Abstract
Background
Robot-assisted thoracoscopic surgery (RATS) thymectomy has been increasingly performed for treating thymic epithelial tumors in recent years. However, there are very limited reports on the long-term oncologic outcomes after RATS thymectomy, particularly in comparison to Video-assisted thoracoscopic surgery (VATS). This study aimed to compare the perioperative and long-term oncological outcomes between RATS and VATS.
Methods
The study was conducted on 180 consecutive patients undergoing RATS or VATS between July 2016 and December 2019, 85 of whom underwent RATS, and 95 of whom underwent VATS. A 1:1 matched propensity score-matched analysis was performed and the perioperative and long-term oncologic outcomes of the two groups compared.
Result
RATS group experienced a shorter operation time (median: 100 min vs. 120 min; P = 0.039) and less blood loss (40.00 ml vs. 50.00 ml, P = 0.011). RATS demonstrated a significantly lower conversion rate to open surgery compared to VATS, with only two patients requiring conversion in the RATS group as opposed to ten patients in the VATS group (3.03% vs. 15.15%, P = 0.030). In the RATS group, the 5-year progression-free survival rate was 87.70%, and the 5-year tumor-related survival rate was 92.31%, demonstrating no statistically significant difference compared to those in the VATS group.
Conclusion
Compared with VATS, robotic thymectomy demonstrated excellent perioperative outcomes, and RATS achieved long-term oncologic outcomes comparable to those of VATS. RATS thymectomy could be considered as an effective alternative approach for treating thymic epithelial tumors.
Keywords: Thymic epithelial tumor, Robot-assisted thoracoscopic surgery, Video-assisted thoracoscopic surgery, Perioperative outcomes, Survival
Background
The open approach through a median sternotomy had ever been considered as the optimal surgical approach for the thymic epithelial tumors. Afterwards, video- and robot-assisted thoracoscopic surgery thymectomy are both becoming more prevalent thanks to advances in fibre-optic lighting and video-endoscopic camera technologies [1]. Several studies have compared the early postoperative outcomes of VATS or RATS with those of a sternotomy approach for thymomas, and both approaches have demonstrated comparable rates of complete tumor removal (R0 resection). Moreover, they have exhibited superior short-term results in comparison to open thymectomy [2–5]. In recent years, several studies have directly compared the short-term outcomes of RATS and VATS for thymomas, highlighting potential advantages of RATS in terms of enhanced three-dimensional visualization and flexible and finer instrument control. However, on account of the indolent nature of thymic epithelial tumors and the relatively recent adoption of the robot-assisted surgery in many centers, there are relatively limited long-term data regarding disease progression and overall survival after RATS or VATS thymectomy. Therefore, a definitive conclusion regarding whether RATS can achieve equivalent or superior short-term surgical outcomes remains elusive, and there is a lack of comprehensive comparisons between these two procedures in terms of long-term survival and recurrence outcomes as well.
In the present study, we conducted a retrospective analysis of patients with thymic epithelial tumors who underwent RATS or VATS thymectomy at our institution to investigate and compare perioperative outcomes and prognoses between the two techniques.
Methods
Patient collection
This study included patients with pathologically confirmed thymic epithelial tumors who were treated with RATS or VATS, including conversion during surgery. Finally, 180 consecutive patients who underwent RATS or VATS at Daping Hospital between July 2016 and December 2019 were included. Since this was a retrospective study, no specific treatment method was deliberately used for patients to obtain a certain sample of data. In our study, the decision to perform VATS or RATS was mostly determined by the patients' decisions. A written consent was obtained from all patients for either RATS or VATS.
All patients were evaluated for biochemical testing, cardiorespiratory function, computed tomography as well as symptoms of myasthenia gravis (MG) prior to surgery, and the results indicated that all patients were eligible for surgery. The Masaoka Koga stage classification system was employed for staging the patients, while pathologists reviewed their histologic characteristics based on the classification provided by the World Health Organization.
Surgical procedures
The Da Vanci Si system (Intuitive Surgical, Inc., USA) was used to perform the robot-assisted thymectomies. The transthoracic surgery was performed under general anaesthesia with a double-lumen intubation, while the subxiphoid approach was performed under single-lumen intubation. The selection of surgical approach (transthoracic or subxiphoid approach) was based on the extent of tumor invasion of surrounding organs according to preoperative imaging examination, and the surgeons’ preferences. The transthoracic surgeries were approached from either the left or right chest based on the predominant laterality of the tumor through three-port method. All surgeries were performed from a phrenic nerve to the other, with maximal excision of superior poles by gradual pulling down and resection, accompanied by removal of anterior mediastinal adipose tissue. The principles of no-touch and en bloc resection were followed during all surgeries. Surgical positioning, incisions, thymus mobilization and dissection, retrieval processes, and chest tube placement were performed essentially as previously described [6].
Postoperative care and adjuvant therapy
The postoperative complications within 30 days, mainly including myasthenia crisis, pneumonia, pneumothorax, deep vein thrombosis, and arrhythmia, were recorded and classified in accordance with the Clavien–Dindo classification [7].
Adjuvant postoperative radiotherapy is considered an important part of the treatment of thymic epithelial tumor. In our institution, patients with B3 thymomas, incomplete surgical resection, and stage III/IV thymomas were suggested to receive adjuvant radiotherapy in accordance with national Chinese guidelines for the clinical diagnosis and treatment of thymic epithelial tumors [8]. Adjuvant postoperative chemotherapy was not adopted as a routine treatment unless combined with radiotherapy for R1 or R2 resected III/IV thymomas and advanced thymic carcinoma [9].
Follow-up method
The recommended protocol entails obtaining an enhanced contrast whole-body CT scan every six months for the initial two years, followed by an annual scan for the next 3 years. All follow-up data were collected by outpatient visits or contacting patients and their relatives by telephone. The last follow-up was implemented in October 2023. Patients lost to follow-up were censored.
Propensity score matching
To minimalize selection bias caused by the retrospective nature and control for potential confounders, we performed propensity score matching between patients who had undergone RATS versus VATS. Specifically, logistic regression was used to calculate propensity scores based on potentially confounding baseline characteristics of patients. Based on literature review of the present topic, We included the variables that could potentially confound perioperative outcomes and patients' prognosis in the logistic regression model. These variables included age, gender, tumor size, WHO classification, and Masaoka stage. Subsequently, patients who underwent RATS and VATS were paired, using the nearest neighbor matching method, a caliper width of 0.05, and no replacement, resulting in a one-to-one matched sample.
Statistical analysis
In descriptive statistics, medians and interquartile ranges (IQRs) or means with standard deviations (SDs) are used. The Categorical variables are reported as counts and percentages of patients and were compared with the χ2 test or Fisher's exact test. Overall survival, disease-specific survival, and recurrence-free survival were calculated by the Kaplan‒Meier method. The log-rank test was used to explore associations between variables and survival time. Prognostic factors associated with survival were estimated by univariate and multivariable Cox proportional hazard models.
Results
Patient characteristics
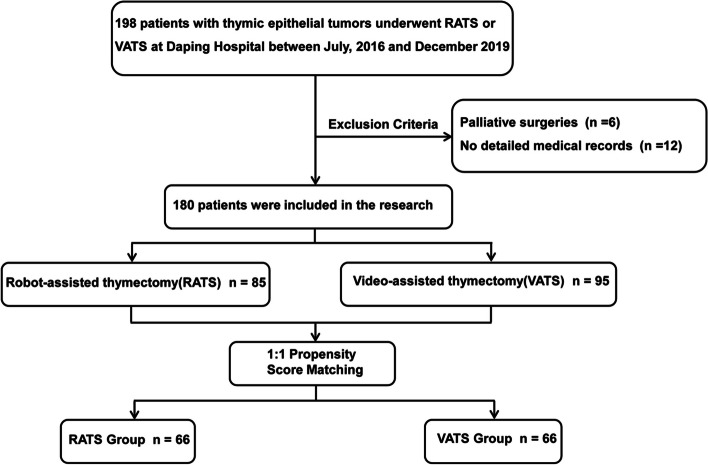
The baseline characteristics of the patient cohorts are presented in Table 1. From July 2016 to December 2019, 198 minimally invasive thymectomies, including 86 RATS and 112 VATS, were performed at Daping Hospital. Six patients who underwent palliative surgery or did not undergo resection of the fat tissue were not included, and 12 patients were also excluded because of a lack of detailed and complete medical records (Fig. 1). Among the included patients, 85 underwent RATS, and 95 underwent VATS. The male population accounted for 57.65% (n = 49) in the RATS group, whereas the VATS group included more female patients (n = 54, 56.84%, P = 0.036). Patients who underwent RATS had larger tumor size than did those in the VATS group (median 4.8 vs. 4.0 cm, P = 0.001). There were no statistically significant differences between the two groups in terms of patient characteristics including mean age, body mass index, smoking history, comorbidities, presence of MG, Masaoka stage, or histological classification.
Table 1.
Demographic characters of patients in RATS group and VATS group before and after 1:1 propensity score matching
| Characteristics | Before propensity score matching | After propensity score matching | ||||
|---|---|---|---|---|---|---|
| RATS group (n = 85) | VATS group (n = 95) | P Value | RATS group (n = 66) | VATS group (n = 66) | P Value | |
| Age (years) | 51.67 ± 13.07 | 52.67 ± 10.42 | 0.573 | 52.95 ± 12.09 | 51.88 ± 10.42 | 0.585 |
| Gender | 0.036 | 0.384 | ||||
| Male | 49 (57.65) | 41 (43.16) | 31 (46.97) | 36 (54.55) | ||
| Female | 36 (42.35) | 54 (56.84) | 35 (53.03) | 30 (45.45) | ||
| BMI (Kg/m2) | 23.32 ± 2.73 | 23.53 ± 3.28 | 0.632 | 23.53 ± 2.57 | 23.64 ± 3.36 | 0.839 |
| Smoking history | 0.481 | 0.455 | ||||
| Yes | 32 (37.65) | 31 (32.63) | 19 (28.79) | 23 (34.85) | ||
| No | 53 (62.35) | 64 (67.37) | 47 (71.21) | 43 (65.15) | ||
| Comorbidity (%) | 0.883 | 0.966 | ||||
| Hypertension | 13 (15.29) | 23 (24.21) | 11 (16.67) | 13 (19.70) | ||
| Diabetes | 3 (3.53) | 5 (5.26) | 3 (4.55) | 3 (4.55) | ||
| Coronary heart disease | 4 (4.71) | 6 (6.32) | 3 (4.55) | 2 (3.03) | ||
| COPD | 2 (2.35) | 1 (1.05) | 1 (1.52) | 1 (1.52) | ||
| Others | 8 (9.41) | 11 (11.58) | 4 (6.06) | 6 (9.09) | ||
| Myasthenia Gravis, n (%) | 27 (31.76) | 31 (32.63) | 0.901 | 19 (28.79) | 16 (24.24) | 0.554 |
| Tumor size (cm), median (IQR) | 4.8 (3.7, 6.5) | 4.0 (2.8, 5.5) | 0.001 | 4.6 (3.5, 5.6) | 4.6 (3.5, 5.9) | 0.689 |
| Pre-operative treatment, n (%) | 1.000 | 1.000 | ||||
| Yes | 1 (1.18) | 1 (1.05) | 1 (1.52) | 1 (1.52) | ||
| No | 84 (98.82) | 94 (98.95) | 65 (98.48) | 65 (98.48) | ||
| WHO Classification, n (%) | 0.524 | 0.865 | ||||
| A | 16 (18.82) | 14 (14.74) | 10 (15.15) | 9 (13.64) | ||
| AB | 21 (24.71) | 24 (25.26) | 18 (27.27) | 19 (28.79) | ||
| B1 | 8 (9.41) | 17 (17.89) | 8 (12.12) | 8 (12.12) | ||
| B2 | 21 (24.71) | 24 (25.26) | 15 (22.73) | 19 (28.79) | ||
| B3 | 8 (9.41) | 9 (9.47) | 8 (12.12) | 4 (6.05) | ||
| Thymic carcinoma | 11 (12.94) | 7 (7.38) | 7 (10.61) | 7 (10.61) | ||
| Masaoka Stage, n (%) | 0.200 | 0.655 | ||||
| I | 48 (56.47) | 60 (63.16) | 38 (57.58) | 39 (59.09) | ||
| IIa | 23 (27.06) | 15 (15.79) | 17 (25.76) | 11 (16.67) | ||
| IIb | 6 (7.06) | 14 (14.74) | 6 (9.09) | 10 (15.16) | ||
| III | 5 (5.88) | 4 (4.21) | 3 (4.54) | 4 (6.05) | ||
| IV | 3 (3.53) | 2 (2.10) | 2 (3.03) | 2 (3.03) | ||
| Adjuvant treatment, n (%) | 15 (17.65) | 18 (18.95) | 0.482 | 11 (16.67) | 12 (18.18) | 0.783 |
| Chemotherapy | 12 (14.12) | 8 (8.42) | 8 (12.12) | 6 (9.09) | ||
| Radiation Therapy | 12 (14.12) | 16 (16.84) | 9 (13.64) | 11 (16.67) | ||
Abbreviations IQR Interquartile range, RATS Robot-assisted thoracoscopic surgery, VATS video-assisted thoracoscopic surgery, COPD Chronic obstructive pulmonary disease, BMI Body mass index
Fig. 1.
Flowchart demonstrating patient inclusion and exclusion criteria. RATS, Robot-assisted thoracoscopic surgery; VATS, Video-assisted thoracoscopic surgery
After PSM, the number of patients in each group was reduced to 66. All the baseline data, such as sex distribution (P = 0.384) and tumor size (P = 0.689), were comparable between the two groups. All further statistical analyses were performed on this population.
Surgical outcomes
The operative characteristics and postoperative outcomes are shown in Table 2. Most patients underwent surgery via a transthoracic approach (n = 54, 81.82% vs. n = 57, 86.36%, P = 0.377) and underwent R0 resection (n = 65, 98.48% vs. n = 65, 98.48%, P = 1.000) in both groups. One patient in each group had a microscopically positive pericardial margin. Significant reductions in the operative time (median: RATS, 100.00 vs. VATS, 120.00 min; P = 0.039) and estimated blood loss (median: RATS, 40.00 vs. VATS, 50.00 mL; P = 0.011) were observed in the RATS group. And no difference in the proportion of patients requiring chest tube insertion was observed between the two cohorts (83.33% vs. 80.30%; P = 0.822).
Table 2.
Surgical outcomes of the matched RATS and VATS groups
| Variables | RATS group | VATS group | P-value |
|---|---|---|---|
| (n = 66) | (n = 66) | ||
| Mini-invasive laterality, n (%) | 0.377 | ||
| Right side | 30 (45.46) | 38 (57.58) | |
| Left side | 24 (36.36) | 19 (28.78) | |
| Sub-xyphoid | 12 (18.18) | 9 (13.64) | |
| Operative time (min), median (IQR) | 100.00 (80.00, 120.00) | 120.00 (90.00, 140.00) | 0.039 |
| Intraoperative blood loss (ml), median (IQR) | 40.00 (20.00, 50.00) | 50.00 (20.00, 100.00) | 0.011 |
| Conversion, n (%) | 2 (3.03) | 10 (15.15) | 0.030 |
| R0 resection, n (%) | 65 (98.48) | 65 (98.48) | 1.000 |
| Concomitant resection, n (%) | 6 (9.09) | 8 (12.12) | 0.464 |
| Lung | 3 (4.55) | 6 (9.09) | |
| Pericardium | 4 (6.06) | 5 (7.58) | |
| Innominate vein | 3 (4.55) | 1 (1.52) | |
| vena cava | 1 (1.52) | 0 | |
| Without chest tube, n (%) | 11 (16.67) | 13 (19.70) | 0.822 |
| Duration of chest tube (days), median (IQR) | 3.00 (3.00, 5.00) | 3.00 (2.00, 4.00) | 0.073 |
| Total drainage volume (ml), median (IQR) | 550.00 (380.00, 920.00) | 460.00 (240.00, 700.00) | 0.015 |
| Post-operative complications, n (%) | 5 (7.58) | 4 (6.06) | 0.987 |
| Myasthenia crisis | 2 (3.03) | 2 (3.03) | |
| Pneumonia | 1 (1.52) | 1 (1.52) | |
| Pneumothorax | 2 (3.03) | 1 (1.52) | |
| Arrhythmia | 1 (1.52) | 1 (1.52) | |
| Clavien-Dindo grade | 0.901 | ||
| I-II | 2 (3.03) | 2 (3.03) | |
| III-IV | 3 (4.55) | 2 (3.03) | |
| Hospital stay (days), median (IQR) | 6.00 (3.00, 10.00) | 6.00 (4.00, 10.00) | 0.454 |
| 30-day mortality | 0 (0) | 0 (0) | - |
| Total costs (¥), median (IQR) | 68,122.00 (61,287.00, 79,467.00) | 37,886.00 (31,281.00, 46,974.00) | < 0.001 |
Abbreviations IQR Interquartile range, RATS Robot-assisted thoracoscopic surgery, VATS Video-assisted thoracoscopic surgery
Notably, ten patients needed conversion in the VATS group, whereas only 2 patients in the robotic group required conversion to open surgeries (RATS, 3.03% vs. VATS, 15.15%; P = 0.030). Specifically, unplanned conversions were performed on one and six patients in the RATS and VATS groups, respectively, due to intraoperative haemorrhage. The other patient in the RATS group had superior vein cava (SVC) involvement, and the tumor also infiltrated in the right innominate vein and was completely removed after conversion to a sternotomy. In three patients in the VATS group with suspected left innominate vein invasion, we made an effort to separate the tumor from the left innominate vein using thoracoscopy, but the attempt failed. Therefore, sternotomies were conducted as a preventive measure against unexpected major internal bleeding. In the remaining patients, VATS was initially performed. Due to the large tumor diameter that hindered safe dissection; subsequently, an elective transition to open thoracotomy was undertaken to improve surgical visualization and facilitate specimen removal.
The concomitant en bloc resection of adjacent structures with the thymus in the matched population with thymic neoplasms was comparable in both groups (9.09% vs. 12.12%; P = 0.464). The pericardium and lung are two of the most resected structures en bloc with the tumor. In the robotic group, three patients required concomitant resection of the innominate vein compared to only one patient in the VATS group.
In the early postoperative period, patients in the RATS group had a larger pleural drainage volume (median: 550.00 vs. 460.00 ml; P = 0.015). The duration of chest tube use appeared to be longer in the RATS group than in the VATS group; however, this difference was not statistically significant (IQR: 3.00–5.00 vs. 2.00–4.00 days; P = 0.073). Although no 30-day mortality occurred after surgery in either group, two patients in each group developed myasthenic crisis. Myasthenic symptom aggravation was managed by large-dose and short-course hormone pulse therapy, intravenous injection of immunoglobulin, and best supportive care. No significant differences were identified in postoperative complications (7.58% vs. 6.06%; P = 0.987), the Clavien–Dindo classification (I–II 3.03% vs. 3.03%; III–IV 4.55% vs. 3.03%; P = 0.901), or the duration of hospital stay (median: 6.00 vs. 6.00 days; P = 0.454) between the two groups. Moreover, the median expense for RATS thymectomy was ¥68,122.00 (IQR ¥61,287.00—¥79,467.00), which was significantly greater than the cost for VATS (median: 37,886.00; P < 0.001).
Oncologic outcomes
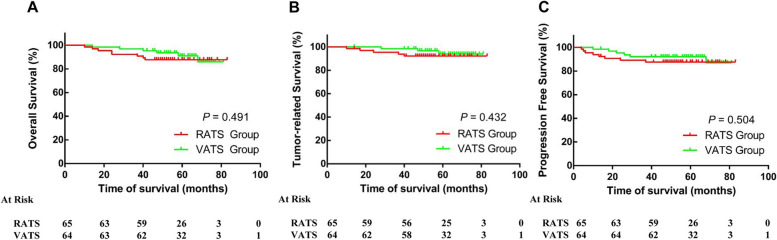
Oncologic follow-up data were available for 129 patients (97.73%), and there was no significant difference in the median follow‐up period between the two groups (54.0 vs. 57.0 months; P = 0.180). In the RATS group, three patients with thymoma died due to non-thymoma-related causes, including myasthenic crisis (which occurred more than half a year after surgery) and pneumonia caused by coronavirus infection. Five patients died from recurrence or progression of the disease. Among the six fatalities observed in the VATS group, three were due to tumor recurrence or progression, while one patient each experienced a myasthenic crisis, lung cancer, or suicide. Overall survival in the RATS and VATS groups is shown in Fig. 2A, with 5-year survival rates of 87.70% and 92.18%, respectively. The tumor-related survival rates in the RATS and VATS groups are shown in Fig. 2B, with 5-year tumor-related survival rates of 92.31% and 95.31%, respectively. In addition, the progression-free survival rates of patients with thymic epithelial tumors were similar between the RATS and VATS approaches (87.70% vs. 90.63%, Fig. 2C). The location of the tumor progression was heterogeneous and included the local recurrence at the previous thymectomy area (n = 2), parietal pleura (n = 9), lung parenchyma (n = 2), the liver (n = 1).
Fig. 2.
Long-term outcomes between robot-assisted thymectomy and video-assisted thymectomy. A. Overall survival. B. Tumor-related survival. C. Progression-free survival. RATS, Robot-assisted thoracoscopic surgery; VATS, Video-assisted thoracoscopic surgery
Multivariate analysis after PSM indicated tumor size ≥ 5 cm, WHO Classification B2 + B3 + thymic carcinoma, and Masaoka Stage IIb + III + IV as independent risk factors for worse tumor-related survival, rather than RATS or VATS (Table 3).
Table 3.
Univariate and multivariate analysis for tumor-related survival rate after PSM
| Variables | Univariate | P Value | Multivariate | P Value | ||
|---|---|---|---|---|---|---|
| HR | 95%CI | HR | 95%CI | |||
| Age ≥ 65 (vs. < 65 years) | 1.37 | 0.90–2.01 | 0.134 | |||
| Male (vs. female) | 1.21 | 0.83–1.79 | 0.451 | |||
| Myasthenia Gravis (vs. NA) | 1.75 | 1.02–2.77 | 0.017 | 1.41 | 0.89–2.69 | 0.148 |
| Tumor size ≥ 5 cm (vs. < 5 cm) | 2.21 | 1.07–3.17 | 0.003 | 2.17 | 1.04–3.12 | 0.001 |
| WHO Classification B2 + B3 + TC (vs. others) | 2.56 | 1.42–4.62 | < .001 | 2.60 | 1.43–4.69 | < .001 |
| Masaoka Stage IIb + III + IV (vs. others) | 2.90 | 1.89–3.82 | < .001 | 2.39 | 1.32–3.20 | < .001 |
| RATS (vs. VATS) | 1.54 | 0.78–2.52 | 0.215 | 1.43 | 0.79–2.13 | 0.191 |
Abbreviations IQR Interquartile range, RATS Robot-assisted thoracoscopic surgery, VATS Video-assisted thoracoscopic surgery, COPD Chronic obstructive pulmonary disease, BMI Body mass index
Discussion
Currently, minimally invasive surgery is extensively employed as a highly efficacious alternative to median sternotomy for the surgical management of thymic epithelial tumors, encompassing not only early-stage neoplasms, owing to its multiple advantages over open surgery. As the earliest applied and most typical minimally invasive approach, VATS thymectomy has been consistently associated with shorter operative time and hospital stay, reduced blood loss, and similar oncological results [4, 10]. However, 2D images of the operative field and physiologic tremors are recognized as inherent limitations of VATS and cannot be completely overcome by proficient operation skills or extensive experience. The implementation of robotic technology has marked a significant advancement in the progression of minimally invasive strategies. In a few previous studies, robotic surgery was believed to not only offer advantages during the perioperative period similar to those of VATS but also provide better visualization and meticulous dissection of remote-to-reach or narrow anatomical regions such as the mediastinum on account of its 3D vision and multiarticulate arm movement [11–13]. However, due to the lack of a direct comparison between the two approaches and significant heterogeneity in the baseline characteristics of the study population, no strong evidence has been shown thus far to demonstrate that these advantages offer superior outcomes compared with those of VATS. Furthermore, most published research studies have primarily focused on the perioperative period, with limited evidence available to support long-term outcomes in thymic epithelial tumors. Therefore, we collected relative data and conducted a propensity score matching analysis to explore and compare the clinical efficacy of RATS and VATS in patients with thymic epithelial tumors.
In the present study, we investigated the perioperative and long-term outcomes of 132 patients with thymomas who underwent minimally invasive thymectomy. With a definite trend towards reductions in the operative time and blood loss, comparable low postoperative complications and zero mortality. We concluded that robotic thymectomy shows more favourable results during the perioperative stage than VATS. In terms of long-term survival, RATS can also achieve results comparable to those of video-assisted procedures.
The total operative time was shorter in the RATS group than in the VATS group, which contradicts previous findings. Rowse et al. reported that the operative time for VATS was much shorter than that for RATS [14], and the same results were obtained by E et al. [15]. Additionally, in a comprehensive systematic review encompassing 11 studies, the operative time was similar between the two groups, with no statistically significant difference between RATS and VATS thymectomy [16]. We speculated that the primary cause of the discrepancy was a complex operative setup, and some patients were still in the early stage of the inevitable learning curve. Furthermore, there is inconsistency in the definition of operative duration across studies as well [17]. Since 2015, RATS thymectomy has been gradually performed in our centre. Therefore, at the onset of this study, more than 40 patients underwent RATS thymectomy, surpassing the robotic learning curve threshold.
It is also important to mention that the VATS group exhibited greater intraoperative blood loss than did the RATS group, which is consistent with findings from previous studies [6, 18]. This can be primarily attributed to its ability to provide surgeons with an enhanced surgical field, particularly in terms of precise localization of the internal mammary veins and innominate veins compared to its counterpart. Based on our experience, advanced optics and precise instrument control, which enable accurate exposure of the complex anatomy around the resection target and facilitate more precise and agile dissection, have significantly increased safety and reduced blood loss.
The high-risk patients for conversion to sternotomy in present study were those with vessel invasion, such as the innominate vein and superior vena cava, or unexpected hemorrhage. This finding is consistent with our earlier study as well [6]. The precise and meticulous mode of operation in RATS contributes significantly to its low incidence of conversion to sternotomy, thus providing an additional advantage. Considering the proficient operation experience and skills acquired by our surgical team through the performance of numerous VATS thymectomies prior to this study, the relatively higher conversion rates in the present study may suggest that there are certain technical limits associated with conventional thoracoscopy during the dissection process. In the 2D view of the operative field and under physiological hand tremor, the upper mediastinum, which has vulnerable large vessels and nerves, becomes a delicate and difficult-to-dissect area [19]. Unfortunately, the fundamental improvement of these inherent defects remains challenging despite the accumulation of experience and advancements in surgical skills. Robotic thymectomy overcomes some of the limitations of VATS, including its magnified 3D view, flexible and fine instrument control, and excellent visualization for exposing the bound ary between the tumor and healthy tissue, and it is advantageous for the dissection of high-risk areas, such as upper mediastinal structures and adjacent areas of the innominate vein.
The potential benefit of RATS thymectomy in terms of postoperative recovery has already been observed in patients with thymomas. Ye et al. analysed the perioperative outcomes and reported that the duration of pleural drainage and length of hospital stay were significantly greater in the VATS group than in the RATS group [20]. However, the postoperative outcomes of patients in both the VATS and RATS groups in the current study were similar. Even the duration of chest tube placement and total drainage volume appeared to be more favourable in the VATS group. We speculated that this discrepancy might be attributed to a greater proportion of patients in the RATS group requiring vessel-related concomitant resection, which implies additional intraoperative manipulations and dissections. Further research is warranted to validate this speculation.
Because of the indolent nature of thymic epithelial tumors and the relatively recent adoption of the robot-assisted surgery in many centers, there are relatively limited long-term data regarding disease progression and overall survival after RATS or VATS thymectomy. In the present study, locoregional recurrence and distal metastasis occurred in 12.3% of patients treated with RATS thymectomy and in 9.37% of patients who underwent VATS thymectomy, with no statistically significant differences. A lower rate of disease progression was observed by Sicolo et al. in their national multicenter study analysing the outcomes of thymic neoplasms, with only nine (4.3%) patients experiencing recurrence after RATS thymectomy [21]. Similarly, Marulli and his colleagues published a retrospective study that described encouraging oncological outcomes, with a single recurrence (0.7%) [19]. In addition, in a cohort of 669 patients who underwent robotic thymectomy for thymoma, with a median follow-up duration of 29 months, only 21 recurrences (3.4%) of thymoma recurrence was reported [22]. Compared to our study, the robotic approach appears to achieve more favourable long-term survival and extremely low recurrence rates based on these findings. The observed discrepancy could be attributed to the predominant presence of high-risk histological classifications (type B2, B3 and thymic carcinoma), as well as the longer follow-up time in this particular study. Kang et al. reported the recurrence and long-term survival of patients with thymic epithelial tumors who underwent robotic thymectomies, revealing that the 5-year recurrence-free survival rate was 93.9% for thymomas and 79.4% for thymic carcinomas [23]. Recently, Marcuse et al. conducted a retrospective follow-up study to investigate long-term oncologic outcomes and reported a recurrence rate of 9.1%, which is consistent with our current study [24]. In terms of long-term outcomes of VATS, Li et al. also reported long term results of 150 cases with VATS thymectomy, and the 5- and 10-year RFS rates for entire group were 96.5% and 94.4%, respectively [25]. Therefore, we believe that the recurrence rate is not greater than the historical outcomes of RATS and VATS thymectomy. In terms of overall survival and tumor-related survival, the analysis of oncological outcomes in our study revealed that VATS and robotic surgery yielded similar results, which are comparable to those of previous studies.
In our study, the total hospitalization costs associated with RATS were significantly greater than those associated with VATS. Therefore, it is necessary to evaluate the cost-effectiveness of RATS over VATS for thymic epithelial tumors from the perspective of health care providers. Imielski and his colleagues conducted a preliminary investigation into this issue and revealed that RATS and VATS might be equally cost-effective surgical approaches for patients with mediastinal masses [26]. The primary reason for this might be that in most western countries, the longer a surgical procedure lasts, the higher the operating room cost will be. However, it seems that the operating time does not have a noticeable effect on the cost of operating rooms in the current Chinese healthcare system. Given that the Price Department of the local government establishes and fixes the cost of a specific procedure, it appears that the duration of operation under realistic circumstances does not have an apparent impact on the price. The primary determinants influencing the likelihood of selecting robotic surgery are the expenses associated with surgical instruments and consumables for robots [27, 28]. It is evident that mitigating these costs holds the key to alleviating financial strain on patients' families during this stage. The expenses associated with the acquisition and maintenance of da Vinci robot equipment are evenly distributed across surgical procedures. With the increased utilization of robotic technology, there will be a proportional reduction in the allocation of costs related to robots for each individual surgical procedure. Therefore, we anticipate that the cost-effectiveness of RATS will be significantly improved with the socioeconomic development, the establishment of a more sound health-insurance policy and the increased utilization of robotic system.
The major limitations of the current study include its retrospective design, a small sample size, and being conducted at a single institution. Although PSM was performed to improve the comparability between the two groups, the results might be affected by the presence of incomplete data and selection bias due to the nonrandomized allocation of patients to the two groups. Secondly, the resection of thymic epithelial tumors with RATS did not begin in our institution until 2016. Besides the incidence rate of thymus epithelial tumor is relatively low. Constrained by these unfortunate factors, although median follow-up time of this study was almost 5 years, it was not long enough considering that 10 years of follow-up is mandatory for thymoma. Therefore, ongoing follow-up is required to assess the accuracy of long-term outcomes.
Conclusion
RATS thymectomy, as a safe and effective procedure, offers some advantages over VATS thymectomy in terms of short-term outcomes for patients with thymic epithelial tumors. From the oncological point of view, long term oncological follow up, whilst still non-sufficient for thymoma, the robotic approach achieved favourable survival and low recurrence rates comparable to those of traditional thoracoscopic procedures. Therefore, RATS thymectomy might be considered an effective alternative surgical approach for treating thymic epithelial tumors.
Acknowledgements
The authors are indebted to all of the donors whose names were not included in the author list but who participated in our study.
Abbreviations
- CT
Computed tomography
- IQRs
Interquartile ranges
- MG
Myasthenia gravis
- PSM
Propensity score matching
- RATS
Robot-assisted thoracoscopic surgery
- SD
Standard deviation
- SVC
Superior vena cava
- VATS
Video-assisted thoracoscopic surgery
- WHO
World Health Organization
Authors’ contributions
Conception and design: Bin Jiang, Long-fei Zhu; Perform research: Bin Jiang, Long-fei Zhu, Ling-min Zhang; Data collection: Long-fei Zhu, Ling-min Zhang, Chun-jian Zuo; Data analysis and interpretation: Long-fei Zhu, Chun-jian Zuo, Nian Cheng; Manuscript writing: All authors; Manuscript revision: Bin Jiang, Long-fei Zhu. Final approval of manuscript: All authors.
Funding
None.
Data availability
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Institutional review board approval from the Ethics Committee of Daping Hospital (Ratification No. 2023(101)) was obtained for this analysis. The requirement for informed consent was waived by the Ethics Committee of Daping Hospital because of the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bin Jiang, Email: ray_miller@126.com.
Nian Cheng, Email: xyx8@tmmu.edu.cn.
References
- 1.Burt BM, Yao X, Shrager J, Antonicelli A, Padda S, Reiss J, et al. Determinants of complete resection of thymoma by minimally invasive and open thymectomy: analysis of an international registry. J Thorac Oncol. 2017;12(1):129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang CH, Hwang Y, Lee HJ, Park IK, Kim YT. Robotic thymectomy in anterior mediastinal mass: propensity score matching study with transsternal thymectomy. Ann Thorac Surg. 2016;102(3):895–901. [DOI] [PubMed] [Google Scholar]
- 3.Marulli G, Comacchio GM, Schiavon M, Rebusso A, Mammana M, Zampieri D, et al. Comparing robotic and trans-sternal thymectomy for early-stage thymoma: a propensity score-matching study. Eur J Cardiothorac Surg. 2018;54(3):579–84. [DOI] [PubMed] [Google Scholar]
- 4.Weng W, Li X, Meng S, Liu X, Peng P, Wang Z, et al. Video-assisted thoracoscopic thymectomy is feasible for large thymomas: a propensity-matched comparison. Interact Cardiovasc Thorac Surg. 2020;30(4):565–72. [DOI] [PubMed] [Google Scholar]
- 5.Li G, Chang H, Wang Z, He D, Qu L, Shao Q, et al. Effect of open versus video-assisted thoracoscopy on perioperative outcomes and survival for cases of thymic carcinomas and thymic neuroendocrine tumors. World J Surg Oncol. 2023;21(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu LF, Zhang LM, Zuo CJ, Sun TY, Jiang B. Robot versus video-assisted thoracoscopic thymectomy for large thymic epithelial tumors: a propensity-matched analysis. BMC Surg. 2023;23(1):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Multidisciplinary Committee of Oncology, Chinese Physicians Association. Chinese guideline for clinical diagnosis and treatment of thymic epithelial tumors. Zhonghua Zhong Liu Za Zhi. 2021;43(4):395–404 Chinese. [DOI] [PubMed] [Google Scholar]
- 9.Xu C, Zhang Y, Wang W, Wang Q, Li Z, Song Z, et al. Chinese expert consensus on the diagnosis and treatment of thymic epithelial tumors. Thorac Cancer. 2023;14(12):1102–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agatsuma H, Yoshida K, Yoshino I, Okumura M, Higashiyama M, Suzuki K, et al. Video-assisted thoracic surgery thymectomy versus sternotomy thymectomy in patients with thymoma. Ann Thorac Surg. 2017;104(3):1047–53. [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Zhang X, Jin R, Xiang J, Han D, Zhang Y, et al. Robot-assisted thoracoscopic surgery for mediastinal masses: a single-institution experience. J Thorac Dis. 2020;12(2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nabe Y, Inoue M, Yoshida J. Perspectives on surgical treatment for thymic epithelial tumors: a narrative review. Gland Surg. 2024;13(2):225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XK, Xu Y, Cong ZZ, Zhou H, Wu WJ, Shen Y. Comparison of the progression-free survival between robot-assisted thymectomy and video-assisted thymectomy for thymic epithelial tumors: a propensity score matching study. J Thorac Dis. 2020;12(8):4033–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowse PG, Roden AC, Corl FM, Allen MS, Cassivi SD, Nichols FC, Shen KR, et al. Minimally invasive thymectomy: the Mayo Clinic experience. Ann Cardiothorac Surg. 2015;4(6):519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.E H, Yang C, Zhang L, Xia L, Xu L, Song N, et al. Perioperative outcomes comparison of robotic and video-assisted thoracoscopic thymectomy for thymic epithelial tumor: a single-center experience. Updates Surg. 2024;76(4):1511–9. [DOI] [PubMed]
- 16.Shen C, Li J, Li J, Che G. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for treatment of patients with thymoma: A systematic review and meta-analysis. Thorac Cancer. 2022;13(2):151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Sullivan KE, Kreaden US, Hebert AE, Eaton D, Redmond KC. A systematic review of robotic versus open and video assisted thoracoscopic surgery (VATS) approaches for thymectomy. Ann Cardiothorac Surg. 2019;8(2):174–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg. 2011;141(3):673–7. [DOI] [PubMed] [Google Scholar]
- 19.Marulli G, Maessen J, Melfi F, Schmid TA, Keijzers M, Fanucchi O, et al. Multi-institutional European experience of robotic thymectomy for thymoma. Ann Cardiothorac Surg. 2016;5(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye B, Tantai JC, Li W, Ge XX, Feng J, Cheng M, et al. Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery in the surgical treatment of Masaoka stage I thymoma. World J Surg Oncol. 2013;17(11):157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sicolo E, Zirafa CC, Romano G, Brandolini J, De Palma A, Bongiolatti S, et al. National multicenter study on the comparison of robotic and open thymectomy for thymic neoplasms in myasthenic patients: surgical, neurological and oncological outcomes. Cancers (Basel). 2024;16(2):406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comacchio GM, Schiavon M, Zirafa CC, De Palma A, Scaramuzzi R, Meacci E, et al. Robotic thymectomy in thymic tumours: a multicentre, nation-wide study. Eur J Cardiothorac Surg. 2024;65(5):ezae178. [DOI] [PubMed] [Google Scholar]
- 23.Kang CH, Na KJ, Park S, Park IK, Kim YT. Long-term outcomes of robotic thymectomy in patients with thymic epithelial tumors. Ann Thorac Surg. 2021A;112(2):430–5. [DOI] [PubMed] [Google Scholar]
- 24.Marcuse F, Hochstenbag M, De Baets MHV, Bootsma G, Maat APWM, Hoeijmakers JGJ, et al. Robotic thymectomy for thymomas: a retrospective follow-up study in the Netherlands. Ann Thorac Surg. 2022;114(5):1886–94. [DOI] [PubMed] [Google Scholar]
- 25.Li JF, Hui BG, Li X, Xiao RX, Jiang GC, Liu J, Wang J. Video-assisted thoracic surgery for thymoma: long-term follow-up results and prognostic factors-single-center experience of 150 cases. J Thorac Dis. 2018;10(1):291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imielski B, Kurihara C, Manerikar A, Chaudhary S, Kosterski S, Odell D, et al. Comparative effectiveness and cost-efficiency of surgical approaches for thymectomy. Surgery. 2020;168(4):737–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D, Kang P, Tao S, Li Q, Wang R, Tan Q. Cost-effectiveness evaluation of robotic-assisted thoracoscopic surgery versus open thoracotomy and video-assisted thoracoscopic surgery for operable non-small cell lung cancer. Lung Cancer. 2021;153:99–107. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Huang Z, Mei H, Rong L, Zhou Y, Guo J, et al. Cost-effectiveness analysis of robot-assisted laparoscopic surgery for complex pediatric surgical conditions. Surg Endosc. 2023;37(11):8404–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.