and better understand the effects of type III-IV recurrence in HCC patients with MaVI.
Nomograms can predict and quantify individual likelihood by integrating multiple clinicopathological parameters, and they are crucial in the area of precision medicine [19]. To recognize those who could benefit from CHR and to conduct an early intervention for individuals at high risk of type III-IV, the current study set out to build type III-IV recurrence predictive models for HCC patients with MaVI.
Materials and methods
Patient cohorts
393 HCC patients who received CHR between January 2012 and May 2020 at two hospitals (Guangxi Medical University’s First Affiliated Hospital and Cancer Hospital) were included in this retrospective cohort. Cohorts for development (290 patients) and validation (103 patients) were created by randomizing the patient population. MaVI includes PVTT, HVTT, and BDTT, described as the presence of tumor thrombus in the portal vein, hepatic vein, and bile duct, respectively. Preoperative imaging should assess for tumor thrombus in the vascular, and the surgeon should confirm it during surgery. The following were the inclusion criteria: (1) pathologically diagnosed as HCC with MaVI; (2) preoperative liver function was Child-Pugh A or initially Child-Pugh B and then returned to Child-Pugh A following supportive preoperative management; (3) treated by CHR, which was outlined as removing the tumor completely and vascular tumor thrombus together (or cutting off) and a negative incisional margin; and (4) no extrahepatic metastasis. The following were the exclusion criteria: (1) co-occurring with another tumor; (2) receiving other anticancer treatments before surgery; and (3) insufficient clinical data. The study was carried out in compliance with the Declaration of Helsinki guidelines and approved by the Ethics Committee of Cancer Hospital of Guangxi Medical University, Nanning, China. The authorized agreement to allow the analysis and publication of the clinical data was signed by the patient or his or her guardian at the time of admission.
Clinicopathologic variables
Clinicopathological data on 393 patients included gender, age, HBsAg status, hepatitis B virus DNA level, serum AFP level, tumor size, Edmondson grade, node number, tumor capsule, surgical margin, MVI, tumor thrombus types, liver fibrosis (Fib-4 score), postoperative complications (Clavien-Dindo: grade II ~ IV), postoperative transarterial chemoembolization (TACE), and the Ki67 index (It is frequently employed in the early screening or prognosis of many malignancies as a well-known sign of tumor stemness and proliferation, although it has not been extensively used in the treatment of hepatocellular carcinoma). The three main categories of tumor thrombus in this study were (1) portal vein tumor thrombus: the portal vein (main or branch) contains a tumor thrombus; (2) hepatic vein tumor thrombus: hepatic vein (main or branch) contains a tumor thrombus; and (3) bile duct tumor thrombus: bile duct (main or branch) contains a tumor thrombus.
Follow-up
For the first year following surgery, follow-up was done every 1–2 months, then every 3 months after that. Ultrasonography, dynamically computed tomography, MRI, and lab tests (measurement of serum AFP) were used for follow-up examinations. The following criteria were used to determine whether recurrence had occurred: (1) AFP ≥ 400 ng/ml and a standard imaging test (contrast-enhanced ultrasound, MRI, or CT); (2) AFP < 400 ng/ml and not less than two standard imaging examinations showing new lesions; and (3) a positive pathological biopsy. According to the Clavien-Dindo grading system, postoperative complications were events that happened within 30 days of the procedure. These events included intra-abdominal infection, massive hydrothorax (> 500 ml), biliary fistula, abdominal bleeding, and post-hepatectomy liver failure (PHLF), etc [20]. Patients who died from serious complications (Clavien–Dindo grade V) during the perioperative period were excluded. The recurrence type and recurrence time were also noted simultaneously. The recurrence types were as follows: type I, solitary-intrahepatic oligo recurrence (Number of tumors = 1); type II, multi-intrahepatic oligo recurrence (1 < Number of tumors ≤ 5); type III, progression recurrence (Recurrence with vascular invasion and/or metastases to lungs, bones, lymph nodes, brain, etc.); and type IV, hyperprogression recurrence (Number of tumors > 5) [18]. The final follow-up was conducted on 31 October 2022.
Analytical statistics
SPSS 26 (IBM, New York, USA) and R (R3.5.1 and the “rms” package, R development core team) were both used for the statistical analysis. Using the random sampling approach, 393 patients were assigned to a development group and a validation group in a 3:1 ratio. The clinicopathologic factors were compared using the exact test of Fisher’s or the test of chi-square. Applying the Kaplan-Meier analysis (log-rank test), the postoperative survival without recurrence (RFS) and long-term survival (OS) of HCC patients with various recurrence types were analyzed for comparison. According to the variables of the development cohort, single and multivariable logistic regression analyses were performed to determine the variables influencing type III-IV recurrence. Two nomogram models were created using R’s “rms” package based on the recognized independent risk variables (all P < 0.05). The area under the curve of ROC was used to assess the predictive strength of the nomograms, and a ROC curve analysis was used to opt for the appropriate cutoff score. The consistency of the observed findings with those predicted by the nomogram was displayed using calibration curves. The clinical impact of each nomogram was evaluated using decision curve analysis (DCA), and the clinical impact curve was created to demonstrate each nomogram’s significance. To demonstrate the stability of the model created in the development cohort, we carried out external verification of the nomogram model utilizing information gathered from the validation group. All two-tailed statistical analyses were considered statistically significant if P < 0.05.
Results
Characteristics of the patients
This retrospective cohort analysis comprised 393 patients with MaVI who undergo CHR, and 279 of these 393 patients (70.9%) experienced type III-IV recurrence after CHR, with 72.9% of patients (209/290) within the developmental group and 68.0% of patients (70/103) within the validation group. Table 1 provides comprehensive details on the clinicopathological characteristics of the 393 HCC patients.
Table 1.
Comparison of the patient features between the validation cohort and the development cohort
| Variable | Development cohort (N = 290) | Validation cohort (N = 103) |
P-Value |
|---|---|---|---|
| Age (years) | 0.002 | ||
| ≤ 45 | 151(52.1) | 35(34.0) | |
| >45 | 139(47.9) | 68(66.0) | |
| Gender | 0.428 | ||
| Female | 26(9.0) | 12(11.7) | |
| Male | 264(91.0) | 91(88.3) | |
| HBsAg | 0.138 | ||
| Positive | 263(90.7) | 88(85.4) | |
| Negative | 27(9.3) | 15(14.6) | |
| HBV-DNA | 0.006 | ||
| < 500 | 89(30.7) | 47(45.6) | |
| ≥ 500 | 201(69.3) | 56(54.4) | |
| Tumor size (cm) | 0.571 | ||
| < 10 | 137(47.2) | 52(50.5) | |
| ≥ 10 | 153(52.8) | 51(49.5) | |
|
Postoperative complications (Clavien-Dindo: grade II ~ IV) |
0.319 | ||
| Yes | 58(20.0) | 16(15.5) | |
| No | 232(80.0) | 87(84.5) | |
| Node number | 0.639 | ||
| 1 | 150(51.7) | 56(54.4) | |
| 2 | 32(11.0) | 8(7.8) | |
| ≥ 3 | 108(37.2) | 39(37.9) | |
| Edmondson grade | 0.019 | ||
| Low | 146(50.3) | 38(36.9) | |
| High/medium | 144(49.7) | 65(63.1) | |
| Tumor capsule | 0.311 | ||
| Incomplete | 188(64.8) | 61(59.2) | |
| Complete | 102(35.2) | 42(40.8) | |
| Resection margin (cm) | 0.594 | ||
| ≥ 1 | 84(29.0) | 27(26.2) | |
| <1 | 206(71.0) | 76(73.8) | |
| MVI | 0.037 | ||
| Positive | 276(95.2) | 92(89.3) | |
| Negative | 14(4.8) | 11(10.7) | |
| AFP (ng/ml) | 0.148 | ||
| ≤ 400 | 90(31.0) | 40(38.8) | |
| >400 | 200(69.0) | 63(61.2) | |
|
Liver fibrosis (Fib-4 score) |
0.901 | ||
| > 3.25 | 77(26.6) | 28(27.2) | |
| ≤ 3.25 | 213(73.4) | 75(72.8) | |
| Tumor thrombus types | 0.314 | ||
| HVTT | 35(12.1) | 13(12.6) | |
| BDTT | 18(6.2) | 11(10.7) | |
| PVTT | 237(73.9) | 79(76.7) | |
|
Postoperative TACE |
0.243 | ||
| Yes | 161(55.5) | 64(62.1) | |
| No | 129(44.5) | 39(37.9) | |
| Ki67 | 0.284 | ||
| <30 | 53(18.3) | 16(15.5) | |
| 30 ~ 60 | 123(42.4) | 53(51.5) | |
| >60 | 114(39.3) | 34(33.0) |
Notes: HBsAg: Hepatitis B surface antigen; HBV-DNA: Hepatitis B virus deoxyribonucleic acid; AFP: Alpha-fetoprotein; MVI: Microvascular invasion; HVTT: Hepatic vein tumor thrombus; BDTT: Bile duct tumor thrombus; PVTT: Portal vein tumor thrombus
Postoperative RFS and OS of HCC patients were compared according to different recurrence patterns
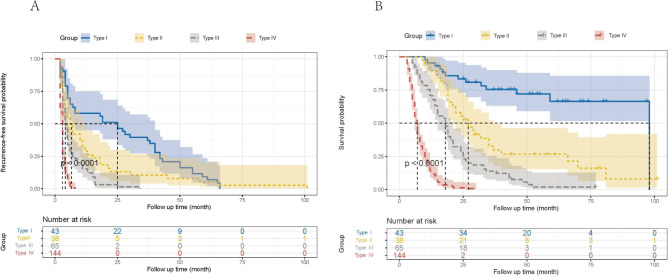
According to the characteristics of four various recurrence modalities [18], the development cohort’s 290 HCC patients were classified into four groups. Estimating the RFS and OS for each of the four groups and analyzing distinctions between survival curves were done using the log-rank test. According to the findings, the median RFS for the four groups was, for types I and II, respectively, 25 months (95% CI: 13.461–36.539) and 6 months (95% CI: 3.584–8.416); for types III and IV, respectively, it was 4 months (95% CI: 3.122–4.878) and 3 months (95% CI: 2.804–3.196). In comparison to the other two types, type III and IV’s median RFS was considerably lower (both P < 0.05) (Fig. 1A). Similar to this, the median OS of the four groups revealed that type I and type II were, respectively, 98 months (95% CI: none) and 27 months (95% CI: 21.183–32.817); type III and type IV were, respectively, 18 months (95% CI: 14.843–21.157) and 7 months (95% CI: 6.391–7.609). When compared to the other two kinds, the median OS of types III and IV was considerably lower (both P < 0.05) (Fig. 1B).
Fig. 1.
The comparison of (A) recurrence-free survival and (B) overall survival for four recurrence patterns in the development cohort (n = 290)
The factors affecting type III-IV recurrence
The univariate logistic analysis revealed that age, AFP, tumor size, Edmondson grade, node number, tumor capsule, MVI, tumor thrombus types, postoperative complications, postoperative TACE, and the Ki67 index were significant risk variables for type III-IV recurrence (all P < 0.05). According to the results of the multivariable logistic regression analyses, these factors—young age (P = 0.001), large tumor size (≥ 10 cm) (P = 0.001), node number (P < 0.001), incomplete tumor capsule (P = 0.001), postoperative complications (P = 0.005), and high Ki67 index (P = 0.001)—were all independent risk factors for type III-IV recurrence (see Table 2).
Table 2.
Clinicopathological variables associated with type III-IV recurrence in the development cohort
| Clinicopathological factors | Odds ratio |
Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|---|
| 95% CI | P | Odds ratio |
95% CI | P | ||
| Age(years) | ||||||
| ≤ 45 VS>45 | 3.985 | 2.282–6.985 | 0.000 | 3.758 | 1.786–7.907 | 0.000 |
| Gender | ||||||
| Female VS Male | 2.032 | 0.891–4.636 | 0.092 | - | - | - |
| HBsAg | ||||||
| Positive VS Negative | 1.096 | 0.460–2.613 | 0.836 | - | - | - |
| HBV-DNA | ||||||
| <500 VS ≥ 500 | 1.614 | 0.940–2.772 | 0.083 | - | - | - |
| Tumor size(cm) | ||||||
| <10 VS ≥ 10 | 3.540 | 2.047–6.120 | 0.000 | 3.277 | 1.589–6.759 | 0.001 |
|
Postoperative complications (Clavien-Dindo: grade II ~ IV) |
||||||
| Yes VS No | 6.706 | 2.343–19.198 | 0.000 | 6.249 | 1.723–22.661 | 0.005 |
| Node number | ||||||
| 1 VS 2 VS ≥ 3 | 2.132 | 1.547–2.941 | 0.000 | 2.334 | 1.526–3.572 | 0.000 |
| Edmondson grade | ||||||
| Low VS High/medium | 1.712 | 1.018–2.880 | 0.043 | 1.011 | 0.502–2.038 | 0.975 |
| Tumor capsule | ||||||
| Incomplete VS Complete | 4.507 | 2.617–7.762 | 0.000 | 3.556 | 1.727–7.322 | 0.001 |
| Resection margin(cm) | ||||||
| <1 VS ≥ 1 | 1.231 | 0.706–2.146 | 0.464 | - | - | - |
| MVI | ||||||
| Positive VS Negative | 39.765 | 5.107-309.598 | 0.000 | 5.315 | 0.516–54.732 | 0.160 |
| AFP (ng/ml) | ||||||
| ≤ 400 VS > 400 | 2.296 | 1.343–3.927 | 0.002 | 1.552 | 0.754–3.197 | 0.233 |
| Liver fibrosis (Fib-4 score) | ||||||
| > 3.25 VS ≤ 3.25 | 1.144 | 0.634–2.063 | 0.655 | - | - | - |
| Tumor thrombus types | ||||||
| HVTT VS BDTT VS PVTT | 1.992 | 1.397–2.841 | 0.000 | 1.171 | 0.708–1.938 | 0.538 |
| Postoperative TACE | ||||||
| Yes VS No | 2.469 | 1.460–4.176 | 0.001 | 1.869 | 0.926–3.774 | 0.081 |
| Ki67 | ||||||
| < 30 VS 30 ~ 60 VS > 60 | 3.596 | 2.398–5.393 | 0.000 | 2.761 | 1.654–4.611 | 0.000 |
Notes: HBsAg: Hepatitis B surface antigen; HBV-DNA: Hepatitis B virus deoxyribonucleic acid; AFP: Alpha-fetoprotein; MVI: Microvascular invasion; HVTT: Hepatic vein tumor thrombus; BDTT: Bile duct tumor thrombus; PVTT: Portal vein tumor thrombus
The construction of preoperative and postoperative nomogram models and multidimensional validations
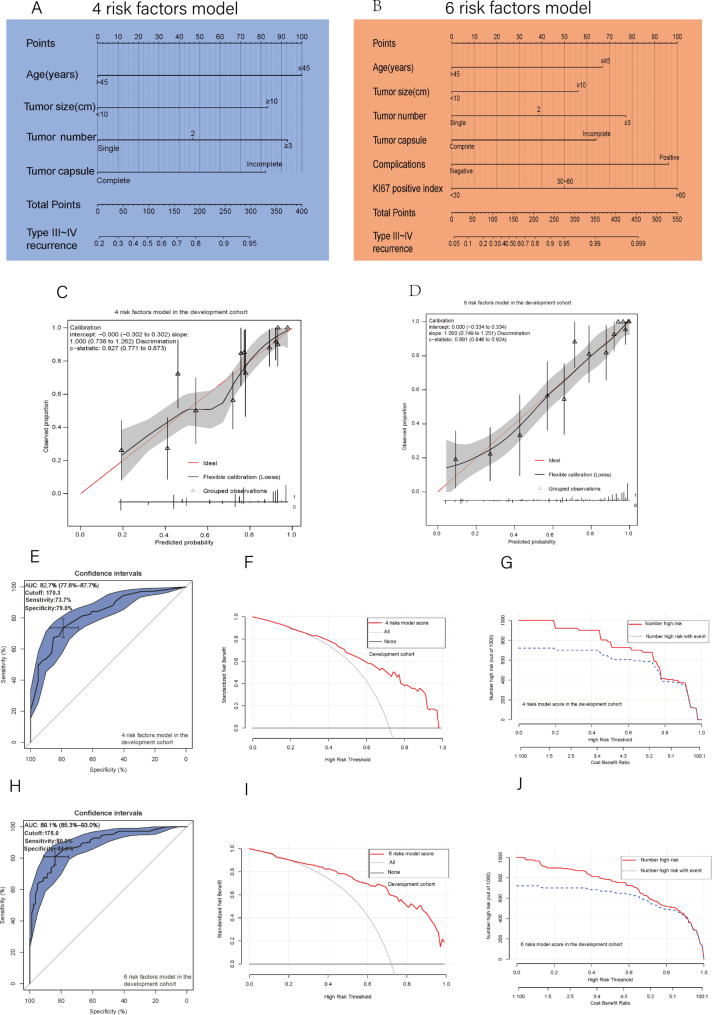
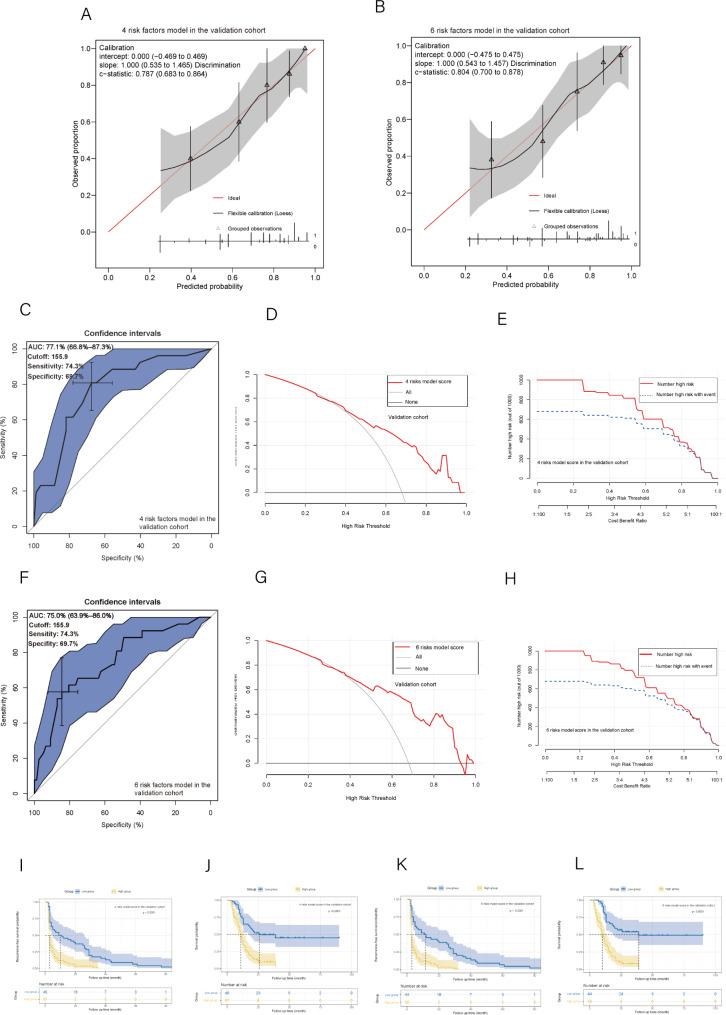
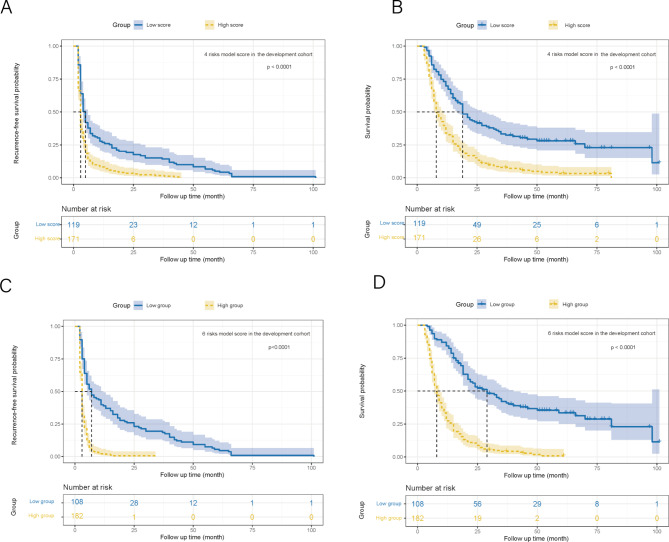
Two nomograms were developed for preoperative and postoperative prediction utilizing 4 and 6 total risk factors, respectively, based on the findings of the multivariable logistic regression analysis (Fig. 2A and B). The nomogram prediction results and actual observation results were well correlated, as shown by the calibration graphs of prediction probabilities (Fig. 2C and D). The verification cohort also witnessed similar encouraging outcomes (Fig. 3A and B). The development cohort’s 4 preoperative risk factor model and 6 overall risk factor model both had AUC values of 0.827 and 0.891, respectively, according to the ROC curve analysis (Fig. 2E and H). The ideal cutoff values for type III-IV recurrence prediction were 170.3 presurgery, with a sensitivity of 73.7% and specificity of 79.0%, and 175.0 postsurgery, with a sensitivity of 80.9% and specificity of 84.0% (Fig. 2E and H). The ROC curve analysis in the verification cohort produced similarly excellent results (Fig. 3C and F). Both nomograms had good prediction accuracy in both cohorts when using decision curve analysis (DCA) (Figs. 2F and I and 3D and G). The two nomogram models in both cohorts had significant predictive power, according to all clinical impact curves (Figs. 2G and J and 3E and H). Furthermore, The threshold score of 170.3 presurgery resulted in considerably shorter RFS and OS for patients with high scores compared to patients with lower values (Fig. 4A and B). The threshold score of 175.0 postsurgery resulted in considerably shorter RFS and OS for patients with high scores compared to patients with lower values (Fig. 4C and D). The validation cohort obtained similar outcomes using the same cutoff score as the development cohort (Fig. 3I, J, K and L).
Fig. 2.
(A, B) The 4 risk factor and 6 risk factor nomograms; (C, D) The 4 and 6 risk factors model’s calibration curve in the development cohort; (E) The 4 risk factors model’s ROC curve, Area under the curve, AUC, and cutoff value in the development cohort; (H) The 6 risk factors model’s ROC curve, Area under the curve, AUC, and cutoff value in the development cohort; (F) The 4 risk factor model’s DCA in the development cohort; (I) The 6 risk factor model’s DCA in the development cohort; (G) The 4 risk factor model’s clinical impact curves in the development cohort; (J) The 6 risk factor model’s clinical impact curves in the development cohort
Fig. 3.
(A, B) The 4 and 6 risk factors model’s calibration curve in the validation cohort; (C) The 4 risk factors model’s ROC curve, Area under the curve, and AUC in the validation cohort; (F) The 6 risk factors model’s ROC curve, Area under the curve, and AUC in the validation cohort; (D) The 4 risk factor model’s DCA in the validation cohort; (G) The 6 risk factor model’s DCA in the validation cohort; (E) The 4 risk factor model’s clinical impact curves in the validation cohort; (H) The 6 risk factor model’s clinical impact curves in the validation cohort; (I) Recurrence-free survival and (J) overall survival between the groups with low and high scores in the validation cohort (The cutoff value comes from the 4 risk factors model); (K) Recurrence-free survival and (L) overall survival between the groups with low and high scores in the validation cohort (The cutoff value comes from the 6 risk factors model)
Fig. 4.
(A) Recurrence-free survival and (B) overall survival between the groups with low and high scores in the development cohort (The cutoff value comes from the 4 risk factors model); (C) Recurrence-free survival and (D) overall survival between the groups with low and high scores in the development cohort (The cutoff value comes from the 6 risk factors model)
Discussion
Previous studies have highlighted the poor prognosis associated with type III-IV recurrence in HCC patients. Our study found that the incidence of postoperative type III-IV recurrence in HCC patients with MaVI was 70.9%, and patients with this recurrence had considerably lower RFS and OS than those with type I-II recurrence. These findings suggest that certain underlying factors may contribute to the development of type III-IV recurrence following surgery. Identifying these risk factors is crucial for improving outcomes in these patients. Six clinicopathologic factors, including four tumor-related factors linked to the malignant behavior of primary tumors (i.e., large tumor size, multiple tumor nodes, incomplete tumor capsule, and high Ki67 index), were found to be associated with type III-IV recurrence through the use of univariate and multivariate analyses. This suggests that patients with potential type III-IV recurrence have a primary tumor with a higher malignant tendency before surgery. Type III-IV recurrence is characterized by vascular invasion, extrahepatic metastasis, or multiple intrahepatic recurrences (Number of tumors > 5), and previous studies have also confirmed the association of these four factors with these conditions [21–26], further supporting our findings. Hepatocellular carcinoma recurrence after surgery is a complicated issue that requires the integration of several treatment modalities. The multidisciplinary team plays a critical role in developing a personalized treatment strategy for each patient [27, 28]. For HCC patients with multiple intrahepatic recurrences and extrahepatic metastases, for instance, both TACE and radiotherapy demonstrate their benefits [29, 30]. In the meantime, research has been done on the operation of TACE with radiation in patients with advanced HCC [31]. Hence, multidisciplinary treatment is the optimal choice, especially for HCC patients who experience type III–IV recurrence following surgery, with the goal of enhancing the patients’ quality of life and extending their survival time.
Ki67 is indeed a valuable biomarker for predicting HCC prognosis and is closely related to tumor proliferation and stemness [22, 32–34]. In addition to its role in predicting the risk of postoperative recurrence, Ki67’s ability to predict the effectiveness of immunotherapy in HCC has also been explored in recent research [35]. According to several preclinical and clinical studies, tumors with high Ki67 expression may be more responsive to immune checkpoint blockade therapy. This may be because these tumors produce more neoantigens and express immune checkpoint molecules like PD-L1 [36, 37]. These results imply that Ki67 may be a valuable biomarker for identifying HCC patients who will probably respond well to immunotherapy. The predictive relevance of Ki67 for the response to immunotherapy in HCC requires further research.
Importantly, while postoperative complications are controllable, they are not the only factor that affects the risk of type III-IV recurrence in HCC patients. As was already noted, other tumor-related variables, such as large tumor size, multiple tumor nodes, and incomplete tumor capsule, have a role in the emergence of aggressive HCC behavior. These factors may not be controllable through surgical techniques or perioperative management alone and may require other treatment strategies, such as targeted therapy or immunotherapy. That being said, the use of laparoscopic liver resection and enhanced recovery after surgery (ERAS) can certainly help to reduce the incidence of postoperative complications and improve patient outcomes [38–43]. The decision to use laparoscopic or open liver resection should be based on a thorough evaluation of each patient’s circumstances, including tumor location, degree of invasion, and degree of cirrhosis. In addition, the use of ERAS can help to reduce the risk of postoperative complications and improve recovery time, regardless of the type of surgical procedure used [38]. Therefore, a comprehensive approach to perioperative management that accounts for both surgical technique and ERAS, can help to optimize patient outcomes after liver resection for HCC.
Tumors occurring in young patients tend to exhibit aggressive behavior and poorer prognosis [44, 45], possibly attributed to delayed detection and distinct tumor characteristics. Notably, Li et al. [46, 47] posited that young HCC patients with vascular invasion demonstrate improved liver function reserve and superior treatment response to hepatectomy. In the current investigation, age represented a critical component of the nomogram model, particularly in the preoperative nomogram, with age ≤ 45 years contributing up to 100 points. Thus, for HCC patients harboring MaVI, surgical management should not be determined solely by age but rather should be integrated with other relevant risk factors.
Several studies have developed nomograms for predicting early relapse or OS in HCC patients with MaVI. An early recurrence nomogram was developed by Zhang et al. [48] in 2019, to identify patients who may benefit from postoperative adjuvant therapy. A C-index of 0.836 (0.784–0.887) indicates that the nomogram was fairly accurate. However, this model only considered preoperative variables and did not evaluate important postoperative variables, such as the Ki67 index, which may limit its ability to accurately recognize patients at high risk of early recurrence. Recent research on HCC patients who had HVTT also revealed comparable limitations [16]. In 2020, to determine which patients would benefit from conservative treatment, Liu et al. [49] developed a nomogram to predict the overall survival of HCC patients with PVTT on conservative treatment. However, it should be highlighted that all patients in this trial underwent conservative treatment, whereas previous studies have shown that some HCC patients with PVTT may benefit from surgical treatment, which highlights the need for precision medicine. Currently, no prognostic model is available for HCC patients with bile duct invasion.
For HCC patients with MaVI, no predictive model has yet been developed to identify the pattern of postoperative recurrence. Because type III-IV recurrence is associated with a very poor prognosis, we created two nomograms in this study to predict recurrence before and after surgery. Our models exhibited good predictive performance and strong diagnostic capabilities, as demonstrated by the calibration plots and ROC analysis. The preoperative and postoperative prediction models had C indices of 0.827 (0.771–0.873) and 0.891 (0.846–0.924), respectively, with corresponding AUCs of 82.7% (77.8 − 87.7%) and 89.1% (85.3 -93.0%). Our nomograms had the excellent predictive ability, as evidenced by the clinical impact curve and DCA, and their inclusion of factors made them simple to apply in practical clinical applications. Based on our preoperative prediction model, high-scoring patients were discouraged from undergoing surgery, while those with high scores in the postoperative prediction model were suggested to undergo systematically monitored and individualized adjuvant therapy to lower the probability of type III-IV recurrence.
This study incorporates several innovative designs. First, two novel prediction models were developed, which is a unique approach, as the pattern of postoperative recurrence is often overlooked in determining patient outcomes. By focusing on this aspect, we created two nomograms that can be used as a guide for treating HCC patients who have MaVI. Second, the models have significant clinical significance, as they can be used to guide both preoperative and postoperative treatment strategies. The preoperative prediction model can effectively predict the likelihood of type III-IV recurrence, allowing for the identification of candidates for surgical therapy. Then again, the postoperative prediction model can help guide the treatment of high-risk patients who have experienced type III-IV recurrence after surgery, reducing their risk of recurrence and achieving long-term prognostic outcomes. Third, the models have a wider range of applications, as they have been designed for HCC patients with hepatic vein, bile duct, or portal vein invasion, thus making them suitable for a broader population of patients.
The following are some of this study’s limitations. First off, since this study’s data are all from China and its sample size is relatively small, some variation in its findings is unavoidable. Additionally, the majority of them have hepatitis B, which is the main factor in Chinese patients’ primary liver cancer. Studies in the future should expand the validation cohort of HCC unrelated to HBV. Lastly, there is currently no method to accurately detect MVI, and the 7-point baseline sample collection protocol adopted in this study may lead to false negatives.
Conclusion
The significant rate of postsurgical progression/hyperprogression recurrence (type III-IV recurrence) in HCC patients with macroscopic vascular invasion (MaVI) is one of the major reasons affecting these patients’ poor prognosis. Independent risk factors associated with type III-IV recurrence include young age, tumor size ≥ 10 cm, node number, incomplete tumor capsule, postoperative complications, and high Ki67 index. To address this issue, we constructed two nomograms (pre- and postoperative) that demonstrated excellent predictive ability. These models offer valuable insights for preoperative decision-making and postoperative treatment guidance and are of utmost importance for the precision treatment of HCC with MaVI.
Acknowledgements
Not applicable.
Author contributions
Y.Y.H and Y.X.S were the major contributors in writing the manuscript. Y.Y.C and J.X.X analyzed and interpreted the patient’s data. L.Z completed the literature search. H.W.W and S.L.Q provided literature revision. Y.C.P and L.N.Q conducted a review and editing. The authors read and approved the final manuscript.
Funding
The National Nature Science Foundation of China (NSFC 81972306,81502533,82273405) and the Guangxi Nature Sciences Grants (2018GXNSFAA138028, 2018GXNSFAA050124) funded this research. This work was also funded in part by the Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor, Ministry of Education/ Guangxi, Independent Research Project (GKE202214).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Guangxi Medical University Cancer Hospital (Approval Number: LW2023078). The clinical data were retrospectively registered.
Consent for publication
We have obtained informed consent from the patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yiyue Huang and Yuexiang Su contributed equally to this work.
Contributor Information
Yuchong Peng, Email: pengyuchong2018@126.com.
Lunan Qi, Email: qilunan_gxmu@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14(9):527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Famularo S, Donadon M, Cipriani F, Giuliante F, Ferri S, Celsa C, et al. Hepatectomy Versus Sorafenib in Advanced Nonmetastatic Hepatocellular Carcinoma. Ann Surg. 2022;275(4):743–52. [DOI] [PubMed] [Google Scholar]
- 6.Liu ZH, Sun JX, Feng JK, Yang SY, Chen ZH, Liu C, et al. Prognostic comparison between liver resection and transcatheter arterial chemoembolization for Hepatocellular Carcinoma patients with bile Duct Tumor Thrombus: a propensity-score matching analysis. Front Oncol. 2022;12:835559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tustumi F, Coelho FF, de Paiva Magalhães D, Júnior SS, Jeismann VB, Fonseca GM et al. Treatment of hepatocellular carcinoma with macroscopic vascular invasion: a systematic review and network meta-analysis. Transplantation Reviews. 2023;37(3). [DOI] [PubMed]
- 8.Gao Y, Wang PX, Cheng JW, Sun YF, Hu B, Guo W, et al. Chemotherapeutic perfusion of portal vein after tumor thrombectomy and hepatectomy benefits patients with advanced hepatocellular carcinoma: a propensity score-matched survival analysis. Cancer Med. 2019;8(16):6933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim D-S, Kim B-W, Hatano E, Hwang S, Hasegawa K, Kudo A, et al. Surgical outcomes of Hepatocellular Carcinoma with bile Duct Tumor Thrombus. Ann Surg. 2020;271(5):913–21. [DOI] [PubMed] [Google Scholar]
- 10.Liu F, Guo X, Dong W, Zhang W, Wei S, Zhang S, et al. Postoperative adjuvant TACE-associated nomogram for predicting the prognosis of resectable Hepatocellular Carcinoma with portal vein tumor Thrombus after liver resection. Int J Biol Sci. 2020;16(16):3210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong TCL, Cheung TT, Chok KSH, Chan ACY, Dai WC, Chan SC, et al. Outcomes of hepatectomy for hepatocellular carcinoma with bile duct tumour thrombus. Hpb. 2015;17(5):401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Zhu Y, Zhao X, Li JH, Xu D, Jia HL, et al. The Prognostic Comparison between Hepatocellular Carcinoma with Portal Vein Tumor Thrombus and bile Duct Cancer Thrombus after liver resection. Cancer Manag Res. 2020;12:12077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding Y, Shaohua L, Qiaoxuan W, Peng S, Qing L, Zhongyuan Y et al. Surgical Strategy for Hepatocellular Carcinoma Patients with Portal/Hepatic vein Tumor thrombosis. PLoS ONE. 2015;10(6). [DOI] [PMC free article] [PubMed]
- 14.Feng J-K, Wu Y-X, Chen Z-H, Sun J-X, Wang K, Chai Z-T, et al. The effect of bile duct tumor thrombus on the long-term prognosis of hepatocellular carcinoma patients after liver resection: a systematic review and meta-analysis. Annals Translational Med. 2020;8(24):1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo L, Wei W, Yan Z, Lei Z, Xie Y, Gong R, et al. Short-term and long-term outcomes of liver resection for HCC patients with portal vein tumor thrombus. Cell Biosci. 2019;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XP, Wang K, Gao YZ, Wei XB, Lu CD, Chai ZT, et al. Prognostic model for identifying candidates for hepatectomy among patients with hepatocellular carcinoma and hepatic vein invasion. Br J Surg. 2020;107(7):865–77. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Wu J-L, Li L-Q. Efficacy comparison of optimal treatments for hepatocellular carcinoma patients with portal vein tumor thrombus. Ann Hepatol. 2022;27(1). [DOI] [PubMed]
- 18.Qi LN, Ma L, Wu FX, Chen YY, Xu JX, Peng YC, et al. Clinical implications and biological features of a novel postoperative recurrent HCC classification: a multi-centre study. Liver Int. 2022;42(10):2283–98. [DOI] [PubMed] [Google Scholar]
- 19.Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu JX, Qin SL, Wei HW, Chen YY, Peng YC, Qi LN. Prognostic factors and an innovative nomogram model for patients with hepatocellular carcinoma treated with postoperative adjuvant transarterial chemoembolization. Ann Med. 2023;55(1):2199219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdou AG, Holah NS, Elazab DS, El-Gendy WG, Badr MT, Al-Sharaky DR. Hepatocellular Carcinoma Score and subclassification into aggressive subtypes using immunohistochemical expression of p53, beta-catenin, CD133, and Ki-67. Appl Immunohistochem. 2021;29(1):20–33. [DOI] [PubMed] [Google Scholar]
- 22.Kim BW, Kim YB, Wang HJ, Kim MW. Risk factors for immediate post-operative fatal recurrence after curative resection of hepatocellular carcinoma. World J Gastroenterol. 2006;12(1):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Huang L, Liu C, Qiu M, Yan J, Yan Y, et al. Risk factors and clinical outcomes of extrahepatic recurrence in patients with post-hepatectomy recurrent hepatocellular carcinoma. ANZ J Surg. 2021;91(6):1174–9. [DOI] [PubMed] [Google Scholar]
- 24.Pan C, Liu X, Zou B, Chin W, Zhang W, Ye Y, et al. A Nomogram Estimation for the risk of Microvascular Invasion in Hepatocellular Carcinoma patients Meeting the Milan Criteria. J Invest Surg. 2022;35(3):535–41. [DOI] [PubMed] [Google Scholar]
- 25.Takeishi K, Maeda T, Tsujita E, Yamashita YI, Harada N, Itoh S, et al. Predictors of Intrahepatic multiple recurrences after curative hepatectomy for Hepatocellular Carcinoma. Anticancer Res. 2015;35(5):3061–6. [PubMed] [Google Scholar]
- 26.Yan Y, Zhou Q, Zhang M, Liu H, Lin J, Liu Q, et al. Integrated Nomograms for Preoperative Prediction of Microvascular Invasion and Lymph Node Metastasis Risk in Hepatocellular Carcinoma patients. Ann Surg Oncol. 2020;27(5):1361–71. [DOI] [PubMed] [Google Scholar]
- 27.Wen T, Jin C, Facciorusso A, Donadon M, Han HS, Mao Y, et al. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr. 2018;7(5):353–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, et al. Guidelines for the diagnosis and treatment of primary Liver Cancer (2022 Edition). Liver Cancer. 2023;12(5):405–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014;19:309–16. [DOI] [PubMed] [Google Scholar]
- 30.He J, Zeng ZC, Tang ZY, Fan J, Zhou J, Zeng MS, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer. 2009;115(12):2710–20. [DOI] [PubMed] [Google Scholar]
- 31.Oh D, Lim DH, Park HC, Paik SW, Koh KC, Lee JH, et al. Early three-dimensional conformal radiotherapy for patients with unresectable hepatocellular carcinoma after incomplete transcatheter arterial chemoembolization: a prospective evaluation of efficacy and toxicity. Am J Clin Oncol. 2010;33(4):370–5. [DOI] [PubMed] [Google Scholar]
- 32.Menon SS, Guruvayoorappan C, Sakthivel KM, Rasmi RR. Ki-67 protein as a tumour proliferation marker. Clin Chim Acta. 2019;491:39–45. [DOI] [PubMed] [Google Scholar]
- 33.Rahmanzadeh R, Huttmann G, Gerdes J, Scholzen T. Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis. Cell Prolif. 2007;40(3):422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng JN, Ma TX, Cao JY, Sun XQ, Chen JC, Li W, et al. Knockdown of Ki-67 by small interfering RNA leads to inhibition of proliferation and induction of apoptosis in human renal carcinoma cells. Life Sci. 2006;78(7):724–9. [DOI] [PubMed] [Google Scholar]
- 35.Xu JX, Xing WT, Peng YC, Chen YY, Qi LN. Outcomes of postoperative adjuvant transarterial chemoembolization for hepatocellular carcinoma according to the Ki67 index. Future Oncol. 2022;18(17):2113–25. [DOI] [PubMed] [Google Scholar]
- 36.Cidado J, Wong HY, Rosen DM, Cimino-Mathews A, Garay JP, Fessler AG, et al. Ki-67 is required for maintenance of cancer stem cells but not cell proliferation. Oncotarget. 2016;7(5):6281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mrouj K, Andres-Sanchez N, Dubra G, Singh P, Sobecki M, Chahar D et al. Ki-67 regulates global gene expression and promotes sequential stages of carcinogenesis. Proc Natl Acad Sci U S A. 2021;118(10). [DOI] [PMC free article] [PubMed]
- 38.Giovinazzo F, Kuemmerli C, Moekotte A, Rawashdeh A, Suhool A, Armstrong T, et al. The impact of enhanced recovery on open and laparoscopic liver resections. Updates Surg. 2020;72(3):649–57. [DOI] [PubMed] [Google Scholar]
- 39.Ivanics T, Claasen MPAW, Patel MS, Rajendran L, Shwaartz C, Raschzok N, et al. Long-term outcomes of laparoscopic liver resection for hepatocellular carcinoma: a propensity score matched analysis of a high-volume north American center. Surgery. 2022;171(4):982–91. [DOI] [PubMed] [Google Scholar]
- 40.Li D-X, Ye W, Yang Y-L, Zhang L, Qian X-J, Jiang P-H. Enhanced recovery nursing and mental health education on postoperative recovery and mental health of laparoscopic liver resection. World J Gastrointest Surg. 2023;15(8):1728–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng Y, Chen K, Li B, Xu H, Wei Y, Liu F. Laparoscopic versus open liver resection for resectable HCC with BCLC stage B: a propensity score-matched analysis. Updates Surg. 2022;74(4):1291–7. [DOI] [PubMed] [Google Scholar]
- 42.Savikko J, Vikatmaa L, Hiltunen A-M, Mallat N, Tukiainen E, Salonen S-M, et al. Enhanced recovery protocol in laparoscopic liver surgery. Surg Endosc. 2020;35(3):1058–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, He X, Wang M, Zhao Y, Wang L, Mao A, et al. Enhanced recovery after surgery in the patients with Hepatocellular Carcinoma Undergoing Hemihepatectomy. Surg Innov. 2022;29(6):752–9. [DOI] [PubMed] [Google Scholar]
- 44.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High alpha-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of Hepatitis virus infection, age, p53 and beta-catenin mutations. Int J Cancer. 2004;112(1):44–50. [DOI] [PubMed] [Google Scholar]
- 45.Yamazaki Y, Kakizaki S, Sohara N, Sato K, Takagi H, Arai H, et al. Hepatocellular carcinoma in young adults: the clinical characteristics, prognosis, and findings of a patient survival analysis. Dig Dis Sci. 2007;52(4):1103–7. [DOI] [PubMed] [Google Scholar]
- 46.Li C, Chen K, Liu X, Liu HT, Liang XM, Liang GL, et al. Analysis of clinicopathological characteristics and prognosis of young patients with Hepatocellular Carcinoma after Hepatectomy. J Clin Transl Hepatol. 2020;8(3):285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeishi K, Shirabe K, Muto J, Toshima T, Taketomi A, Maehara Y. Clinicopathological features and outcomes of young patients with hepatocellular carcinoma after hepatectomy. World J Surg. 2011;35(5):1063–71. [DOI] [PubMed] [Google Scholar]
- 48.Zhang XP, Chen ZH, Zhou TF, Li LQ, Chen MS, Wen TF, et al. A nomogram to predict early postoperative recurrence of hepatocellular carcinoma with portal vein tumour thrombus after R0 liver resection: a large-scale, multicenter study. Eur J Surg Oncol. 2019;45(9):1644–51. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Xue D, Zhang X, Gao F, Sun L, Yang X, et al. Nomogram for Individualized Prediction of Hepatocellular Carcinoma with Portal Vein Tumor thrombosis on conservative treatment. Biomed Res Int. 2020;2020:1473718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.