Abstract
The close spatial and temporal connection between osteogenesis and angiogenesis around type H vasculature is referred as “osteogenesis-angiogenesis coupling”, which is one of the basic mechanisms of osteogenesis. Endothelial cells (ECs), bone marrow mesenchymal stem cells (BMSCs), and their specific lineage constitute important cluster that participate in the regulation of osteogenesis and angiogenesis in bone microenvironment. However, the regulatory mechanism of osteogenesis-angiogenesis coupling under the condition of bone healing has not been unveiled. In this study, we demonstrated that the exosome derived from ECs (EC-exo) is an initiator of type H blood vessels formation, and EC-exo acts as a mediator in orchestrating osteogenesis-angiogenesis coupling by enhancing BMSC osteogenic differentiation and EC angiogenesis both in monolayer and stereoscopic co-culture system of primary human cells. The transcriptome array indicated that zinc finger and BTB domain containing 16 (ZBTB16) is a key gene in EC-exo-mediated osteogenesis, and ZBTB16 is indispensable in EC-exo-initiated osteogenesis-angiogenesis coupling. Mechanistically, EC-exo up-regulated the expression of ZBTB16 in BMSCs, thereby promoting osteoprogenitor phenotype transformation; the osteoprogenitors further promote ECs which constitute type H vessel (H-ECs) generation by activating HIF-1α pathway; and the H-ECs conversely promotes osteogenic differentiation of BMSCs. The crosstalk between BMSCs and ECs triggered by EC-exo constitutes a positive feedback loop that enhances osteogenesis-angiogenesis coupling. This study demonstrates that EC-exo can become an effective therapeutic tool to promote bone regeneration and repair.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-03002-5.
Keywords: Osteogenesis-angiogenesis coupling, Endothelial cell derived exosomes, Type H blood vessel, Human bone marrow mesenchymal stem cell, Human umbilical vein endothelial cell, ZBTB16, Bone regeneration and repair
Introduction
Bone is highly vascularized. In addition to the common consensus dynamic balance between osteoblasts and osteoclasts, blood vessels in bone tissue are also indispensable in maintaining bone homeostasis, which plays a key role in bone development, remodeling, and regeneration [1]. Vascular networks are densely and complex distributed in bone, they provide essential nutrients for bone tissue and discharge metabolic waste, providing a favorable and stable microenvironment for bone homeostasis [2]. Both bone tissue and vessels have endocrine functions, which can regulate the adjacent or distal tissues or organs, especially the bidirectional promoting effect between each other [3, 4]. During the regeneration process after bone injury, the process of osteogenesis and angiogenesis will exhibit a close spatiotemporal coordination [5]. In bone development and injury repair, the close spatial and temporal connection between osteogenesis and angiogenesis is referred as “osteogenesis-angiogenesis coupling”, which actively directs bone formation by producing factors that relate to proliferation and differentiation of osteoprogenitors [6].
In the process of bone regeneration or remodeling, bone marrow mesenchymal stem cells (BMSCs) mobilize, recruit, and differentiate into osteoblasts, which are the cellular basis for bone development and regeneration [7]. At the same time, vascular endothelial cells (ECs) are also recruited and rapidly form vascular structures to transport necessary nutrients and oxygen, discharge metabolic waste, and provide a dynamic niche for bone regeneration and homeostasis maintenance, constructing an favorable niche for osteogenesis [8]. Since the osteogenic differentiation ability of BMSCs is strictly regulated by multiple factors that rely on vascular transportation, including hormones, cytokines, and metabolite, the regulation of osteogenesis by vascularization is particularly important [9]. Type H blood vessels, which located near the growth plate in the metaphysis and both the periosteum and endosteum of the diaphysis [6], are the most prominent vascular structure with specific functions associated with osteogenesis [10]. They are densely surrounded by osteoprogenitor cells expressing the transcription factor OSX, a potent promoter of bone formation [11, 12], and the specific cellular structure of type H vessels facilitates the vigorous intracellular crosstalk that regulates bone homeostasis. Type H vessels can actively guide bone formation by producing factors that stimulate the proliferation and differentiation of osteoprogenitor cells, and the molecular interactions between BMSCs and endothelial cells can also promote angiogenesis, thus forming a unique “osteogenesis-angiogenesis coupling” mode with bidirectional regulatory effects [11–13].
The ECs which constitute type H blood vessel (H-ECs) have dynamic relation to osteogenesis in repairing processes [14]. In the early stage after bone injury, the damaged area is enveloped by hematoma to form a hypoxic microenvironment. Chronic hypoxia activates the HIF-1α pathway in H-ECs and promotes their proliferation, making abundant type H blood vessels widely distributed throughout the injury area [15, 16]. It is well demonstrated that H-ECs promote the proliferation and differentiation of osteoprogenitors and the maturation and hypertrophy of chondrocytes by secreting soluble protein NOG [15]. In turn, osteoprogenitor cells, osteocytes, and chondrocytes can guide the sprouting and formation of blood vessels, as well as the generation of H-ECs through the abundant secretion of SLIT3 and VEGF [6]. Overall, osteogenic and vascular growth factors and pathways in BMSCs and H-ECs control the interaction between osteogenesis and angiogenesis. In addition to the above-mentioned secreted proteins, exosome is another important mediator for intercellular communication [17]. Although significant progresses have made in the field of exosome-based bone regeneration, the regulatory roles of exosomes in type H vessel generation and the underlying mechanisms associated to osteogenesis and angiogenesis coupling are not fully clarified.
Exosomes are extracellular vesicles that range 30–150 nm in diameters and contain nucleic acids, proteins, and lipids [18]. They are vital bioinformation carrier that play a significant role in intracellular crosstalk as signaling messenger [18, 19]. Previous studies have shown that exosomes derived from mesenchymal stem cells accelerate bone repair by enhancing angiogenesis [20, 21]. Recent studies have further revealed that ECs also participate in the regulation of bone microenvironment and promote osteogenesis through exosomes [22–27]. Most studies mainly discussed the solitary effects of exosomes on osteogenesis or angiogenesis, however, osteogenesis and angiogenesis often work in an integrated coupling manner, fully demonstrating of the exosome-mediated effects and the mechanisms on cell coupling is important as well. Therefore, it is essential to elucidate the key exosome that specifically regulates the content or activity of H-ECs, thereby enhancing the osteogenesis-angiogenesis coupling, which will help guide interventions and therapies for bone regeneration and repair.
In this study, we demonstrated that among the candidate exosome mediators, the exosome derived from ECs (EC-exo) is a key initiator of type H blood vessels formation and a mediator in orchestrating osteogenesis-angiogenesis coupling. Mechanistically, EC-exo upregulates the expression of zinc finger and BTB domain containing 16 (ZBTB16) in BMSCs, thereby promoting OSX+ osteoprogenitor phenotype transformation. The osteoprogenitors further promote H-ECs generation by activating HIF-1α pathway in ECs. And in turn, the H-ECs promotes osteogenic differentiation of BMSCs. The crosstalk between BMSCs and ECs triggered by EC-exo induces a positive feedback loop and enhancing osteogenesis-angiogenesis coupling. As the key exosome that regulates of osteogenesis-angiogenesis coupling, EC-exo can become an effective therapeutic tool to promote bone regeneration and repair.
Results
BMSCs and ECs formed osteogenesis-angiogenesis coupling in the co-culture system
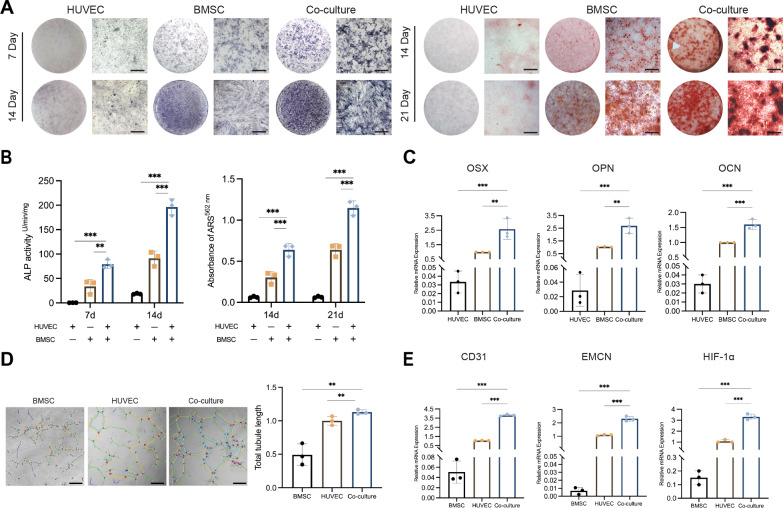
Previous studies have mostly focused on the coupling between osteoblasts and H-ECs [6, 11, 12]. Here we traced whether there is the same coupling effect between BMSCs and ECs. After confirming the optimal co-culture ratio (Fig. S1) [28], the primary human BMSCs (hBMSCs) and human umbilical vein ECs (HUVECs) were cultured exclusively or co-cultured in a 1:1 ratio system. First, the osteogenic ability of hBMSCs was evaluated in co-culture system. alkaline phosphatase (ALP) staining and quantitative showed that co-culture significantly increased the ALP activity of hBMSCs at the 7th and 14th days of osteogenic induction compared with hBMSCs independently (Fig. 1A, B). Alizarin red S (ARS) staining and calcium content assay detected that the mineralization level of hBMSCs at the 14th and 21st days of osteogenic induction was significantly increased in co-culture system (Fig. 1A, B). Real-time quantitative polymerase chain reaction (RT-qPCR) of osteogenesis related genes also showed the same results, that is, compared with hBMSCs independently, the expression of OSX, OPN and OCN was significantly up-regulated after 7 days of osteogenic induction in co-culture system (Fig. 1C).
Fig. 1.
The hBMSCs and HUVECs co-culture system improves osteogenic differentiation potential and induces CD31hi EMCNhi endothelium formation. A Representative images of ALP and ARS staining in hBMSCs, HUVECs and co-culture system after osteogenic induction. B ALP quantitative and relative mineralization in hBMSCs, HUVECs and co-cultured system after osteogenic induction. C Relative mRNA expression of OSX, OPN and OCN in hBMSCs, HUVECs and co-culture system after osteogenic induction. D Representative images and relative quantification of tubule branches length in hBMSCs, HUVECs and co-culture system. The tubules are marked with lines. Scale bar represents 200 μm. E Relative mRNA expression of CD31, EMCN and HIF-1α in hBMSCs, HUVECs and co-culture system after osteogenic induction. The statistical data are represented as the mean ± SD. n = 3, *p < 0.05, **p < 0.01, ***p < 0.001
Meanwhile, the angiogenic ability of HUVECs was evaluated in co-culture system. According to the tubule formation experiment, the number of capillary-like hollow structures and the length of tubule branches in the co-culture group were significantly increased compared with HUVECs independently (Fig. 1D). Further, the phenotypic analysis of ECs by RT-qPCR showed that CD31, EMCN and HIF-1α, which are critical for H-ECs, was significantly increased after co-culture, which indicated that neovascularization in the co-culture system tends to H phenotype (Fig. 1E). These results suggested that the coupling of BMSCs and ECs has a bidirectional promoting effect on osteogenic differentiation and the increase of H-EC phenotype.
Characterization of EC‑exo and BMSC-exo
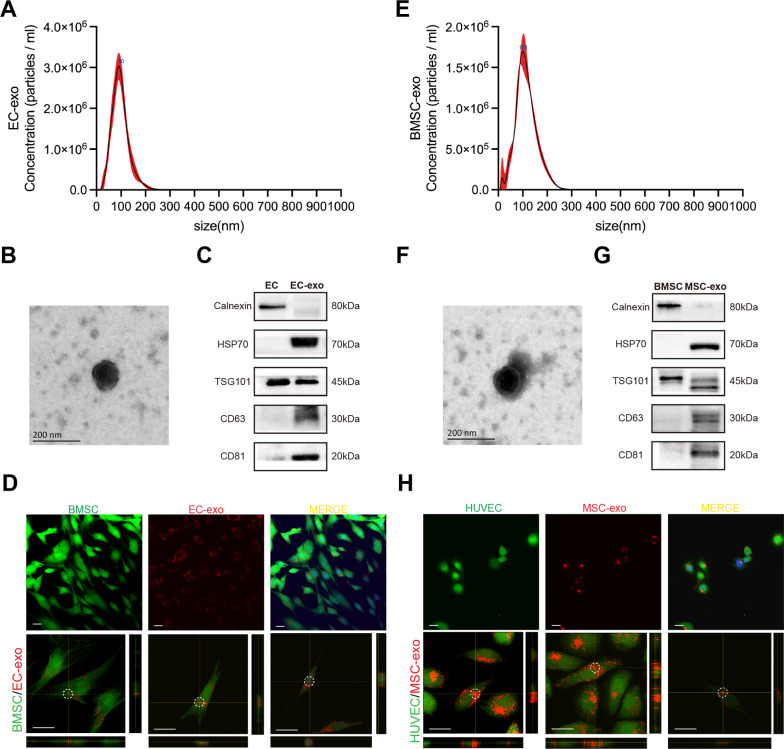
The morphology, size distribution, and specific surface markers of exosomes were characterized, and the internalizing ability of hBMSCs/HUVECs to EC-exo/BMSC-exo was verified. First, exosomes from hBMSCs/HUVECs were isolated by ultracentrifugation strategy [29]. Nanoparticle Tracking Analysis (NTA) was employed to measure the size distribution of exosomes (Fig. 2A, E). Then, the morphology of individual exosomes was observed using transmission electron microscopy (TEM). The isolated exosomes were saucer-shaped and had a double-layer structure (Fig. 2B, F). In addition, western blot was used to detect the presence of classical exosome markers (HSP70, TSG101, CD63, and CD81) and the absence of negative markers (Calnexin), with hBMSCs/HUVEC cells as controls (Fig. 2C, G). The above results showed that the isolated exosomes conform to the broad consensus of exosome [30]. Finally, the fluorescence images of exosomes internalized by recipient cells were taken with a Laser Confocal Microscope. It could be observed that the exosomes stained by DiI were fully taken up by recipient cells, most of which stayed in the cytoplasm, and a small part entered the nucleus (Fig. 2D, H).
Fig. 2.
Characterization of EC‑exo and BMSC-exo. A NTA results of EC-exo. B TEM image of EC-exo. C Exosome specific markers western blot result of EC-exo. D The fluorescence images of EC-exo internalized by hBMSCs, and the z-stack showing EC-exo (circle) within hBMSCs. E NTA results of BMSC-exo. F TEM image of BMSC-exo. G Exosome specific markers western blot result of BMSC-exo. (H) The fluorescence images of BMSC-exo internalized by HUVECs, and the z-stack showing MSC-exo (circle) within HUVECs
EC-exo is a key mediator in initiating osteogenesis-angiogenesis coupling
To investigate whether the osteogenesis-angiogenesis coupling between BMSCs and ECs is mediated via exosomes, the co-culture system of hBMSCs and HUVECs was treated with an inhibitor of exosome generation (GW4869). The results showed that, the osteogenic ability (Fig. 3A, B) and osteogenic markers of hBMSCs (Fig. 3C), as well as the angiogenic ability (Fig. 3D) and type H phenotype of HUVECs (Fig. 3E) were significantly inhibited by GW4869. Although GW4869 partially inhibited the osteogenic differentiation of BMSCs, when comparing BMSCs with or without GW4869 treatment and co-culture systems with or without GW4869 treatment, this difference was overwhelmed by the differences between the two co-culture systems (Fig. S2). This indicates that GW4869 has a more significant impact on the osteogenic process during the coupling of two types of cells, rather than solely inhibiting the osteogenic differentiation of BMSCs. These evidences suggest that, the osteogenesis-angiogenesis coupling effect of BMSCs and ECs is largely achieved through exosome.
Fig. 3.
EC-exo improves osteogenic differentiation potential and induces CD31hi EMCNhi endothelium formation in co-cultured hBMSCs and HUVECs. A Representative images of ALP staining and quantitative analysis in co-culture system after osteogenesis induction treated with GW4869, BMSC-exo or EC-exo. B Representative images of ARS staining and relative mineralization assay in co-culture system after osteogenesis induction treated with GW4869, BMSC-exo or EC-exo. C Relative mRNA expression of OSX, OPN and OCN in co-culture system after osteogenesis induction treated with GW4869, BMSC-exo or EC-exo. D Representative images and relative quantification of tubule branches length in co-culture system after osteogenesis induction treated with GW4869, BMSC-exo or EC-exo. The tubules are marked with lines. Scale bar represents 200 μm. E Relative mRNA expression of CD31, EMCN and HIF-1α in co-culture system after osteogenesis induction treated with GW4869, BMSC-exo or EC-exo. The statistical data are represented as the mean (SD). n = 3, *p < 0.05, **p < 0.01, ***p < 0.001
In order to further clarify whether exosome derived from mesenchymal stem cells (MSC-exo) or EC-derived exosome (EC-exo) dominated the coupling effect, supplemental MSC-exo or EC-exo were introduced into the co-culture system, respectively. It can be concluded from the results that both MSC-exo and EC-exo can significantly promote the osteogenic differentiation of hBMSCs and the angiogenesis of HUVECs in the co-culture system, and there is no significant difference between them (Fig. 3A–D). It is worth noting that EC-exo can significantly improve the H-EC markers and related gene expression (CD31, EMCN and HIF-1α), but MSC-exo has no contribute to this (Fig. 3E). The above results suggest that osteogenesis-angiogenesis coupling depends on the mediation of exosomes, and EC-exo is decisive in H phenotype angiogenesis of ECs in the co-culture system.
EC-exo enhances BMSCs osteogenic differentiation, and promotes ECs angiogenesis
To explore the effect of EC-exo on hBMSCs and HUVECs, EC-exo was applied to hBMSCs and HUVECs respectively, and its effects on proliferation, migration, osteogenic differentiation, and angiogenesis were detected. First, hBMSCs were treated with 20 μg/ml of EC-exo. CCK8 results showed that the number of hBMSCs after EC-exo treatment was significantly higher than that of the control group at 72 h, and the disparity widened further at 96 h (Fig. 4A). ALP and ARS staining showed that EC-exo treatment significantly increased the osteogenic differentiation ability of hBMSCs (Fig. 4B, C). Transwell assay revealed that more hBMSC cells migrated through the micropores after EC-exo treatment for 18 h (Fig. 4D). RT-qPCR revealed that the expression of osteogenic markers (OSX, OPN and OCN) was significantly up-regulated in hBMSCs treated with EC-exo (Fig. 4E). The above results indicated that EC-exo could directly promote the proliferation, migration, and osteogenic differentiation of hBMSCs.
Fig. 4.
EC-exo enhances hBMSCs osteogenic differentiation, and promotes HUVECs angiogenesis. A Proliferation of hBMSCs after osteogenesis induction treated with or without EC-exo. B Representative images of ALP staining and quantitative analysis of BMSCs after osteogenesis induction treated with or without EC-exo. C Representative images of ARS staining and relative mineralization assay of BMSCs after osteogenesis induction treated with or without EC-exo. D Representative images of BMSCs migration and the number of migrating cells after osteogenesis induction treated with or without EC-exo. Scale bar represents 200 μm. E Relative mRNA expression of OSX, OPN and OCN in BMSCs after osteogenesis induction treated with or without EC-exo. F Proliferation of HUVECs treated with or without EC-exo. G Representative images and relative quantification of wound healing assays of HUVECs with or without EC-exo, and the co-culture system with or without EC-exo. The edges of the wounds are marked with lines. Scale bar represents 200 μm. H Representative images and relative quantification of tubule branches length of HUVECs with or without EC-exo, and the co-culture system with or without EC-exo. The tubules are marked with lines. Scale bar represents 200 μm. I Relative mRNA expression of CD31, EMCN and HIF-1α in HUVECs with or without EC-exo, and the co-culture system with or without EC-exo. The statistical data are represented as the mean (SD). n = 3, *p < 0.05, **p < 0.01, ***p < 0.001
Meanwhile, the ability of EC-exo to promote HUVECs angiogenesis was also investigated. CCK8 results showed that the number of HUVECs after EC-exo treatment was significantly higher than that of the control group at 48 h, and the disparity widened further at 96 h (Fig. 4F). Wound healing experiments revealed that EC-exo treatment significantly enhanced the migration ability of HUVECs (Fig. 4G). The tube forming experiment showed that EC-exo treatment significantly promoted the angiogenic ability (Fig. 4H). After observing the positive effect of EC-exo on angiogenesis, RT-qPCR was used to detect whether HUVECs had the type H phenotype after EC-exo treatment. RT-qPCR results showed that although the expression of CD31 increased compared with the control group, the expression of the other two key genes of type H phenotype (HIF-1α and EMCN) showed no significant variation (Fig. 4I). It is worth noting that compared with pure HUVEC, these functions were enhanced after co-culture, and further significantly improved after the addition of EC-exo was added. The above results indicated that EC-exo could significantly enhance the angiogenic ability of HUVECs, but it did not contribute to the H-EC phenotype. These clues implied that although EC-exo promotes osteogenesis-angiogenesis coupling through independent effect on BMSCs or ECs, the type H vessel formation is more dependent on the BMSC-EC communication enhanced by EC-exo.
EC-exo promotes the osteogenesis-angiogenesis coupling in BMSC/EC constructs
In order to further verify the effect of EC-exo on osteogenesis-angiogenesis coupling, hBMSCs and HUVECs were applied to fabricate BMSC/EC construct to simulate the in vivo crosstalk between blood vessels and surrounding stem cells through a three-dimensional structure more similar to the osteogenic microenvironment in vivo (Fig. 5A). It could be clearly observed by light microscope that after being cultured in osteogenic conditions for 10 days, only vessel budding structures could be observed in cell spheres containing HUVECs independently, and no vascular network was formed. The cell spheres containing both hBMSCs and HUVECs formed obvious capillary-like structures. When 50 μg/ml EC-exo was introduced into the culture system, a longer length and more tightly connected vascular structure was formed (Fig. 5B). These results reiterated that the coupling between BMSCs and ECs was a critical factor in promoting angiogenesis, and EC-exo significantly enhance this effect. In addition to the angiogenic, the ability of EC-exo to promote osteogenesis was also evaluated. The results showed that EC-exo enhanced the ALP activity of hBMSCs (Fig. 5C). The phenotype of ECs was also detected. Immunofluorescence (IF) results showed that coupling with hBMSCs enhanced the expression of CD31 and EMCN in HUVECs to some extent. When EC-exo was introduced into the culture system, the vascular network formed was almost all composed of H-ECs (Fig. 5D). These results re-emphasized that EC-exo could promote osteogenesis-angiogenesis coupling of osteogenic BMSC/EC constructs and predispose ECs to a type H phenotype.
Fig. 5.
EC-exo induces CD31hi EMCNhi endothelium formation in BMSC/EC constructs. A Diagrammatic sketch of fabricating BMSC/EC construct. B Light microscopic appearance of BMSC/EC construct after 10 days of osteogenic culture and relative quantification of tubule branches length in constructs. Scale bar represents 200 μm. C Representative images of ALP staining of BMSC/EC construct after 10 days of osteogenic culture and relative quantification of ALP staining areas. Scale bar represents 200 μm. D Representative images of IF staining of BMSC/EC construct after 10 days of osteogenic culture for CD31 (green) and EMCN (red). 3D surface plot showing the fluorescence intensity. Histogram showing the quantitation of CD31 and EMCN immunostaining signal intensity. Scale bar represents 200 μm. The statistical data are represented as the mean ± SD. n = 3, *p < 0.05, **p < 0.01
ZBTB16 is a key gene in EC-exo-mediated osteogenesis
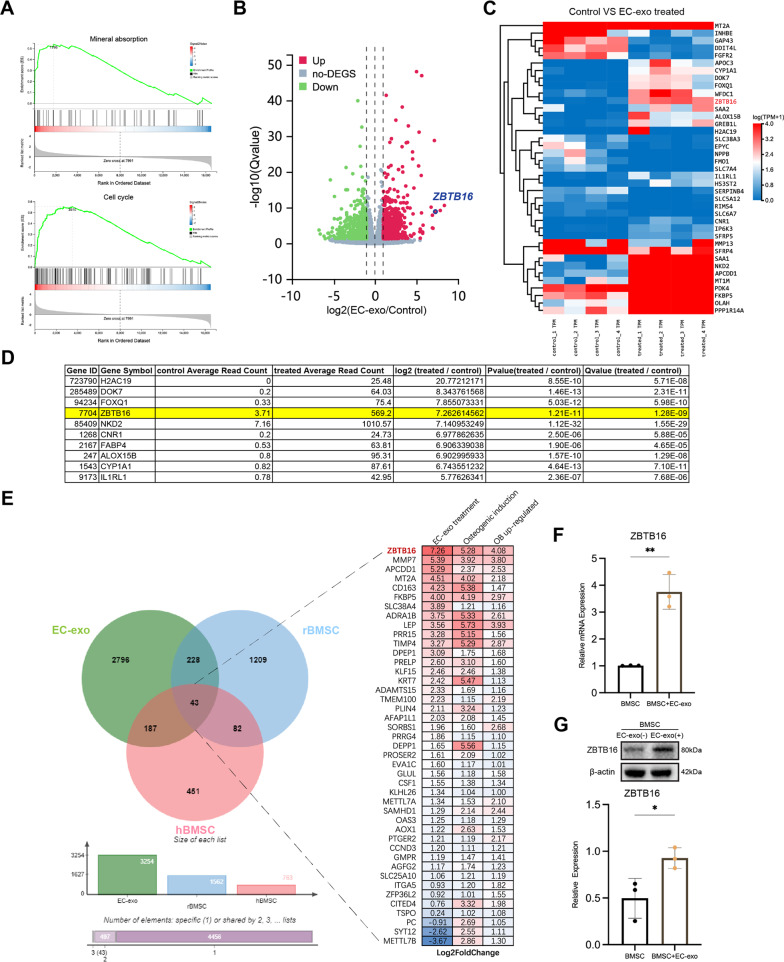
To determine the molecular mechanism by which EC-exo affects BMSCs, RNA-seq was administered to 4 hBMSCs treated with EC-exo and their corresponding untreated hBMSCs, then their transcriptome changes relative to untreated hBMSCs were analyzed. Gene Ontology (GO) enrichment analysis showed that some terms related to cell activity and cell–cell interaction were enriched (Fig. S3A). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis enriched some pathways related to cell activity or osteogenesis with significant differences, such as cell cycle, mineral absorption. (Fig. S3B). The Mineral Absorption pathway and Cell Cycle pathway were enriched by Gene Set Enrichment Analysis (GSEA) and found to be up-regulated by EC-exo treatment (Fig. 6A). The volcano diagram shows the up/down-regulated genes after EC-exo treatment (Fig. 6B). The results showed that 631 genes were up-regulated and 571 genes were down-regulated (|log2FC|≥ 1 and Qvalue < 0.05) in hBMSCs after EC-exo treatment (Table S1). The heatmap further clustered the genes with the largest up/down-regulated expression (|log2FC|≥ 4 and Q value < 0.01) (Fig. 6C). The top 10 up-regulated genes were listed in Fig. 6D. In order to investigate the effect of these differentially expressed genes on the osteogenic differentiation of BMSCs, RNA-seq analysis was performed on Sprague–Dawley (SD) rat BMSCs (rBMSCs) before and after osteogenic induction (Table S2), and it was compared with the RNA-seq data of hBMSCs before and after osteogenic induction published by Yu et al. [31]. It was found that 43 genes were significantly dysregulated in all groups (Fig. 6E, Table S3). Among these 43 genes, ZBTB16 was the most up-regulated in hBMSCs either after EC-exo treatment or osteogenic induction. Studies have demonstrated that ZBTB16 is a dominant component of limb and axial skeletal formation and an important osteogenic marker independent of BMP and Wnt pathways during the later stages of stem cell differentiation [32, 33]. The activation of its expression has a preventive effect on osteoporosis [31]. RT-qPCR and western blot results also confirmed that EC-exo treatment increased the expression level of ZBTB16 in hBMSCs (Fig. 6F, G). In summary, ZBTB16, which is up-regulated by EC-exo in hBMSCs, is a key gene in EC-exo mediated osteogenesis.
Fig. 6.
ZBTB16 is a key gene in EC-exo-mediated osteogenesis. A GSEA of Mineral Absorption pathway and Cell Cycle pathway in hBMSCs treated with or without EC-exo. NES, normalized enrichment score. FDR false discovery rate. B Volcano plot showing the differentially expressed genes in hBMSCs treated with or without EC-exo. C The heatmap displayed the expression ratios of different genes in hBMSCs treated with or without EC-exo. D The list showing top 10 up-regulated genes. E Venn diagram showing the intersection of significantly up-regulated genes in hBMSCs treated with EC-exo treatment (green), osteogenic induced rBMSCs (blue), and osteogenic induced hBMSCs (pink). F Relative mRNA expression of ZBTB16 in hBMSCs with or without EC-exo treatment. G Relative protein expression of ZBTB16 in hBMSCs with or without EC-exo treatment. The statistical data are represented as the mean ± SD. n = 3, *p < 0.05, **p < 0.01
ZBTB16 in BMSCs is indispensable in EC-exo-initiated osteogenesis-angiogenesis coupling
Based on transcriptome analysis, ZBTB16 was found to be a key factor promoting the osteogenic phenotype differentiation of hBMSCs. This section will focus on its impact on the coupling of hBMSCs and HUVECs. The expression of ZBTB16 in hBMSCs was knocked-down by siRNA and co-cultured with HUVECs. (Fig. S4) Then, EC-exo was introduced into the co-culture system as previously described. After 7 and 14 days of osteogenic differentiation induction, ALP staining and activity detection showed that ALP enzyme activity was significantly reduced after ZBTB16 was knocked-down, and it could not be reversed by EC-exo treatment (Fig. 7A). The ARS staining showed that after 14 and 21 days of osteogenic differentiation induction, the calcium content decreased with the knockdown of ZBTB16, which was also not improved by EC-exo treatment (Fig. 7B). RT-qPCR and western blot results showed that with the knockdown of ZBTB16, the expression levels of its downstream gene OSX also decreased significantly, and EC-exo treatment was also unable to rescue their expression (Fig. 7C, D). These results indicated that EC-exo promotes the differentiation of hBMSCs into OSX+ osteogenic phenotype through ZBTB16, thereby promoting the coupling between osteogenesis and angiogenesis.
Fig. 7.
ZBTB16 in hBMSCs is indispensable in EC-exo-initiated osteogenesis-angiogenesis coupling. A Representative images of ALP staining and quantitative analysis of co-cultured ZBTB16-knockdown hBMSCs and HUVECs after osteogenesis induction treated with or without EC-exo. B Representative images of ARS staining and relative mineralization assay of co-cultured ZBTB16-knockdown hBMSCs and HUVECs after osteogenesis induction treated with or without EC-exo. C Relative mRNA expression of ZBTB16 and OSX in co-cultured ZBTB16-knockdown hBMSCs and HUVECs after osteogenesis induction treated with or without EC-exo. D Relative protein expression of ZBTB16 and OSX in co-cultured ZBTB16-knockdown hBMSCs and HUVECs after osteogenesis induction treated with or without EC-exo. E Representative images and relative quantification of tubule branches length in co-cultured ZBTB16-knockdown hBMSCs and HUVECs with or without EC-exo. Scale bar represents 200 μm. F Relative mRNA expression of CD31, EMCN and HIF-1α in co-cultured ZBTB16-knockdown hBMSCs and HUVECs after osteogenesis induction treated with or without EC-exo. G Relative protein expression of CD31, EMCN and HIF-1α in co-cultured ZBTB16-knockdown hBMSCs and HUVECs after osteogenesis induction treated with or without EC-exo. H Relative miR-200c-3p expression in EC-exo and BMSC-exo. I Relative miR-200c-3p expression in hBMSCs treated with or without EC-exo. J Relative mRNA expression of NOG and SLIT3 in co-cultured hBMSCs and HUVECs with or without EC-exo. K A model depicting the proposed mechanism of EC-exo-initiated osteogenesis-angiogenesis coupling. The statistical data are represented as the mean ± SD. n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ns, no significance
In addition, tube forming experiment showed that ZBTB16 knockdown in hBMSCs led to a significant reduction in the angiogenic ability of HUVECs in the co-culture system, and it could not be reversed by EC-exo treatment (Fig. 7E). RT-qPCR and western blot detection also revealed that as a putative regulator of type H vessels, HIF-1α was significantly decreased in co-culture system with the knockdown of ZBTB16 in HUVECs, and it could not be reversed by EC-exo treatment. The expression levels of H-EC markers (CD31 and EMCN) also showed the same trend (Fig. 7F, G). These results suggested that the activation of H-EC phenotype by EC-exo in co-culture system is achieved via BMSC. EC-exo first promotes the osteogenic phenotype of BMSCs by up-regulating ZBTB16, BMSCs with osteogenic phenotype further activate the HIF-1α pathway in ECs, promoting their phenotypic switch into H-EC, thereby activating the osteogenesis-angiogenesis coupling. ZBTB16 is a vital link in this series of processes.
EC-exo activates the positive feedback loop of BMSCs and ECs
The above results have revealed the role of BMSCs in the phenotypic swift of ECs in the co-culture system. This section will further investigate the impact of ECs on BMSCs, and complement the osteogenesis-angiogenesis coupling chain by checking key molecules of intercellular communication. Previous studies have found that miR-200c-3p can promote the expression of ZBTB16 in a non-canonical pathway [34]. RT-qPCR results showed that miR-200c-3p was enriched in EC-exo (Fig. 7H). When hBMSCs were treated with EC-exo, the content of miR-200c-3p in hBMSCs was significantly increased (Fig. 7I).
At the same time, several key molecules of osteogenesis coupled with type H blood vessels were detected in the osteogenic co-culture system. RT-qPCR results showed that after EC-exo treatment, the expression levels of SLIT3, which should be produced by osteoprogenitor to promote type H EC epitope conversion, were significantly increased in the osteogenic co-culture system (Fig. 7J). Similarly, the expression level of NOG secreted by H-ECs to promote osteogenic differentiation was also significantly increased (Fig. 7J). The above results revealed that EC-exo delivered miR-200c-3p to BMSCs, up-regulated the expression of ZBTB16, triggered the canonical NOG and SLIT3 crosstalk between BMSCs and ECs, and induced a positive feedback loop.
ZBTB16 maintains type H vessels during bone development
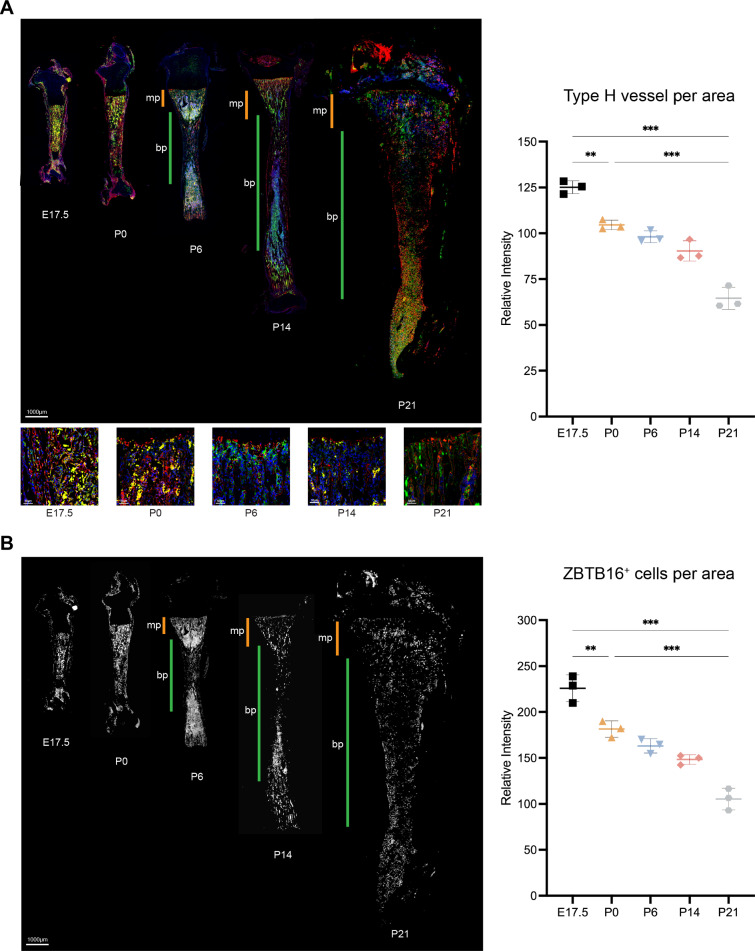
To address the pivotal role of ZBTB16 in regulating osteogenesis-angiogenesis coupling, we investigated the spatiotemporal relationship between the expression of ZBTB16 during bone development, which is another type H vessels expression peak other than bone healing. At the 17.5 day of embryonic (E17.5), the primary ossification center of the tibia had formed a highly branched vascular plexus with endothelial cells highly expressing CD31 and EMCN. On the day of birth (P0), the type H vessels in the diaphysis are reduced relative to the embryonic period. Subsequently, the long bones expanded longitudinally and radially, accompanied by expansion of the local vasculature. From the 6th day of postnatal (P6) onwards, the metaphyseal and diaphysis vessels formed different vascular groups. The endothelial cells in the metaphysis were highly expressed CD31 and EMCN, and still showed type H vascular phenotype, while the diaphysis was replaced by type L vessels of lowly expression of CD31 and EMCN. At P14 and P21, the reduction in type H vessels in the tibial metaphyseal region can be clearly observed (Fig. 8A). Similarly, ZBTB16+ osteoprogenitors were found throughout the primary ossification centers during embryonic period, converged to the metaphysis at P0, and decreased rapidly in the diaphysis area from P6. At P14 and P21, it was evident that ZBTB16+ osteoprogenitors in the metaphysis also began to decrease, forming a spatiotemporal association with type H vessels (Fig. 8B).
Fig. 8.
ZBTB16 maintains type H vessels in the tibia during rat development. A Representative IF staining of E17.5, P0, P6, P14, P21 and P28. Rat tibia sections for CD31 (green) and EMCN (red) and the quantitative analysis of type H vessels. Yellow fluorescence indicates H-type vessels. Orange bars mark metaphysis (mp), green bars indicate diaphysis (dp). Scale bar represents 1000 μm. B Representative IF staining of E17.5, P0, P6, P14, P21 and P28 Rat tibia sections for ZBTB16 (white) and the quantitative analysis of ZBTB16+ cells. Orange bars mark metaphysis (mp), green bars indicate diaphysis (dp). Scale bar represents 1000 μm
EC-exo promotes osteogenesis-angiogenesis coupling in vivo
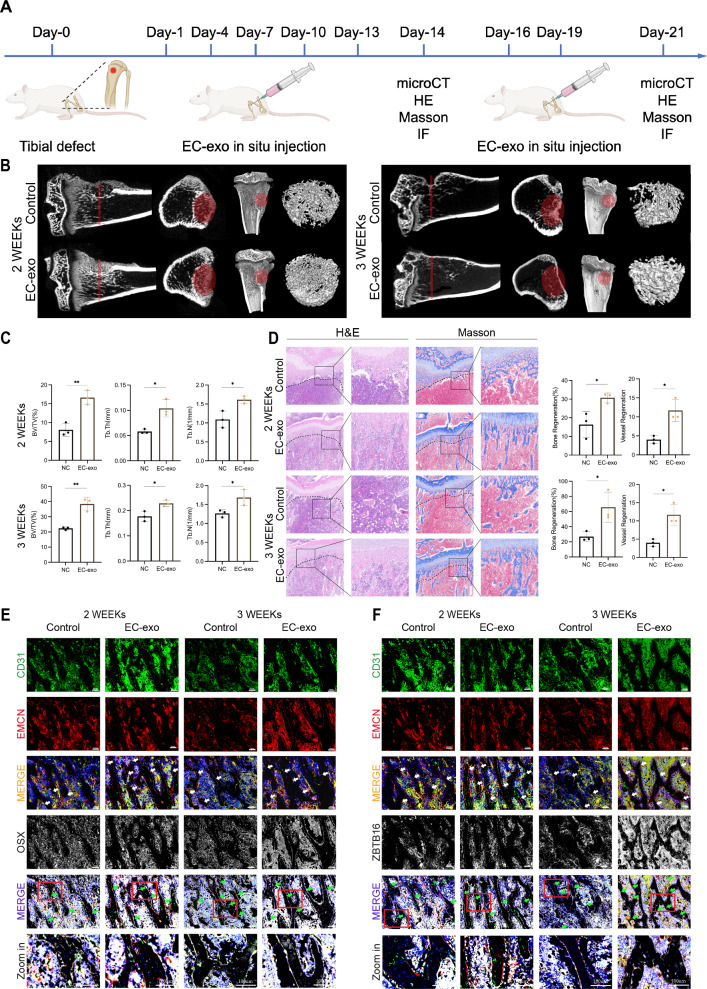
To further verify the role of EC-exo in promoting the coupling of osteogenesis and angiogenesis in vivo and to investigate the therapeutic effects of EC-exo in bone repair, a bone defect with a diameter of 3 mm and a depth of 3 mm was created in the tibial metaphysis of 6-week-old SD rats. Injection 50 μg EC-exo into the soft tissue around the injury site, once every 3 days for 2 and 3 weeks, to observe the bone healing and vascular regeneration (Fig. 9A). In terms of osteogenesis, micro-CT results showed that more new generated bone was formed at the injury site after EC-exo injection (Fig. 9B). The volume of new generated bone, number and thickness of new generated bone trabeculae were significantly higher than those in the control group (Fig. 9C). Hematoxylin and eosin (H&E) and Masson staining showed that the new generated bone, blood vessels and collagen fibers in the defect of rats injected with EC-exo were significantly increased (Fig. 9D). In terms of angiogenesis, IF staining showed that type H blood vessels around the bone defect area injected with EC-exo were significantly increased. Meanwhile, the content of OSX+ osteoprogenitors and ZBTB16+ osteoprogenitors surrounding type H vessels also increased significantly, which colocalized with type H blood vessels (Fig. 9E, F). EC-exo did not cause damage to important organs (Fig. S5). The above results indicated that EC-exo was a potential exosome therapy for bone repair by promoting osteogenesis-angiogenesis coupling.
Fig. 9.
EC-exo promotes osteogenesis-angiogenesis coupling in vivo. A Diagrammatic sketch of EC-exo treatment protocol for tibial defects in rats. B Representative micro-CT images showing 3D-reconstruction of the regeneration area. C Quantitative analysis of trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), and trabecular number (Tb.N). D Representative H&E staining and Masson's trichrome staining of the tibia sections and analysis of bone and vessels regeneration. Magnified areas of dashed boxed sections are shown in right panels. Scale bar represents 200 μm. Below the dotted line is the defect area. E Representative IF staining of tibia sections for CD31 (green), EMCN (red), and OSX (white). Scale bar represents 100 μm. F Representative IF staining of tibia sections for CD31 (green), EMCN (red), and ZBTB16 (white). Scale bar represents 100 μm. The statistical data are represented as the mean ± SD. n = 3, *p < 0.05, **p < 0.01
Discussion
Vascularization is the environmental basis of the maintenance of bone homeostasis [8]. Based on their distribution, function, and molecular expression characteristics in bone tissues, the vessels can be divided into type H blood vessels with high expression of CD31 and EMCN and type L vessels with low expression of CD31 and EMCN [10]. Type H blood vessels are widely distributed in the metaphyseal and subperiosteal region during the developmental stage, with more than 82% of RUNX2+ and 70% of OSX+ osteoprogenitors selectively surrounded, which significantly decrease with age [10, 11]. The proliferative capacity of ECs in the bone marrow is mainly determined by H-ECs, although they account for only 1.77% of total bone ECs and 0.015% of total bone marrow ECs [35]. During the process of bone healing, a large number of H-ECs are gradually generated in the injury area, indicating that H-EC is an effective promoter of bone formation and regeneration [6].
H-EC and the nearby osteogenesis cell lineage have subtle intercellular crosstalk that orchestrate the bone and vessel formation, constitutes a complex osteogenic-angiogenic regulatory network [12, 13, 36, 37]. However, there is no knowledge of whether this coupling exists at an earlier stage, such as between BMSCs and ECs. In this study, we employed primary human BMSCs and human umbilical vein ECs as the research tools in order to increase the evidentiality. A co-culture system was established by hBMSC and HUVEC to simulate the in vivo osteogenic microenvironment. The reason why we chose HUVEC as the in vitro research object for H-shaped blood vessels is mainly due to the following considerations: (1) It is difficult to extract blood vessel ECs from human bone tissue and the culture condition is really harsh; (2) HUVEC is a kindle of poorly differentiated EC that is homologous to bone derived EC and is suitable for simulating the ECs in bones [38]; (3) Among the numerous commercialized endothelial cells, there have been many previous studies that have chosen HUVEC as the in vitro research object for type H blood vessels [39, 40]. We found that there indeed existing a coupling effect between BMSCs and ECs, which are the cells in the previous stage of osteoblasts and H-ECs. We also observed a strong coupling effect through organoid formation, which is more closely related to in vivo and purer interaction between the two cell lineages.
In addition to widely recognized secreted proteins and cytokines, exosomes have recently been found to play a prominent signaling role in paracrine signaling as an important carrier of intercellular crosstalk [18, 41]. To confirm whether exosome is involved in osteogenesis-angiogenesis coupling, we employed GW4869 to inhibit the production of exosome in the co-culture system and found that this coupling effect was greatly weakened, which indicates that the osteogenesis-angiogenesis coupling effect of BMSCs and ECs is largely achieved through exosome. It was found that tensile stress stimulates ECs in the bone marrow to secrete exosomes that composing the positive feedback pathway, which promotes segmental bone defect healing by promoting osteogenesis-angiogenesis coupling [24, 42]. Whilst exogenous MSC-derived exosomes can also promote osteogenesis-angiogenesis coupling in vivo [43, 44]. However, which cell-derived exosome dominates the coupling effect? What are its main recipient cells? What mechanism mediates osteogenesis-angiogenesis coupling? There are still many unresolved issues that need further exploration.
To unravel these mysteries, we introduced additional MSC-exo and EC-exo into the co-culture system, and found that EC-exo is the key mediator of osteogenesis-angiogenesis coupling. Previous studies have identified that EC-exo has positive effects on angiogenesis, cardiac repair, neural regeneration, fracture healing, and immunology regulation [22, 26, 45–48]. It can further enhance its specific functions through engineering modifications of the surface or cargo [48]. Interestingly, we found in our study that EC-exo-induced phenotypic transformation of H-ECs only occurred in the co-culture system, while EC-exo alone could not stimulate HUVECs to become H-ECs. Therefore, we speculate that EC-exo stimulates BMSCs first, and then the activated BMSCs induce HUVEC to transform into H-ECs.
To uncover the mechanism underlying this effect, we conducted transcriptome analysis on hBMSCs treated with EC-exo. We also selected the dataset (GSE192962) that is most similar to our experimental conditions from all available GEO datasets [31]. Although it comes from rats, most of the genes with practical significance are conserved and homologous to humans ZBTB16 attracted our attention due to its highest expression change after osteogenic induction and EC-exo treatment in hBMSCs. ZBTB16 (also known as PLZF or ZNF145) is a dominant component of limb and axial skeletal formation and an important osteogenic marker independent of BMP and Wnt pathways during the later stages of stem cell differentiation [32, 33]. We found that ZBTB16+ cells are preferentially located near type H vessels during development and essential for the formation of type H vessels during bone development and homeostasis. These findings suggest a functional framework in which ZBTB16+ BMSCs and osteoprogenitors drive the formation of a coupled specialized type H vascular system in tissue homeostasis for promoting generation and repair. Yu et al. disclosed that ZBTB16 is regulated by super enhancers, which can activate the expression of ZBTB16 during osteogenesis to prevent osteoporosis [31]. Our results elucidated that EC-exo first promotes the osteogenic phenotype of BMSCs by up-regulating ZBTB16, BMSCs with osteogenic phenotype further activate the HIF-1α pathway in ECs, promoting their phenotypic switch into H-EC, thereby activating the osteogenesis-angiogenesis coupling. ZBTB16 in BMSCs is indispensable in EC-exo-initiated osteogenesis-angiogenesis coupling.
To better demonstrate the strong relation between ZBTB16 and type H vessel formation, we investigated the expression of ZBTB16 and H-EC markers using 2 in vivo models, which are known by being rich in type H vessels in their bone tissue. In rat bone development, we noticed remarkable correlation between ZBTB16 expression and type H vessel formation in a time manner, and the co-localization of the ZBTB16 and the H-EC markers showed a spatial coordination between ZBTB16 and type H vessels. In bone defect model, the expression of ZBTB16, CD31, and EMCN were up-regulated by the administration of EC-exo, which as well displayed strong spatial and temporal correlation. Dahal et al. found that ZBTB16 is regulated by miR-200c-3p in an atypical manner [34]. We also found a significant enrichment of miR-200c-3p in EC-exo. The expression levels of miR-200c-3p and ZBTB16 are also positively correlated, indicating that EC-exo transports miR-200c-3p to BMSCs, upregulating ZBTB16 expression. Further experiments showed that ZBTB16 can promote BMSC differentiation into OSX+ osteoprogenitors. Osteoprogenitors promote the transformation of ECs into H-EC phenotype through activating the HIF-1α pathway by secretory SLIT3. H-ECs in turn promote the differentiation of BMSCs into osteoprogenitors by secreting NOG. Thereby, EC-exo trigger a positive feedback loop between BMSCs and ECs and dramatically promote osteogenesis-angiogenesis coupling.
This study elucidated the positive role of EC-exo in osteogenesis-angiogenesis coupling, and suggested that EC-exo can be used as a therapeutic strategy for bone regeneration and repair. Therefore, we administered EC-exo in a rat bone defect model for treatment, and the results showed that EC-exo significantly promoted the bone healing and increased the co-localization of type H blood vessels and osteoprogenitors at the bone injury site. However, we still need to face some unresolved issues in this study. Firstly, this study focused only on the interaction between BMSCs and ECs, while there are also multiple components such as osteoblast lineage cells, hematopoietic cells, and extracellular matrix in the bone microenvironment, which require more extensive research to fully elucidate their complex interactions. Secondly, this study explained the regulatory role of EC-exo on BMSCs and ECs from the perspective of miRNA, but whether there are more diverse regulatory pathways remains to be further explored. Finally, although there are gaps between the strategy employed in this study and the clinical practice, however, these gaps do not stand in the way of clinical application of exosome therapy once concerns about treatment efficacy and safety have been addressed.
In conclusion, we revealed that exosomes derived from different cells have their own independent functions in the process of bone tissue repair. EC-exo has advantages in osteogenesis-angiogenesis coupling that MSC-exo does not possess. Osteogenesis and angiogenesis are mutually reinforcing processes, but require an initiating factor to trigger their positive feedback loop. EC-exo can precisely serve as the initiating factor to activate and dramatically amplify this process. Our study demonstrates that EC-exo can become an effective therapeutic tool to promote bone regeneration and repair. We hope that this study can provide a novel and valuable therapeutic strategy for bone regeneration and repair.
Materials and methods
Primary hBMSC isolation
The obtention of primary hBMSCs has been approved by the Ethics Committee of the First Hospital of Jilin University (No. 22K095-002) and obtained informed consent from the participants. The bone marrow blood that inevitably bleeds during the surgery was collected. After being filtered through a 70 μm sterile filter, the bone marrow blood was centrifuged at 1000g for 10 min to remove plasma, and resuspend the cell pellet with Hank’s solution (Solarbio, China). The resuspension was slowly dropped into the upper layer of Ficoll lymphocyte separation solution (TBD science, China) and centrifuged at 2000g for 20 min to stratify the cells according to density gradient. The mononuclear cell layer was transferred to a new centrifuge tube, mixed with phosphate buffered saline (PBS) for resuspension and washing, then centrifuged at 1000g for 10 min and the supernatant was discarded. The cell pellet was resuspended with MEM-α complete medium and transferred into a culture flask for adherent growth. After 72 h, non-adherent cells were removed, the remaining cells were hBMSCs. The hBMSCs were identified by flow cytometry to exhibit typical features of MSCs, namely negative hematopoietic stem cell markers (CD34 and CD45) and positive MSC markers (CD73 and CD90) (Fig. S6). The hBMSCs were obtained from a total of 33 donors (age 40.6 ± 6.6), including 15 male donors (age 40.4 ± 8.3) and 18 female donors (age 40.7 ± 5.1).
hBMSC and HUVEC independent/co-culture and exosome treatment
hBMSCs were cultured in MEM-α basic medium (Gibco, USA) supplemented with 1% penicillin–streptomycin (Gibco, USA) and 10% FBS (Gibco, USA). Cells in passage 3–5 were used for in vitro experiments. HUVEC (iCELL Bioscience, China) were cultured in ECM complete medium (ScienCell, USA). In the co-culture system. hBMSCs and HUVECs were mixed at the ratio of 1:1 and seeded in the culture dishes. MEM-α complete medium was mixed with ECM complete medium at a ratio of 1:1 [39, 40]. The cells were cultured at 37 °C in humidified air containing 5% CO2.
To fabricate the hBMSC/HUVEC constructs, 500 μl hBMSCs suspension (5 × 105 cells/ml), 500 μl HUVECs suspension (5 × 105 cells/ml), and 200 μl Matrigel matrix (Corning, USA) were mixed and then dropped 200 μl into ultra-low attachment 96-well plate (Corning, USA) each well. The plate was centrifuged at 300g for 10 min. The spherical gel wrapping the cells at the bottom of the well were hBMSC/HUVEC structures. The hBMSC/HUVEC structures were cultured in the osteogenic medium at 37 °C in humidified air containing 5% CO2 for 10 days, the medium was renewed every 2 days.
When treating with exosomes, hBMSC-exo or EC-exo was added to the cell culture system at a concentration of 20 μg/ml or 50 μg/ml respectively, which were added along with the culture medium was exchanged in 2D and 3D experiments. The exosome production inhibitor GW4869 (Yeasen Biotechnology, China) was added to the cell culture system at a concentration of 10 μM, also added along with the culture medium was exchanged.
Animal experiments
The animal experiment was approved by the Animal Protection and Utilization Committee of Changchun Weishi Testing Technology Service (No. 20230109–01). The experimental animals were purchased from SPF biotechnology co., Ltd (China). To detect the therapeutic effect of EC-exo, a rat tibial defect model was established to evaluate the bone integration effect. Twelve 6-week-old SD rats were randomly divided into four groups, and anesthetized with isoflurane. A dental drill was used to make a 3 mm-diameter circular bone defect near the proximal end of the tibia, then the incision was sutured. The experimental groups rats were injected in situ at the injury site with EC-exo (50 μg) every 3 days (Fig. S7), while the control group rats were injected with the same volume PBS. After 2 and 3 weeks, the rats were euthanized. The tibias were stripped and fixed with 4% paraformaldehyde for micro-CT and histological detection. To clarify the spatial and temporal relationship between Zbtb16 and type H blood vessels during bone development in rats, the embryos on day 17.5 (E17.5), infant mice on postnatal day 0 (P0), 6 (P6), 14 (P14), and 21 (P21) were humanly killed. The tibias were stripped and fixed with 4% paraformaldehyde for histological detection.
Isolation and characterization of exosomes
According to the previous research[49], ultracentrifugation was utilized to remove exosomes from FBS to obtain exosome-depleted FBS, which is used to prepare complete culture medium. hBMSCs were cultured in exosome-depleted MEM-α complete medium, while HUVECs are cultured in exosome-depleted ECM complete medium. The supernatant of the medium was collected and centrifuged at 300g for 10 min to remove cells. The supernatant was further centrifuged at 2000 g for 30 min to remove dead cells. Centrifuge again at 10,000g for 30 min to remove cell debris. and larger diameter extracellular vesicles. After being filtered with a 0.22 μm filter (Millipore, USA), the supernatant was centrifuged at 100,000g for 70 min. Resuspend and washed the pellet with PBS and centrifuge again at 100,000g for 70 min. The pellet is purified exosome. Exosome concentration was quantitatively detected by BCA detection [50, 51].
The morphology of individual exosomes was observed using TEM (ThermoFisher Scientific, USA). Then, NTA (NanoSight, UK) was employed to measure the size distribution of exosomes. In addition, western blot was used to detect the presence of classical exosome markers (HSP70, TSG101, CD63, and CD81) and the absence of negative markers (Calnexin), with hBMSCs/HUVEC cells as controls.
Exosomes labeling and in vitro internalization
The exosomes were stained by 10 mg/ml DiI (Beyotime, China) at room temperature for 10 min in the dark. Centrifuge at 100,000g for 70 min to remove residual fluorescent dye. hBMSCs or HUVECs were seeded in 20 mm glass bottom cell culture dishes and incubate overnight with DiI-labeled exosomes. The cells were stained with Calcein-AM (Beyotime, China) and DAPI (Beyotime, China) before detection. Laser confocal microscopy was used to observe exosomes internalized by cells (Zeiss, Germany).
Osteogenic induction in vitro
When the cells reached 80% confluence, the conventional complete medium was switched to osteogenic medium, which is MEM-α basic medium (Gibco, USA) supplemented with 10% exosome-depleted FBS, 50 μg/ml ascorbic acid (Sigma, USA), 10 mM β-glycerophosphate disodium (BBI, China), and 0.1 μM dexamethasone (Sigma, USA). The osteogenic medium was renewed every 3 days. The osteogenic effect was detected after 7, 14, and 21 days, respectively.
ARS and ALP assays
After osteogenic induction, cells were washed with PBS and fixed with 4% paraformaldehyde (Solarbio, China) for 30 min. For ARS staining, 2% (w/v) ARS solution (Beyotime, China) was used for staining, and ddH2O was used to remove non-specific staining. Detected by stereomicroscopy and invert microscopy. Then, ARS was extracted by 10% cetylpyridinium chloride monohydrate (Aladdin, China) for quantification by microplate reader (BioTek, USA). For ALP staining, BCIP/NBT ALP kit was used for staining (Beyotime, China) then detected by stereomicroscopy and invert microscopy. For ALP activity assay, cells were lysed in RIPA buffer (Beyotime, China) and measured by ALP activity detection kit (Beyotime, China) with microplate reader at 405 nm.
Cell proliferation assay
Cells were seeded in 96-well plates (1 × 103 cells/well) and detected after 24, 48, 72, and 96 h. The CCK-8 cell proliferation kit was utilized (Beyotime, China), 10 μl CCK-8 solution was added into each well, incubated at 37 °C for 1 h, and the absorbance was measured at 490 nm using microplate reader (BioTek, USA).
Transwell assay
The migration of hBMSCs was evaluated using the transwell (Corning, USA) with 8 μm pores. The hBMSCs were seeded at a density of 1 × 104 cells/well in the upper chamber, supplemented with 1% exosome-depleted FBS, while the complete medium supplemented with 10% exosome-depleted FBS was added to the lower chamber. The cells were incubated for 24 h, then non-migrating cells were erased with a cotton swab. The migrated cells were fixed with 4% paraformaldehyde, washed with PBS, stained with 0.1% crystal violet staining solution, dried, and observed by invert microscopy. The number of migrated cells was analyzed using ImageJ software.
Wound healing assay
The HUVECs were seeded at a density of 2 × 105 cells/well in the 6-well plate. When the cells reached 90% confluence, linear scratches were made using tips. After 12 h, the cell migration rate was observed by invert microscopy, and analyzed using ImageJ software.
Angiogenesis assay
The HUVECs were seeded at a density of 1 × 104 cells/well in the 96-well plate precoated with Matrigel (Beyotime, China). After 6 h, the capillary-like structure was observed by invert microscopy. The number of cell network nodes was analyzed using the ImageJ software.
RNA sequencing and transcriptome analysis
A certain amount of RNA samples was denatured at suitable temperature to expose the secondary structure, and mRNA is enriched by oligo (dT)-attached magnetic beads. After RNAs are fragmented, synthesize the first-strand and the second-strand cDNA, double-stranded cDNA fragments are subjected to end-repair, and then a single ‘A’ nucleotide is added to the 3’ ends of the blunt fragments. PCR-amplified cDNA fragments were purified with AMPure XP Beads, and EB solution was added to dissolve the products. The double-stranded PCR products were heated, denatured, and circularized to generate the final library. Single-stranded circle DNA molecules are replicated via rolling cycle amplification, and a DNA nanoball which contain multiple copies of DNA is generated. Sufficient quality DNA nanoballs are then loaded into patterned nanoarrays using high-intensity DNA nanochip technique and sequenced through BGISEQ-500 platform (BGI-Shenzhen, China). The sequencing data was filtered with SOAPnuke[52] by first, removing reads containing sequencing adapter; second removing reads whose low-quality base ratio (base quality less than or equal to 15) is more than 20%; third, removing reads whose unknown base ('N' base) ratio is more than 5%, afterwards clean reads were obtained and stored in FASTQ format. To take insight to the change of phenotype, GO (http://www.geneontology.org/) and KEGG (https://www.kegg.jp/) enrichment analysis of annotated different expression gene was performed by Phyper based on Hypergeometric test. The significant levels of terms and pathways were corrected by p < 0.05 and |log2FoldChange|≥ 1 with a rigorous threshold. GSEA was performed using Dr.TOM II delivery platform at https://biosys.bgi.com.
Transfection of siRNA
The ZBTB16 siRNA was designed and synthesized by GenePharma (China). When the confluence of hBMSCs reached 80%, Lipofectamine 2000 (ThermoFisher, USA) was used to transfect ZBTB16 siRNA with Opti-MEM medium (Gibco, USA). After 4 h, the transfect medium was replaced by complete medium. The transfection efficiency was observed under a fluorescence microscope 24 h later.
RT-qPCR assay
The total RNA was extracted from the samples using TRIzol (ThermoFisher, USA). Reverse transcription was performed using BlazeTaq RTase Mix (GeneCopoeia, China). Real-time quantitative PCR was performed using BlazeTaq SYBR Green qPCR Mix (GeneCopoeia, China) in a CFX Connect Real Time System (Bio-Rad, USA). GAPDH was used as an internal reference gene for mRNAs, while U6 was used as an internal reference gene for miRNAs. RT-qPCR data were analyzed using 2−ΔΔCt. The primers are listed in Table S4.
Western blotting
The RIPA lysis buffer containing protease inhibitor (Beyotime, China) was utilized to lyse exosomes and cells. The lysate was centrifuged at 12,000g for 15 min to extract total proteins. The protein was separated in SDS–polyacrylamide gel (Epizyme Biotech, China) and transferred to polyvinylidene fluoride (PVDF) membrane (Merck, USA). Incubate at room temperature for 15 min by protein-free rapid blocking buffer (Epizyme Biotech, China). Incubate the PVDF membrane with the primary antibody overnight at 4 °C. The next day, the PVDF membrane was wash thrice and incubated with horseradish peroxidase-coupled secondary antibody. After another triple washes, the ECL assay kit (Proteintech, Chian) was used to induce chemiluminescence. The Bio-Rad VersaDoc imaging system (Bio-Rad, USA) was used to detect target bands. The β-actin was served as an internal reference control, and the target protein is normalized to the control group for statistical analysis. The antibodies are listed in Table S5.
Micro-CT analysis
To evaluate bone regeneration, the rat tibia was scanned using a high-resolution micro-CT scanning system (PerkinElmer, USA). Images were acquired at the following settings: 6.5 μm voxels, medium resolution, 85 kV, 200 μA, 1 mm Al filter, and 384 ms for integration. The three-dimensional image reconstruction was reconstructed from a series of 2D images (CTvox; version 3.3.0). The bone volume fraction (BV/TV), trabecular thickness (Tb. Th), trabecular number (Tb. N), and trabecular separation (Tb. Sp) parameters were analyzed using Inveon Research Workplace.
Histological assessment
The rat tibia was stripped and fixed in 4% paraformaldehyde, embedded in paraffin, decalcified, and stained with H&E and Masson trichrome according to the manufacturer's instructions. Representative images of stained slices were observed under a microscope (Olympus, Japan). The bone tissue slices used for IF staining were dewaxed and rehydrated, and then the antigen was repaired through a 10 mM citric acid buffer (pH 6.0). Soak the slices in citric acid buffer and microwave for 15 min. After being permeabilized with 0.5% Triton X-100 for 20 min, the slices were blocked with PBS containing 10% FBS for 1 h. The slices were incubated overnight with primary antibodies at 4 °C. The next day, the slices were washed and incubated with secondary antibodies at room temperature for 1 h. The nuclei were stained with DAPI (Beyotime, China). Representative images of stained slices were observed under a fluorescence microscope (Olympus, Japan). The integrated option density was divided by the selected area and then multiplied by 100%, to calculate the percentage of positive area marked. All parameters were measured using ImageJ. The antibodies are listed in Table S6.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA). We conducted three separate replicates with cell from different donors for each experiment, which was technically replicated at least 3 times. Results were expressed as mean ± SD. Multiple group comparisons were performed using One-way ANOVA, and two-tailed unpaired Student's test was used for comparisons between two groups. p values < 0.05 were considered statistically significant.
Supplementary Information
Acknowledgements
We appreciate the technical help from the Medical Research Center, the Second Hospital of Jilin University during performing experiments. Schematic diagrams were created with Biorender.com.
Abbreviations
- BMSCs
Bone marrow mesenchymal stem cells
- ECs
Endothelial cells
- OSX
Osterix
- H-ECs
Endothelial cells from type H blood vessels
- HIF-1α
Hypoxia inducible factor 1 subunit alpha
- SLIT3
Slit guidance ligand 3
- VEGF
Vascular endothelial growth factor
- EC-exo
Endothelial cell-derived exosomes
- ZBTB16
Zinc finger and BTB domain containing 16
- hBMSCs
Human bone marrow mesenchymal stem cells
- HUVECs
Human umbilical vein endothelial cells
- ALP
Alkaline phosphatase
- ARS
Alizarin red S
- RT-qPCR
Real-time quantitative polymerase chain reaction
- OPN
Osteopontin
- OCN
Osteocalcin
- EMCN
Endomucin
- NTA
Nanoparticle tracking analysis
- TEM
Transmission electron microscopy
- HSP70
Heat shock protein 70
- TSG101
Tumor susceptibility gene 101
- DiI
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- IF
Immunofluorescence
- PBS
Phosphate buffered saline
- CCK8
Cell counting kit-8
- GO
Gene ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- GSEA
Gene set enrichment analysis
- SD rats
Sprague–Dawley rats
- rBMSCs
Rat bone marrow mesenchymal stem cells
- H&E
Hematoxylin and eosin
- PBS
Phosphate buffered saline
- PVDF
Polyvinylidene fluoride
- BV/TV
Bone volume fraction
- Tb. Th
Trabecular thickness
- Tb. N
Trabecular number
- Tb. Sp
Trabecular separation
Author contributions
Y.W. and Q.Y. conceived and designed the study. Y.W. and X.W. acquired funding and oversaw the project. L.L. S.F. and L.W. performed the experiments. N.Z. and Y.W. supervised the study. S.F., Y.L., F.X. and C.F. provided human samples. W.G., Y.W. provided advice. J.C. and J.D. analyzed the data. L.L. and Q.Y. wrote the paper. All authors reviewed and edited the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82372498), the joint open project of Beijing Key Laboratory of Bone Malformation Genetics Research and Key Laboratory of Big Data for Spinal Deformities of Chinese Academy of Medical Sciences (BKJORT202401), the Jilin Provincial Department of Science and Technology (20230204049YY), the Excellent Youth Science Foundation of Changchun (23YQ07), the Genetic precision medicine discipline development public welfare project of Peking Union Medical Foundation (PUMFO1010075), the Chunlei project of China-Japan Union Hospital of Jilin University (2024CL07).
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Declarations
Ethics approval and consent to participate
Obtaining samples of hBMSCs was reviewed and approved by Ethics Committee of the First Hospital of Jilin University (No. 22K095-002). The animal experiment was reviewed and approved by the Animal Protection and Utilization Committee of Changchun Weishi Testing Technology Service (No. 20230109-01).
Consent for publication
All authors agree to be published.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaofeng Wang, Email: wangxiaofeng@jlu.edu.cn.
Qiwei Yang, Email: yangqw@jlu.edu.cn.
Yuanyi Wang, Email: wangyuanyi@jlu.edu.cn.
References
- 1.Chen M, Li Y, Huang X, Gu Y, Li S, Yin P, Zhang L, Tang P. Skeleton-vasculature chain reaction: a novel insight into the mystery of homeostasis. Bone Res. 2021;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stegen S, Carmeliet G. The skeletal vascular system—breathing life into bone tissue. Bone. 2018;115:50–8. [DOI] [PubMed] [Google Scholar]
- 3.Deng AF, Wang FX, Wang SC, Zhang YZ, Bai L, Su JC. Bone-organ axes: bidirectional crosstalk. Mil Med Res. 2024;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Maggio N, Banfi A. The osteo-angiogenic signaling crosstalk for bone regeneration: harmony out of complexity. Curr Opin Biotechnol. 2022;76: 102750. [DOI] [PubMed] [Google Scholar]
- 6.Peng Y, Wu S, Li Y, Crane JL. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10:426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salhotra A, Shah HN, Levi B, Longaker MT. Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 2020;21:696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prisby RD. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J Endocrinol. 2017;235:R77-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivan U, De Angelis J, Kusumbe AP. Role of angiocrine signals in bone development, homeostasis and disease. Open Biol. 2019;9: 190144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langen UH, Pitulescu ME, Kim JM, Enriquez-Gasca R, Sivaraj KK, Kusumbe AP, Singh A, Di Russo J, Bixel MG, Zhou B, et al. Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat Cell Biol. 2017;19:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu R, Yallowitz A, Qin A, Wu Z, Shin DY, Kim JM, Debnath S, Ji G, Bostrom MP, Yang X, et al. Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 2018;24:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Pan J, Jing W. Motivating role of type H vessels in bone regeneration. Cell Prolif. 2020;53: e12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Xie L. Unique bone marrow blood vessels couple angiogenesis and osteogenesis in bone homeostasis and diseases. Ann N Y Acad Sci. 2020;1474:5–14. [DOI] [PubMed] [Google Scholar]
- 16.Shao J, Liu S, Zhang M, Chen S, Gan S, Chen C, Chen W, Li L, Zhu Z. A dual role of HIF1α in regulating osteogenesis-angiogenesis coupling. Stem Cell Res Ther. 2022;13:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu M, Liu W, Li J, Lu J, Lu H, Jia W, Liu F. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Yang Q, Fang D, Sun L, Zheng L, Dou D. Progress in exosome-related research supported by the National Natural Science Foundation of China. Fundam Res. 2023. 10.1016/j.fmre.2023.05.018.38933842 [Google Scholar]
- 19.Du WW, Li X, Ma J, Fang L, Wu N, Li F, Dhaliwal P, Yang W, Yee AJ, Yang BB. Promotion of tumor progression by exosome transmission of circular RNA circSKA3. Mol Ther Nucleic Acids. 2022;27:276–92. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Zhang Y, Hao Z, Wang P, Xia Y, Wu J, Xia D, Fang S, Xu S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52: e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, Li L, Xu S, Xia Y. Umbilical mesenchymal stem cell-derived exosome-encapsulated hydrogels accelerate bone repair by enhancing angiogenesis. ACS Appl Mater Interfaces. 2021;13:18472–87. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Wu Y, Guo L, Yuan S, Sun J, Zhao K, Wang J, An R. Exosomal Lnc NEAT1 from endothelial cells promote bone regeneration by regulating macrophage polarization via DDX3X/NLRP3 axis. J Nanobiotechnol. 2023;21:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang RZ, Xu WN, Zheng HL, Zheng XF, Li B, Jiang LS, Jiang SD. Exosomes derived from vascular endothelial cells antagonize glucocorticoid-induced osteoporosis by inhibiting ferritinophagy with resultant limited ferroptosis of osteoblasts. J Cell Physiol. 2021;236:6691–705. [DOI] [PubMed] [Google Scholar]
- 24.Jia Y, Zhu Y, Qiu S, Xu J, Chai Y. Exosomes secreted by endothelial progenitor cells accelerate bone regeneration during distraction osteogenesis by stimulating angiogenesis. Stem Cell Res Ther. 2019;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song H, Li X, Zhao Z, Qian J, Wang Y, Cui J, Weng W, Cao L, Chen X, Hu Y, Su J. Reversal of osteoporotic activity by endothelial cell-secreted bone targeting and biocompatible exosomes. Nano Lett. 2019;19:3040–8. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Wang J, Xie X, Chen Y, Zheng Q, He J, Lu Q. Exosome-derived miR-5p-72106_14 in vascular endothelial cells regulates fate determination of BMSCs. Toxicol Appl Pharmacol. 2024;482: 116793. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Xie X, Li H, Zheng Q, Chen Y, Chen W, Chen Y, He J, Lu Q. Vascular endothelial cells-derived exosomes synergize with curcumin to prevent osteoporosis development. iScience. 2024;27:109608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665–7. [DOI] [PubMed] [Google Scholar]
- 29.Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Möller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28. [DOI] [PubMed] [Google Scholar]
- 31.Yu W, Xie Z, Li J, Lin J, Su Z, Che Y, Ye F, Zhang Z, Xu P, Zeng Y, et al. Super enhancers targeting ZBTB16 in osteogenesis protect against osteoporosis. Bone Res. 2023;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barna M, Hawe N, Niswander L, Pandolfi PP. Plzf regulates limb and axial skeletal patterning. Nat Genet. 2000;25:166–72. [DOI] [PubMed] [Google Scholar]
- 33.Marofi F, Vahedi G, Solali S, Alivand M, Salarinasab S, ZadiHeydarabad M, FarshdoustiHagh M. Gene expression of TWIST1 and ZBTB16 is regulated by methylation modifications during the osteoblastic differentiation of mesenchymal stem cells. J Cell Physiol. 2019;234:6230–43. [DOI] [PubMed] [Google Scholar]
- 34.Dahal S, Chaudhary P, Kim JA. Induction of promyelocytic leukemia zinc finger protein by miR-200c-3p restores sensitivity to anti-androgen therapy in androgen-refractory prostate cancer and inhibits the cancer progression via down-regulation of integrin α3β4. Cell Oncol (Dordr). 2023;46:1113–26. [DOI] [PubMed] [Google Scholar]
- 35.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. [DOI] [PubMed] [Google Scholar]
- 36.Fu R, Lv WC, Xu Y, Gong MY, Chen XJ, Jiang N, Xu Y, Yao QQ, Di L, Lu T, et al. Endothelial ZEB1 promotes angiogenesis-dependent bone formation and reverses osteoporosis. Nat Commun. 2020;11:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, Li C, Xie L, Crane J, Wan M, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20:1270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan PH, Chan C, Xue SA, Dong R, Ananthesayanan B, Manunta M, Kerouedan C, Cheshire NJ, Wolfe JH, Haskard DO, et al. Phenotypic and functional differences between human saphenous vein (HSVEC) and umbilical vein (HUVEC) endothelial cells. Atherosclerosis. 2004;173:171–83. [DOI] [PubMed] [Google Scholar]
- 39.Gao L, Chen W, Li L, Li J, Kongling W, Zhang Y, Yang X, Zhao Y, Bai J, Wang F. Targeting soluble epoxide hydrolase promotes osteogenic-angiogenic coupling via activating SLIT3/HIF-1α signalling pathway. Cell Prolif. 2023;56: e13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Liu H, Liu S, Li B, Liu Y, Luo E. SIRT1 activation promotes bone repair by enhancing the coupling of type H vessel formation and osteogenesis. Cell Prolif. 2024;57: e13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YY, Cheng J, Liu YD, Wang YP, Yang QW, Zhou N. Exosome-based regenerative rehabilitation: a novel ice breaker for neurological disorders. Biomed Pharmacother. 2023;169: 115920. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Li S, Kong L, Feng K, Zuo R, Zhang H, Yu Y, Zhang K, Cao Y, Chai Y, et al. Tensile stress-activated and exosome-transferred YAP/TAZ-Notch circuit specifies type H endothelial cell for segmental bone regeneration. Adv Sci (Weinh). 2024;11: e2309133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang S, Liu Z, Wu S, Chen X, You M, Li Y, Yang F, Zhang S, Lai Y, Liu P, et al. Pro-angiognetic and pro-osteogenic effects of human umbilical cord mesenchymal stem cell-derived exosomal miR-21-5p in osteonecrosis of the femoral head. Cell Death Discov. 2022;8:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu GD, Cheng P, Liu T, Wang Z. BMSC-derived exosomal miR-29a promotes angiogenesis and osteogenesis. Front Cell Dev Biol. 2020;8: 608521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel DM, Stoorvogel W, Würdinger T, Verhaar MC. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006, s3991-3915. [DOI] [PubMed] [Google Scholar]
- 46.Yue Y, Wang C, Benedict C, Huang G, Truongcao M, Roy R, Cimini M, Garikipati VNS, Cheng Z, Koch WJ, Kishore R. Interleukin-10 deficiency alters endothelial progenitor cell-derived exosome reparative effect on myocardial repair via integrin-linked kinase enrichment. Circ Res. 2020;126:315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J, Zhang G, Li S, Li J, Wang W, Xue J, Wang Y, Fang M, Zhou N. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J Nanobiotechnology. 2023;21:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mi B, Chen L, Xiong Y, Yang Y, Panayi AC, Xue H, Hu Y, Yan C, Hu L, Xie X, et al. Osteoblast/osteoclast and immune cocktail therapy of an exosome/drug delivery multifunctional hydrogel accelerates fracture repair. ACS Nano. 2022;16:771–82. [DOI] [PubMed] [Google Scholar]
- 49.Shelke GV, Lässer C, Gho YS, Lötvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014. 10.3402/jev.v3.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang J, Li J, Li S, Yang X, Huo N, Chen Q, Wang W, Yang N, Wang Y, Zhou N. Netrin-1-engineered endothelial cell exosomes induce the formation of pre-regenerative niche to accelerate peripheral nerve repair. Sci Adv. 2024;10:eadm8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, Mu J, Zhang Y, Zhang C, Ma T, Chen L, Huang T, Wu J, Cao J, Feng S, et al. Stimulation by exosomes from hypoxia preconditioned human umbilical vein endothelial cells facilitates mesenchymal stem cells angiogenic function for spinal cord repair. ACS Nano. 2022;16:10811–23. [DOI] [PubMed] [Google Scholar]
- 52.Li R, Li Y, Kristiansen K, Wang J. SOAP: short oligonucleotide alignment program. Bioinformatics. 2008;24:713–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.