Abstract
Cold temperatures have been shown to slow skin wound healing. However, the specific mechanisms underlying cold-induced impairment of wound healing remain unclear. Here, we demonstrate that small extracellular vesicles derived from cold-exposed mouse plasma (CT-sEVs) decelerate re-epithelialization, increase scar width, and weaken angiogenesis. CT-sEVs are enriched with miRNAs involved in the regulation of wound healing-related biological processes. Functional assays revealed that miR-423-3p, enriched in CT-sEVs, acts as a critical mediator in cold-induced impairment of angiogenic responses and poor wound healing by inhibiting phosphatase and poly(A) binding protein cytoplasmic 1 (PABPC1). These findings indicate that cold delays wound healing via miR-423-3p in plasma-derived sEVs through the inhibition of the ERK or AKT phosphorylation pathways. Our results enhance understanding of the molecular mechanisms by which cold exposure delays soft tissue wound healing.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-03009-y.
Keywords: Cold exposure, Wound healing, Angiogenesis, Extracelluar Vesicles, miR-423-3p, PABPC1
Introduction
As with other environmental factors such as pressure, heat, and oxygen, cold exposure has been regarded for centuries as both a ‘hero’ and a ‘villain’, having beneficial and detrimental effects, depending on the combined effects of the environment and the body. Open wounds on the skin exposed to cold environments are dangerous and pose a significant threat to the health of individuals working outdoors. Observations suggest that cold temperatures lead to slower wound healing. This is believed to be due to cold causing blood vessels to constrict, thereby reducing blood flow. Cold stress poses serious health risks, especially to young children, the elderly, and those with underlying health conditions. Although the human body can maintain homeostasis and prevent harm through self-regulation, such as reducing heat loss and increasing heat production [1], prolonged exposure to cold environments still poses a threat to human life. Acute or short-term exposure to cold can affect various physiological indicators in humans and animals within hours, including blood viscosity, cholesterol, blood pressure, heart rate, and muscle control [2–4]. Even more concerning, long-term exposure to cold affects metabolism and immune function [5], causes abnormal glucose metabolism [6], and increases the risk of various diseases such as cardiovascular [7], respiratory [8], and gastrointestinal dysfunction [9]. It can also severely damage organ including the pancreas [10], blood vessels [11], heart [12], liver [13], and muscles [14]. The skin, the largest organ in the human body, is one of the tissues with the fastest self-renewal rates. It provides a natural barrier against external environmental damage and plays a crucial protective role. When acute or chronic injury disrupts skin integrity, a multi-step dynamic repair process initiates in the injured area, promoting local tissue healing and repairing the skin’s barrier function [15–17]. The natural wound healing process comprises four overlapping but distinct stages: hemostasis, inflammation, proliferation, and remodelling [18–21]. Angiogenesis plays a significant role in skin wound healing [22]. The external microenvironment plays a crucial role in the wound healing process. Compared to normal temperatures, exposure to large areas of trauma in cold environments poses a higher risk [23, 24].
According to the International Society for Extracellular Vesicles (ISEV) 2018, extracellular vesicles (EVs) are divided into two subgroups: small EVs (sEVs or exosomes, < 100 nm or < 200 nm) and medium/large EVs (m/l EVs, > 200 nm) [25]. EVs are nanoscale bilayer membrane structures secreted by living organisms, ranging in size from nanometers to micrometers. EVs derived from eukaryotic organisms are mainly categorized into exosomes, microparticles/vesicles, and apoptotic bodies. Among these, exosomes have the most distinctive characteristics and are the most extensively studied. Exosomes have a diameter of 50–150 nm and form a double concave disc shape. They are secreted by nearly all cells in the body (including neurons and glial cells) and are found in nearly all bodily fluids (including urine [26], blood [3], ascites [27], and cerebrospinal fluid [28]). sEVs are transport carriers that encapsulate proteins, lipids, DNA, and RNA (mRNA, miRNA, lncRNA and circRNA) [29–33], facilitating intercellular interactions (cross talk). Research indicates that cold exposure affects the production and function of sEVs. Our previous study found that exposing mice to low temperatures promotes the release and activity of EVs and alters the composition of sEVs [3].
Cold exposure affects the production, composition, and function of sEVs, and may be involved in the body’s immune regulation process. However, further research is needed to elucidate the mechanism underlying the relationship between cold exposure and sEVs. Studies have found that sEVs play various roles in wound healing [34, 35]. We therefore hypothesize that sEVs may serve as communication vesicles and impact wound healing at an ambient temperature.
While creating a 4℃ chronic cold exposure mouse model, skin damage occurred during fighting between the mice. We founnd that cold exposure delays wound healing in mice. We then further explored this phenomenon to explain why wound healing is delayed in mice under cold exposure. Given the pivotal role of sEVs, we aimed to investigate whether the secretion of CT-sEVs constitutes a critical mechanism mediating the anti-angiogenic and anti-wound healing effects observed following cold exposure.
In this study, we unravelled a unique mechanism responsible for delayed wound healing in cold temperatures and investigated the function of plasma-derived sEVs from cold exposure (CT-sEVs) as messengers, transferring specific miRNAs to human microvascular endothelial cells (HMEC-1). First, we isolated plasma-derived sEVs from mice exposed to cold and room temperature and evaluated the identity of these vesicles. We then determined the effects of CT-sEVs on the activities of cultured wound healing-related cells and profiled miRNA expression in CT-sEVs and RT-sEVs to screen the candidate molecules that mediate CT-sEVs function. In vivo, we investigated the effect of CT-sEVs on angiogenesis and chronic wound repair. Additionally, we assessed the role of candidate miRNA in CT-sEVs-induced regulation of wound healing. Our study aimed to determine the function of sEVs in wound healing and elucidate the underlying molecular mechanisms.
Materials and methods
Cell culture and treatments
HMEC-1 were cultured in complete MCDB131 medium (Gibco, USA) containing 10% fetal bovine serum (FBS) (Gibco, USA) and 1% Penicillin-Streptomycin (PS) (P1400, Solarbio, China). The human keratinocytes cell line HaCaT and skin fibroblasts (HSFs) were maintained in complete high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA) with 10% FBS. All cells were incubated at 37ºC with 5% CO2.
Isolation, identification and uptake of sEVs
Whole blood was collected in EDTA-coated tubes and centrifuged at 1,900 × g for 10 min to collect the plasma. This was followed by sequential centrifugation at 300 × g for 10 min and 2,000 × g for 10 min to remove cell debris. The supernatant was then centrifuged at 10,000 × g for 30 min and ultracentrifugation at 100,000 × g for 120 min. The pelleted sEVs were washed twice with a large volume of PBS and centrifuged again at 100,000 × g for 120 min, then re-suspended in 15 mL of PBS. The sEVs suspension was filtered through a 0.22 μm filter (Millipore, USA) and centrifuged to about 200 µL by ultra-filtration at 4,000 × g using a 15 mL Amicon Ultra-15 Centrifugal Filter Unit (Millipore, USA). All procedures were performed at 4ºC. The sEVs were either stored at -80ºC or used for the downstream assays.
The morphology of sEVs was observed by a transmission electron microscope (TEM) (Hitachi, Japan), and their size distribution was measured by nanoparticle tracking analysis (NTA) with a ZetaView PMX 110 (Particle Metrix, Germany) as previously described in detail [36]. Western blotting was used to assess sEVs surface marker expression [37].
The red dye PKH26 (Sigma-Aldrich) was used to stain membranes and track purified sEVs. After labeling, 20 mL of PBS was used to wash the sEVs, which were then recollected via ultracentrifugation. The uptake of the labeled particles by HaCaT, HSFs, and HMEC-1 cells was assessed by combining the cells with sEVs and using immunofluorescence to measure uptake. The signals were analyzed with a fluorescence microscope (Nikon Instruments Korea, Seoul, Korea).
Flow cytometry
First, 10 µg of sEVs were mixed with 5 µL of 4 μm aldehyde/sulphate latex beads (Invitrogen, Carlsbad, USA) and rotated at room temperature for 15 min. Then, the suspension was diluted to 1 mL with 1 × PBS and continued rotating at room temperature for 30 min. The reaction was terminated by adding 100 mM glycine and 2% bovine serum albumin (BSA) to the 1×PBS and rotating at room temperature for 30 min. The sEVs-bound beads were washed once with 2% BSA/1 × PBS and centrifuged at 10,000 rpm for 1 min. They were then blocked with 10% BSA and rotated at room temperature for 30 min. After another wash with 2% BSA/1 × PBS and centrifuge at 10,000 rpm for 1 min the beads were incubated with primary antibodies at 4ºC for 30 min. The primary antibodies used were anti-CD63 (SC5275, Santa cruz, 1:5) and anti-TSG101 (14497-1-AP, Proteintech, 1:10). The beads were centrifuged at 10,000 rpm for 1 min, the supernatant was discarded, and the beads were washed with 2% BSA/1×PBS before being centrifuged again at 10,000 rpm for 1 min. Finally, the beads were incubated with secondary antibodies at 4ºC for 30 min. The secondary antibodies used were Alexa Fluor 488-conjugated goat anti-mouse IgG (ab150117, Abcam, 1:2000) or goat anti-rabbit IgG (ab150077, Abcam, 1:2000). Light exposure was avoided. Unbound fluorescence and impurities were washed off with 5% BSA/1×PBS as the flow wash buffer. The samples were loaded as required and analysed using the FACSCANTO II (BD Biosciences) flow cytometer. The results were analyzed using the Flowjo software.
Proliferation assay
The cells were seeded in 96-well plates at a cell density of 5,000 cells per well and cultured at 37ºC in 5% CO2. After 6 h of cell adhesion, the cell counting kit-8 reagent (CCK-8; 7Sea Biotech, Shanghai, China) was prepared by adding 10 µL CCK-8 reagent to 100 µL of serum-free medium for each well. The CCK-8 mixture was added to each well (110 µL/well) and incubated at 37ºC and 5% CO2 for 3 h. Absorbance values were measured at a wavelength of 450 nm using a microplate reader (NanoDrop 2000) and denoted as day 1 (D1). The solution in the wells was then removed, and 100 µL of serum-free medium containing 100 µg/mL of sEVs, 100 µg/mL of sEVs + 200 nM of antagomiR-423-3p, 100 µg/mL of sEVs + 200 nM of antagomiR-NC, or an equal volume of PBS was added according to the experimental design. The cells were then returned to the incubator to continue culturing. A total of four replicates were made for each group. Fresh CCK-8 mixture (100 µL serum-free medium with 10 µL CCK-8 reagent) was added on the 2nd, 3rd and 4th day, and the OD value was measured after incubation for 3 h. A group with no cells and only CCK-8 mixture was used as blank control. A cell growth curve was plotted according to the OD values for each day. The experiment was repeated three times to improve the accuracy of the results.
Scratch wound healing assay
The HMEC-1 cells were seeded in 12-well plates at a density of 2 × 105 cells per well. Serum-free medium containing 100 µg/mL sEVs, 100 µg/mL sEVs + 200 nM antagomiR-423-3p, 100 µg/mL sEVs + 200 nM antagomiR-NC, or an equal amount of PBS was used to culture the cells in each group for 24 h. The monolayer was scratched with a p200 pipette tip and washed with serum-free medium to remove detached cells. Mitomycin-C (5 µg/mL, Sigma) was present throughout the migration assay to rule out the effect of cell proliferation on wound closure. The cells were immediately photographed under a normal light microscope and recorded at 0 h. The cells were photographed at 12 h and 24 h after the scratch. Cell mobility (%) was calculated as = (A0-An) /A0 × 100, where A0 represents the scratch area at 0 h and an represents the scratch area at n h. The experiment was repeated three times to improve the accuracy of the results.
Transwell migration assay
The cells were suspended in low serum (0% FBS) medium and added to the upper chamber of transwell inserts (3422, Corning, USA) with an 8 μm pore size (3 × 104/well) in 200 µL of serum-free media. The lower chamber was filled with 500 µL of complete medium containing 20% FBS, along with sEVs or antagomiR. After 12 h, non-migrated cells attached to the surface of the filter were removed with a cotton swab, and the migrated cells on the bottom side of the filter were stained with 0.5% crystal violet for 10 min. The number of migrating cells was evaluated using light microscopy.
Tube formation assay
The Growth Factor Reduced Matrigel (354263, BD Biosciences, USA) was dissolved at 4ºC. The 96-well plate and 200 µL gun head were frozen at -20ºC. The next day, the 96-well plate was placed on ice, and Matrigel was quickly absorbed into the 96-well plate at 50 µL per well. The culture plate was moved to a 37ºC incubator for 30 min to solidified Matrigel. HMEC-1 cells were digested by centrifuged at 1,000 rpm for 5 min, the supernatant was removed, and the cells were resuspended in serum-free MCDB131 medium. HMEC-1 cells were then implanted on a 96-well plate with Matrigel at a cell density of 8,000 cells/well and 100 µL per well. The plates were photographed under an ordinary optical microscope for 4 h after planting. The indicators (total branching points and total tube length) of ability to form tubes were measured using Image-Pro Plus 6.0 software.
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was isolated from the cells using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. For miRNA detection, miRNA was reverse transcribed and analyzed using TB Green® Premix Ex Taq™ II (Tli RNaseH Plus, RR820A, Takara, Japan) according to the manufacturer’s protocol, with U6 serving as the normalization control. U6 (MmiRQP9001) and miR-423-3p (MmiRQP0491) primers were purchased from GeneCopoeia (Guangzhou, China).
RNA interference
Small interfering RNAs (siRNAs) and the negative control RNA duplex (siRNA-NC) were purchased from GenePharma Biotech (Shanghai, China). The miR-423-3p mimics or miR-423-3p inhibitor and scrambled oligonucleotides (mimics NC or inhibitor NC) were also purchased from GenePharma Biotech. Transfection was performed using the GP-transfect-Mate transfection reagent (GenePharma Biotech) according to the manufacturer’s protocol. The transfected sequences of the miR-423-3p mimics/inhibitor and siRNA oligonucleotides are shown in Additional file 1, Table S1. AntagomiRs were purchased from GenePharma Biotech. sEVs were transfected with antagomiR-423-3p or antagomiR-NC at a concentration of 200 nM for 60 min at 37ºC. Excess antagomiRs were removed by centrifugation at 4,000 × g for 5 min using a 100 kDa Amicon Ultra-4 Centrifugal Filter Unit (Millipore) [38]. sEVs treated with other antagomiRs (genetically engineered sEVs) were used for subsequent experiments.
Western blotting
SDS-PAGE (6 -12% gel) was used to separate 40 µg of protein per sample extract before transferring it to PVDF membranes (Millipore, USA). The membranes were then stained overnight at 4ºC with primary antibodies, followed by incubation with the HRP-conjugated secondary antibodies (SA00001-1 or SA00001-2, Proteintech, 1:5000) at 37ºC for 1 h. The following antibodies were used: anti-CD31 (11265-1-AP, Proteintech, 1:1600), anti-VEGF (sc-7269, Santa cruze, 1:600), anti-PABPC1 (10970-1-AP, Proteintech, 1:1000), anti-p-AKT (66444-1-Ig, Proteintech, 1:5000), anti-t-AKT (60203-2-Ig, Proteintech, 1:5000), anti-p-ERK (28733-1-AP, Proteintech, 1:1000), anti-t-ERK (67170-1-Ig, Proteintech, 1:1000), anti-BAX (60267-1-Ig, Proteintech, 1:10000) and anti-GAPDH (10494-1-AP, Proteintech, 1:5000). Prestained color protein ladders were obtained from ThermoFisher Scientific (26616, USA) or Beyotime (P0079, China). The immunoreactive bands were visualized with chemiluminescence reagent (WBKLS0100, Millipore, Billerica, MA) and then analyzed with an Amersham Imager 600 analyser (General Electric, USA) or Tanon 5200 Chemiluminescence Imaging Analysis System (China). Densitometric quantification of band intensity was carried out using Image-Pro Plus 6.0 software.
Mouse wound model and treatment
All experiments were reviewed and approved by the Ethics Committee of the Second Xiangya Hospital, Central South University. All of the procedures conform to the Guide for the Care and Use of Laboratory Animals, NIH publication, 8th edition, 2011. The experiments were formally approved by the Ethics Committee of the Second Xiangya Hospital, Central South University (No.20230007).
Mice were housed in the Animal House of the Second Xiangya Hospital with a 12-hour daylight/darkness cycle. The room temperature exposure group (RT) of mice was kept at 22–25ºC for 30 days, while the cold exposure group (CT) was initially acclimated to 18ºC for 7 days and then exposed to 4–8ºC for another 30 days. The cold room was equipped with a ventilation system to circulate cold air.
Male C57BL/6 mice (8 weeks old) were obtained from Hunan SJA Laboratory Animal Co., LTD. (Changsha, China). To explore the effect of ambient temperature on wound healing in mice, we directly placed the mice in CT (4–8ºC) or RT (22–25ºC) for 30 days to create wound models and took photos on days 0, 3, 6, 9, and 12 after modeling. The hair in the treatment region was shaved, and 1.0 × 1.0 cm full-thickness excision skin wounds were generated on the dorsum for evaluation of the effects of the sEVs on wound healing. The animals were randomized into several groups that received a total of 100 µL injection of 200 µg/mL samples (PBS, RT-sEVs, CT-sEVs, CT-sEVs + NC and CT-sEVs + antagomiR-423-3p) around the wounds at four injection sites (25 µL per site) on days 0, 2, 4, 6, 8, and 10 post wounding (n = 6).
Wound-size reduction was calculated using the following formula: wound-size reduction (%) = (A0 –At)/A0 × 100, where A0 represents the original size of wound and at indicates the diameter of wounds at the indicated times [39, 40]. Mice were sacrificed and skin samples were collected 12 days after surgery. The lower surface of the skin was observed and photographed to detect newly formed blood vessels.
Luciferase reporter assay
For the luciferase reporter assay, HMEC-1 were co-transfected with a luciferase reporter carrying the wild-type poly (A) binding protein cytoplasmic 1 (PABPC1) 3′-untranslated regions (UTR), a mutant PABPC1 3′-UTR and miR-423-3p mimics or scramble oligonucleotides. 48 h after transfection, luciferase activity was quantified using the luciferase assay system (Promega, Madison, WI, USA). Renal luciferase activity was used to standardize the relative luciferase activity of cells.
Histological and immunofluorescence analysis
The collected mouse samples, including the wound bed and surrounding healthy skin, were fixed in 4% paraformaldehyde and then sliced into 10 μm thick sections after dehydration and embedding. The wound sections were stained with hematoxylin and eosin (H&E), and the percentage of re-epithelial cells (E%) was evaluated according to the previously described method: E% = Wn/Wo × 100, where Wo is the original wound distance and Wn is the length of newly formed epithelium on the wound surface [41]. Masson trichrome staining was used to evaluate the maturity of collagen. Masson’s average dyeing intensity was measured in at least three random fields of vision for each part using Image Pro Plus 6 software.
Immunofluorescence staining of ki67 and CD31 was performed to estimate the range of newly formed capillaries during wound healing. The skin samples on the 8th day after the trauma were fixed in 4% paraformaldehyde. The sections were rehydrated and subjected to immunofluorescence staining for ki67 and CD31. The sections were heated in a microwave in citrate buffer (0.01 M, pH 6.0) for 15 min to retrieve the antigen. The sections were then blocked in 5% BSA at room temperature for 30 min, and the primary antibodies anti-ki67 (1:100, Servicebio, China) or anti-CD31 (1:50, Servicebio, China) were incubated overnight at 4ºC. The sections were then incubated with the corresponding secondary antibodies (1:250, Servicebio, China) at room temperature for 1 h while protected from light. The nuclei were stained with 0.5 µg/mL 4′,6-diamidino-2-phenylindole (DAPI) (C0065, Solarbia, China). The signals were analyzed with a fluorescence microscope (Leica DMI6000B, Solms, Germany). Three random areas of each slice near the wound edge were counted using the Image Pro Plus 6 software.
Statistical analysis
Data are presented as mean ± SD. Multiple-group comparisons were made by one-way ANOVA. The independent-sample t-test was used to compare means between two different groups. Analyses were performed using the GraphPad Prism software. Differences were judged to be statistically significant when p was < 0.05. All experiments were repeated at least three times. In the figures, statistical significance is indicated as ns > 0.05; *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001.
Results
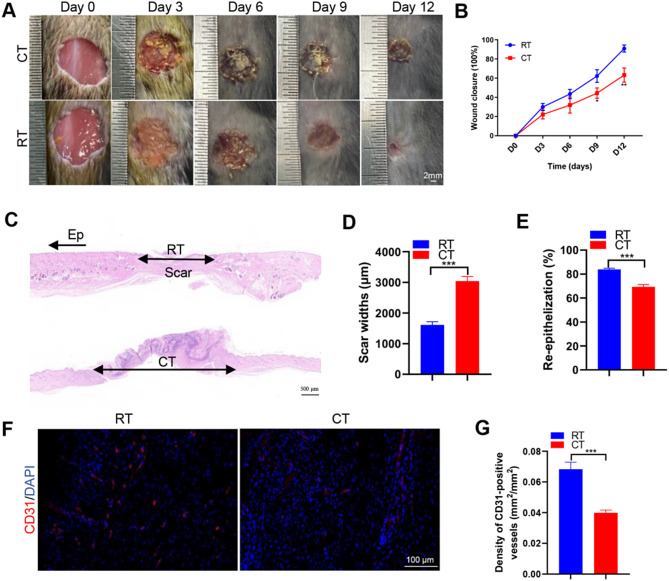
Wound healing was delayed in CT environments
To determine the effects of CT or RT on wound healing, mice were placed in RT (22–25 °C) or CT (4–8 °C) environments for 30 days. Two full-thickness cutaneous wounds were created on the back of each mouse, followed by continuous placement in RT or CT environments. Digital photographs of the wounds showed much slower wound closure in mice exposed to CT, as determined by the larger wound areas measured at days 9 and 12 post-wounding compared to the RT group (Fig. 1A-B). H&E staining revealed that on day 12 post-injury, wounds subjected to prolonged chronic cold exposure at 4 °C for 30 days displayed shorter new epidermis and dermis, with fewer regenerating hair follicles and adipocytes, compared to wounds treated at RT (Fig. 1C). Quantification of the epithelial reformation rate and scar width further confirmed that cold exposure inhibited epidermal regeneration and increased scar formation in the wounds (Fig. 1D-E). As shown in Fig. 1F, few blood vessels were observed in the wounds treated with cold exposure (CT), while abundant blood vessels appeared in the wounds treated with RT. Quantification of new vessel density, defined as the area of CD31-positive stained cells per square millimeter, validated the reduction in vessel numbers induced by CT (Fig. 1G). These data suggest that cold exposure can inhibit the vascular response and wound healing processes at the wound site in mice.
Fig. 1.
Delayed wound healing in the CT environment. (A) Gross view of wounds treated with RT or CT at day 3, 6, 9 and 12 post-wounding. (B) The rate of wound closure in wounds receiving different RT or CT treatments, with n = 6 per group. (C) Representative images of H&E-stained wound sections at day 12 post-wounding. Double-headed black arrows indicate the edges of the scars. Ep: epithelium. Scale bar: 500 μm. (D) Quantification of the scar widths, n = 4 per group. (E) Quantification of the rate of re-epithelialization, n = 4 per group. (F) CD31 immunofluorescence staining of wound sections at day 12 post-wounding. Scale bar: 100 μm. (G) Quantitative analysis of the density of blood vessels in (F), n = 4 per group. Two-group comparison was performed using unpaired, two tailed student’s t-test. *p < 0.05; **p < 0.01; ***p < 0.001
CT-sEVs decelerated the healing of wounds
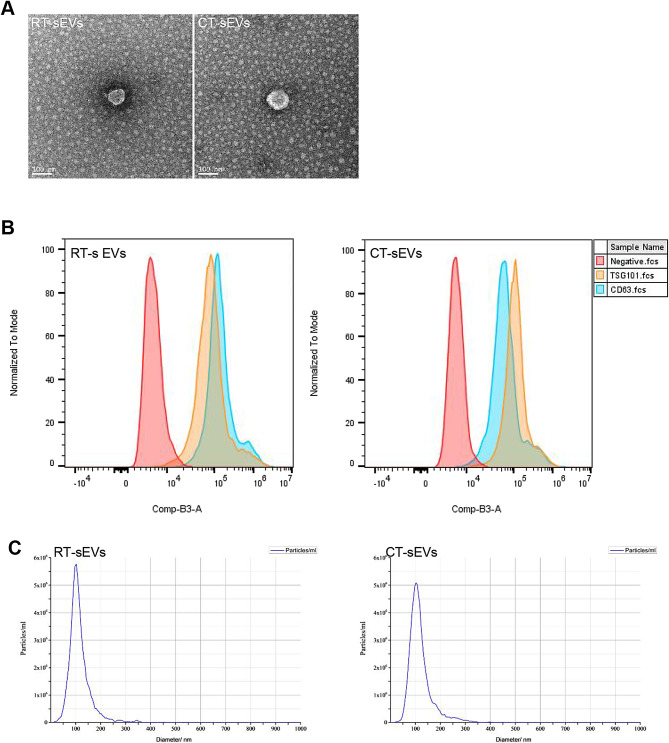
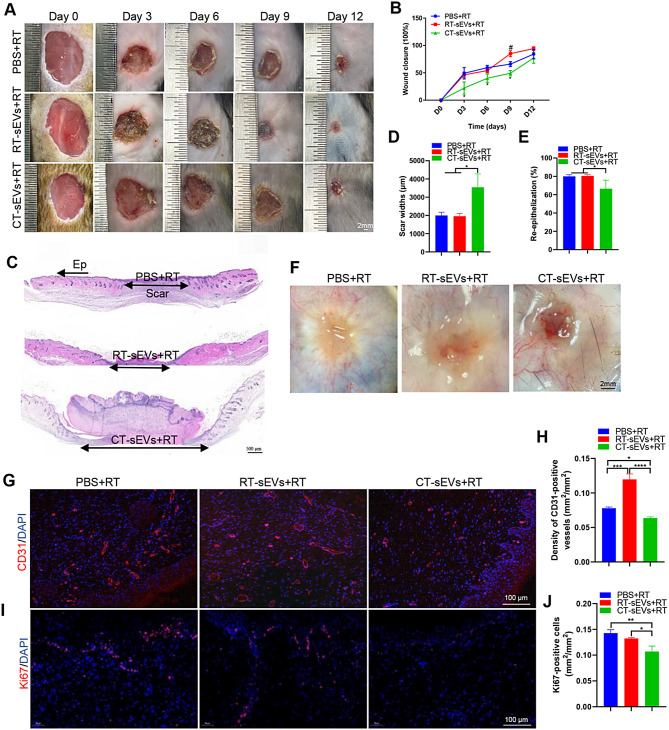
To investigate the potential mechanism involved in delayed wound healing in a cold environment, we isolated sEVs from the plasma of mice raised in room temperature (RT) or cold temperature (CT) environments. We performed TEM, NTA analysis, and Western blotting to identify the purified nanoparticles derived from plasma. As shown in Fig. 2A and C, the vesicles exhibited a cup- or sphere-shaped morphology with a diameter ranging predominantly from 50 nm to 150 nm, similar to previously reported exosomes [38, 42]. Western blotting further confirmed that these particles contained exosomal surface markers such as CD9, CD81 and TSG101 (Fig. 2B). Together, these results confirmed that the isolated circulating nanoparticles were mainly exosomes. To assess the effects of sEVs on wound healing, we transplanted PBS, RT-sEVs, or CT-sEVs into mice placed in RT. As indicated by the representative images of wounds at days 3, 6, 9 and 12 post-wounding in Fig. 3A and the quantification of the wound closure rates in Fig. 3B, the CT-sEVs-transplanted group showed profoundly delayed wound closure at all tested time points compared to mice injected with PBS or RT-sEVs. H&E staining showed that the mice receiving PBS or RT-sEVs treatments had much shorter scar tissues (with an absence of cutaneous fat and hair follicles in the dermis) compared to CT-sEVs mice (Fig. 3C). Quantification of the rate of re-epithelialization and scar width further confirmed that CT-sEVs transplantation weakened epidermal regeneration and induced scar formation of wounds (Fig. 3D-E). The wound tissues of RT-sEVs-treated mice also exhibited more regularly and densely arranged collagen fibers than those of CT-sEVs-treated mice, as revealed by Masson’s trichrome staining (Fig. S1A-B). Skin images from the undersurface revealed that RT-sEVs or PBS-treated wounds exhibited many more newly formed blood vessels compared to the CT-sEVs group wounds at day 12 post-wounding (Fig. 3F). As shown in Fig. 3G-H, a larger number of blood vessels were observed in RT-sEVs-treated wounds compared to the wounds treated with CT-sEVs. We also performed ki67 staining to test the proliferation of skin cells in the wound sites. Immunofluorescence staining for ki67 indicated a much lower number of proliferating cells in the wound sites of CT-sEVs-treated mice compared with RT-sEVs or PBS-treated mice (Fig. 3I-J). These data indicate that CT-sEVs induce anti-wound healing effects, while RT-sEVs have a certain promoting effect on wound healing, but there was no significant statistical difference compared to the PBS group. Subsequently, we placed the mice in a cold exposure environment for 30 days followed by subcutaneous injection of RT-sEVs, CT-sEVs, or an equal volume of PBS. The entire injection cycle was kept at 4–8℃ until the mouse was sacrificed. As shown in Fig. S2A-B, RT-sEVs significantly induced rapid closure of cold exposure wounds compared with the CT-sEVs or PBS groups.
Fig. 2.
Identification of sEVs. (A) Morphology of RT-sEVs or CT-sEVs under transmission electron microscopy. Scale bar: 100 nm. (B) Flow cytometry analysis of the cell surface markers on RT-sEVs or CT-sEVs (n = 4). (C) Diameter distribution of RT-sEVs or CT-sEVs
Fig. 3.
CT-sEVs reduced cutaneous wound healing in mice. Representative images (A) and closure rate (B) of wounds treated with PBS, RT-sEVs and CT-sEVs were observed at days 3, 6, 9 and 12 post-wounding. n = 6 per group. (C) Representative images of H&E-stained wound sections at day 12 post-wounding. The double-headed black arrows indicate the edges of the scars. Ep: epithelium. Scale bar: 500 μm. (D) Quantification of the scar widths, n = 4 per group. (E) Quantification of the rate of re-epithelialization, n = 4 per group. (F) Gross view of wounds treated with PBS, RT-sEVs and CT-sEVs at day 12 post-wounding from the undersurface. Newly formed blood vessels were detected in the wound sites. Scale bar: 2 mm. (G) CD31 immunofluorescence staining of wound sections treated with PBS, RT-sEVs and CT-sEVs at day 12 post-wounding. Scale bar: 100 μm. (H) Quantitative analysis of the density of blood vessels in (G), n = 4 per group. (I) Ki67 immunofluorescence staining of wound sections treated with PBS, RT-sEVs and CT-sEVs at day 12 post-wounding. Scale bar: 100 μm. (J) Quantitative analysis of the density of blood vessels in (I), n = 4 per group. One-way ANOVA combined with Bonferroni post hoc test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. (*) Significant difference CT-sEVs group vs. RT-sEVs group, (#) Significant difference RT-sEVs group vs. PBS (control) group
CT-sEVs inhibited the proliferation, migration and angiogenic activities in vitro
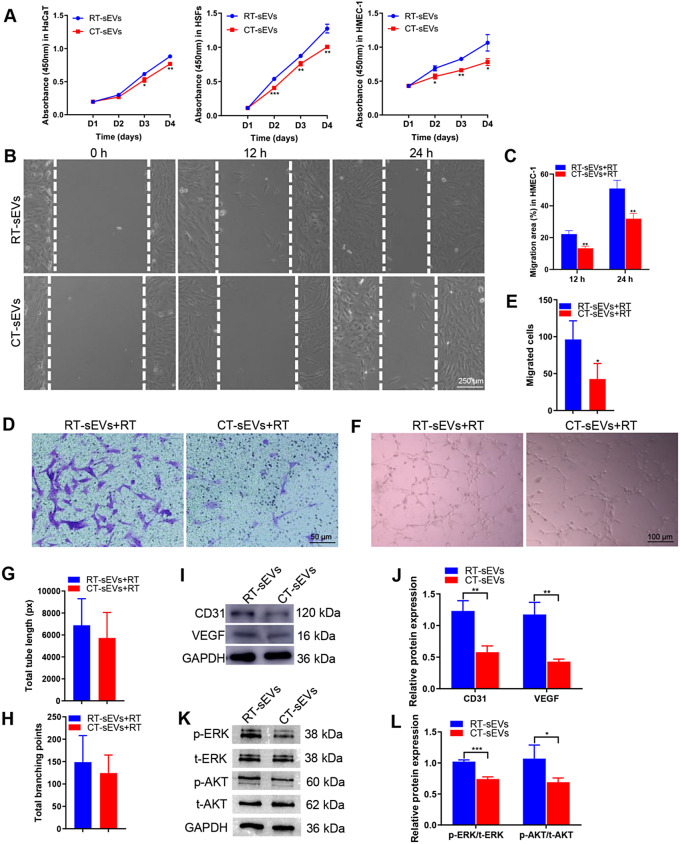
Next, we determined whether sEVs could be internalized into keratinocytes, fibroblasts, and endothelial cells, which is a prerequisite for subsequent exosomal miRNA transfer. As shown in Fig. S3A, PKH26-labeled sEVs were transferred to the perinuclear region of HaCaT, HSFs and HMEC-1 after 3 h of incubation. Fluorescence microscopy analysis revealed that the PKH26-labeled sEVs had been transferred to the perinuclear region of HaCaT, HSFs, and HMEC-1. CCK-8 analysis was applied to measure the effect of sEVs on the proliferation of HSFs, HaCaT, and HMEC-1. The proliferation of these cells was markedly decreased in response to CT-sEVs stimulation compared with the cells incubated in RT-sEVs-containing medium (Fig. 4A). CT-sEVs treatment also caused a remarkable decrease in HMEC-1 and HSFs migration compared to the RT-sEVs groups, as evidenced by the HMEC-1 and HSFs scratch wound assay (Fig. 4B-C and Fig. S4A-B) and HMEC-1 transwell migration assay (Fig. 4D-E). The tube formation assay on Matrigel was used as an in vitro model of angiogenesis. As shown in Fig. 4F, HMEC-1 cells treated with CT-sEVs exhibited a lower number of capillary-like structures compared to the RT-sEVs group. Quantitative measurements showed significant decreases in the total tube length and total number of branching points after CT-sEVs stimulation (Fig. 4G-H). Consistent with these results, there was a decrease in the expression of angiogenesis-related proteins CD31 and VEGF (Fig. 4I-J). These findings indicate that CT-sEVs reduce the angiogenic activities of endothelial cells. Cold stress (around 5℃) usually causes cell apoptosis [43], and HMEC-1, which is crucial for the healing process, may also suffer under these conditions. Through Western blotting detection, we found that CT-sEVs treatment activated BAX expression and exhibited pro-apoptotic effects, suggesting that cold environments may further affect wound healing by inducing HMEC-1 cell apoptosis (Fig. S5A-B). Previous studies have reported a significant correlation between the activation of the ERK and AKT pathways and wound healing and angiogenesis [40, 44]. The Western blotting images and the quantitative data of the relative band intensities revealed that CT-sEVs induced significant decreases in the phosphorylation of ERK and AKT (Fig. 4K-L).
Fig. 4.
CT-sEVs inhibited the proliferation and migration and angiogenic activities in vitro. (A) HaCaT, HSFs and HMEC-1 exhibited a much weaker proliferative ability when exposed to CT-sEVs, as tested by CCK-8 analysis, n = 4 per group. (B) CT-sEVs inhibited HMEC-1 migration as analyzed by scratch wound assay. Scale bar: 250 μm. (C) Quantitative analysis of the migration rates in (B), n = 4 per group. (D) The migratory ability of HMEC-1 receiving different treatments was further confirmed by the transwell assay. Scale bar: 50 μm. (E) Quantitative analysis of the migrated cells in (D), n = 4 per group. (F) Representative images of HMEC-1 tube formation. Scale bar: 100 μm. (G and H) Quantification of the total tube length and total branching points, n = 4 per group. (I and J) CT-sEVs incubation reduced the protein levels of CD31, VEGF, p-ERK and p-AKT in HEMC-1. (K and L) Densitometric quantification of the relative band intensity in (J and L), n = 4 per group. Two-group comparison was performed using unpaired, two tailed student’s t-test. *p < 0.05; **p < 0.01; ***p < 0.001
mir-423-3p mediated the anti-angiogenic effect of CT-sEVs by targeting PABPC1
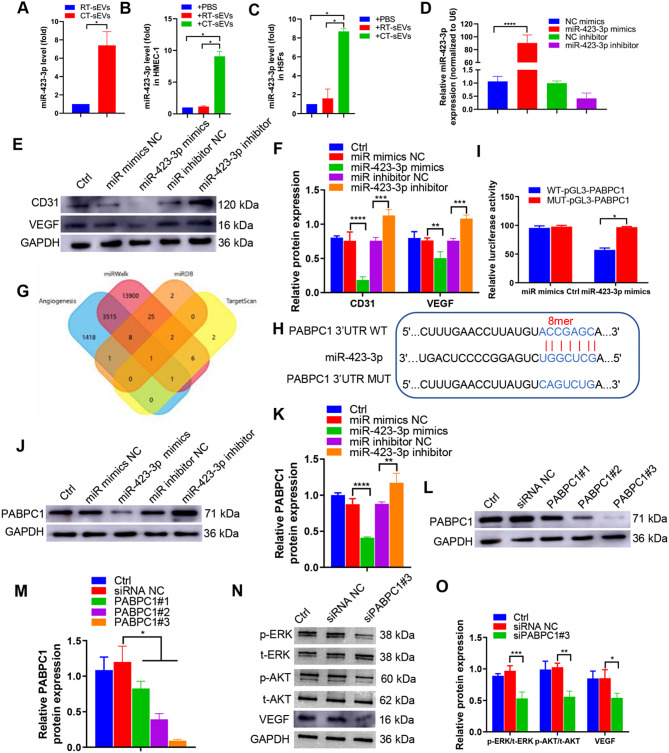
To explore the mechanism involved in the CT-sEVs-induced anti-angiogenic effect, we employed miRNA sequencing to compare the miRNA expression profiles of CT-sEVs and RT-sEVs from mouse plasma. The sequencing results can be found in our previously published studies [3]. Based on the miRNA sequencing results, we selected miR-423-3p, which was the more abundant miRNA in CT-sEVs compared with RT-sEVs (Fig. 5A). Moreover, miR-423-3p expression was significantly increased in HMEC-1 or HSFs after intervention with CT-sEVs compared to HMEC-1 or HSFs from intervention with RT-sEVs (Fig. 5B-C). In qRT-PCR assays, after transfection with miR-423-3p mimics, miR-423-3p expression in HMEC-1 was significantly higher than in HMEC-1 transfected with NC mimics, and miR-423-3p expression in HMEC-1 treated with miR-423-3p inhibitor was significantly lower than in HMEC-1 treated with the NC inhibitor (Fig. 5D). To verify the role of miR-423-3p in the sEVs-induced regulation of HMEC-1, we first determined the effects of miR-423-3p overexpression or knockdown in HMEC-1. miR-423-3p overexpression reduced the expression of CD31 and VEGF (Fig. 5E-F). In contrast, miR-423-3p knockdown promoted the expression of CD31 and VEGF.
Fig. 5.
miR-423-3p mediated the anti-angiogenic effect of CT-sEVs by targeting PABPC1. (A) qRT-PCR analysis of miR-423-3p expression in sEVs from the plasma of the RT or CT mice (n = 6). (B-C) qRT-PCR analysis of miR-423-3p expression in HMEC-1 (B) and HSFs (C) from RT-sEVs or CT-sEVs (n = 6). (D) qRT-PCR was performed to evaluate the expression of miR-423-3p in HMEC-1 transfected with specific miR-423-3p mimics or inhibitor (n = 4). (E) Western blotting was performed to determine the protein expression levels of CD31 and VEGF in HMEC-1 cells transfected with specific miR-423-3p mimics or inhibitors (n = 4). (F) The data are presented as densitometric ratios, normalised to GAPDH. (G) A Venn diagram showing bioinformatics analysis of miR-423-3p target genes. (H) Schematic representation of miR-423-3p putative target sites in the PABPC1 3′-UTR and the alignment of miR-423-3p with wild type and mutant PABPC1 3′-UTR showing pairing. (I) Luciferase reporter assays were performed using luciferase constructs carrying a wild type or mutant PABPC1 3′-UTR co-transfected into HMEC-1 with miR-423-3p mimics compared with empty vector control. Firefly luciferase activity was normalised to Renilla luciferase activity. (J and K) PABPC1 protein expression in HMEC-1 transfected with miR-423-3p mimics or miR-423-3p inhibitor was determined by Western blotting (n = 4). (L and M) The efficiency of PABPC1 knockdown in HMEC-1 by siRNA was measured by Western blotting (n = 4). (N and O) p-ERK, p-AKT and VEGF expression was measured in the HMEC-1 cells treated with siPABPC1#3 or a siRNA control (n = 4). Two-group comparison was performed using unpaired, two tailed student’s t-test. One-way ANOVA combined with Bonferroni post hoc test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
To understand the mechanism by which miR-423-3p restrained wound healing, we used the online bioinformatics tools TargetScan (Version 7.2, http://www.targetscan.org/vert_72/) and miRDB (http://mirdb.org/mirdb/index.html) and miRWalk (http://mirwalk.umm.uni-heidelberg.de/) to predict potential target genes of miR-423-3p (Fig. 5G). Among them, PABPC1, encoded within the chromosome region 8q22.2–23, is a cytoplasmic-nuclear shuttling protein expressed in most eukaryotes. It has been implicated in promoting angiogenesis [45], atherosclerosis [46] and the occurrence and development of tumors [47]. The sequence alignment results indicate that miR-423-3p has a complementary pairing relationship with the 3′-UTR region of PABPC1 (Fig. 5H), indicating that PABPC1 may be a target gene of miR-423-3p. A luciferase reporter assay also demonstrated that miR-423-3p overexpression reduced the activity of wild type PABPC1 promotor but not mutant PABPC1 promoter (Fig. 5I). In addition, Western blotting showed that PABPC1 protein was downregulated by miR-423-3p mimics and upregulated by miR-423-3p inhibitor (Fig. 5J-K). These data suggest that PABPC1 may be a target of miR-423-3p in HMEC-1.
To determine whether PABPC1 mediates the inhibitory effect of miR-423-3p on angiogenesis, we also used PABPC1-specific siRNA (siPABPC1 #1, 2, and 3) to block its expression. Western blotting showed that all three siPABPC1-sequences could suppress > 70% of PABPC1-protein expression; the third siRNA sequence was the most effective (Fig. 5L-M). Hence, we used this siRNA in subsequent experiments. PABPC1 downregulation reduced the expression of VEGF and decreased the level of ERK and AKT phosphorylation (Fig. 5N-O), indicating that PABPC1 plays a crucial role in HMEC-1 angiogenesis.
CT-sEVs mediated the anti-angiogenic effects involving miR‑423-3p‑PABPC1-ERK/AKT
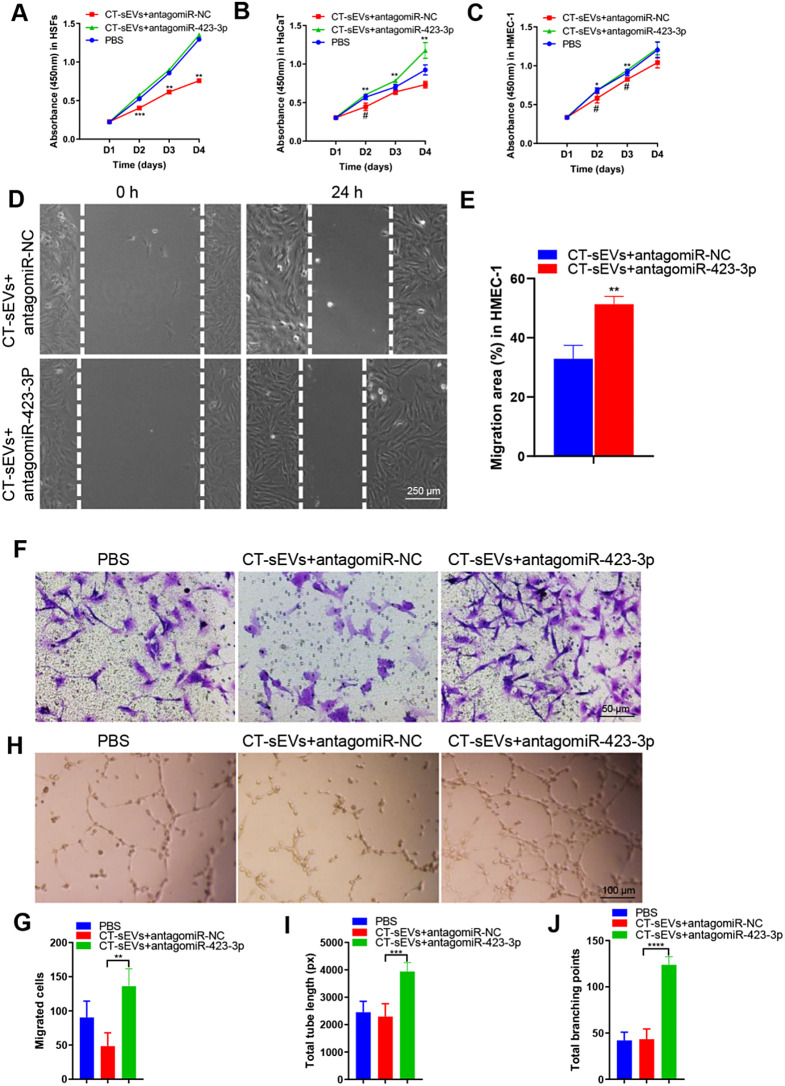
We then investigated whether miR-423-3p derived from CT-sEVs could influence the angiogenic responses of endothelial cells. To produce genetically engineered CT-sEVs with low miR-423-3p levels, we used specific antagomiRs to silence miR-423-3p in CT-sEVs. A CCK-8 assay showed that CT-sEVs + antagomiR-423-3p enhanced the proliferative activity of HMEC-1, whereas treatment with CT-sEVs reduced the proliferation of HMEC-1(Fig. 6A-C). The scratch wound assay and the transwell assay indicated that CT-sEVs remarkably down-regulated the migration of HMEC-1 and HSFs. However, CT-sEVs + antagomiR-423-3p impaired the anti-migratory effect of CT-sEVs (Fig. 6D-G and Fig. S6A-B). The tube formation assay showed a greater number of capillary-like structures on Matrigel in the HMEC-1 treated with CT-sEVs + antagomiR-423-3p group compared with the CT-sEVs group (Fig. 6H). Quantitative analysis of the total tube length and total branching points confirmed that downregulation of miR-423-3p in CT-sEVs blocked the negative effects of CT-sEVs on tube formation (Fig. 6I-J). Collectively, our findings suggest that miR-423-3p is required for CT-sEVs-induced inhibition of endothelial angiogenesis.
Fig. 6.
Genetically engineered CT-sEVs + antagomiR-423-3p facilitated the proliferation, migration and angiogenic effects. (A- B) CCK-8 assay showed that CT-sEVs + antagomiR-NC inhibited HSFs, HaCaT and HMEC-1 proliferation, whereas this effect was attenuated by miR-423-3p inhibition, n = 4 per group. (D) CT-sEVs + antagomiR-NC suppressed HMEC-1 migration as analyzed by scratch wound assay, but this effect was reduced by miR-423-3p inhibitor. Scale bar: 250 μm. (E) Quantitative analysis of the migration rates in (D), n = 4 per group. (F) The migratory ability of HMEC-1 receiving different treatments was further confirmed by the transwell assay. Scale bar: 50 μm. (G) Quantitative analysis of the migrated cells in (F), n = 4 per group. (H) Representative images of the tube formation assay on Matrigel in HMEC-1 treated with PBS, CT-sEVs + antagomiR-NC or CT-sEVs + antagomiR-423-3p. Scale bar: 100 μm. (I -J) Quantitative analyses of the total tube length and total branching points in (B), n = 4 per group. Two-group comparison was performed using unpaired, two tailed student’s t-test. One-way ANOVA combined with Bonferroni post hoc test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. (*) Significant difference CT-sEVs + antagomiR-423-3p group vs. CT-sEVs + antagomiR-NC group, (#) Significant difference CT-sEVs + antagomiR-NC group vs. PBS (control) group
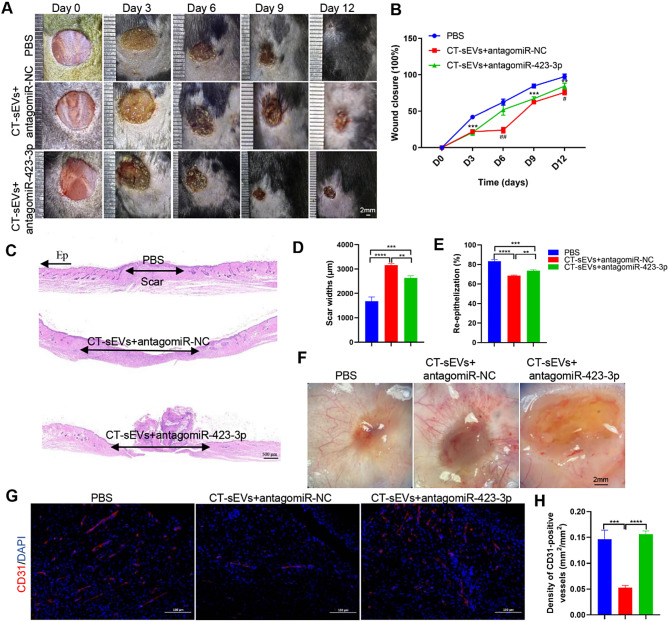
We then aimed to confirm the anti-wound healing effects of CT-sEVs in vivo. Two full-thickness cutaneous wounds were established on the back of each mouse as described above, and the wounds were subcutaneously injected with CT-sEVs + antagomiR-423-3p, CT-sEVs + antagomiR-NC, or an equal volume of PBS. As shown in Fig. 7A-B, wound closure in mice receiving CT-sEVs + antagomiR-423-3p treatment was significantly faster than in those treated that CT-sEVs + antagomiR-NC or PBS. Histological analysis by H&E staining indicated that CT-sEVs + antagomiR-423-3p induced comparable beneficial effects on dermal and epidermal regeneration and reduced scar formation relative to the CT-sEVs + antagomiR-NC mice. (Fig. 7C-E). We next determined whether angiogenesis in the wound sites was enhanced after CT-sEVs + antagomiR-423-3p transplantation. As evidenced by the gross view of skin tissues from the undersurface (Fig. 7F) and CD31 immunostaining (Fig. 7G-H), the wounds treated with CT-sEVs + antagomiR-423-3p therapy had significantly more blood vessel formation compared with the CT-sEVs + antagomiR-NC or PBS- treated control wounds. These data suggest that CT-sEVs or exosomal miR-423-3p ultimately impeded angiogenesis and delayed wound healing. Our finding indicate that miR-423-3p mediated, at least in part, the CT-sEVs-induced anti-wound healing effects. The results suggest that CT-sEVs represent a new mechanism to explain the delayed healing caused by cold exposure, involving miR-423-3p-PABPC1-ERK/AKT-induced anti-angiogenic and anti-wound healing effects (Fig. 8).
Fig. 7.
Exosomal miR-423-3p decelerated cutaneous wound healing in mice. (A) Gross view of wounds treated with PBS, CT-sEVs + antagomiR-NC and CT-sEVs + antagomiR-423-3p at days 3, 6, 9 and 12 post-wounding. (B) The rate of wound-closure in wounds receiving different treatments at the indicated times, n = 6 per group. (C) H&E staining of wound sections treated with PBS, CT-sEVs + antagomiR-NC and CT-sEVs + antagomiR-423-3p at 12 days after operation. Scale bar: 500 μm. (D- E) Quantitative analysis of scar widths and the extent of re-epithelialization in (C), n = 6 per group. (F) Gross view of wounds treated with PBS, CT-sEVs + antagomiR-NC and CT-sEVs + antagomiR-423-3p at day 12 post-wounding from the undersurface. Newly formed blood vessels were detected in the wound sites. Scale bar: 2 mm. (G) Representative images of CD31 staining of wound sections. Scale bar: 100 μm. (H) Quantification of the number of ki67-positive cells in (G), n = 4 per group. One-way ANOVA combined with Bonferroni post hoc test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. (*) Significant difference CT-sEVs + antagomiR-423-3p group vs. CT-sEVs + antagomiR-NC group, (#) Significant difference CT-sEVs + antagomiR-NC group vs. PBS (control) group
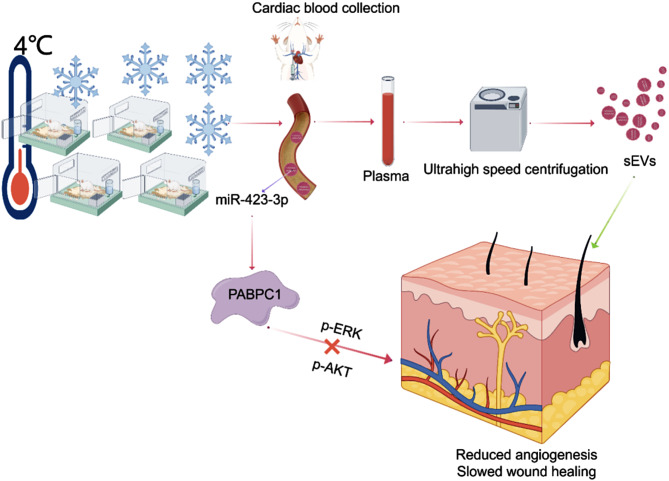
Fig. 8.
CT-sEVs enrichment of miR-423-3p under CT exposure can delay wound healing through the ERK or AKT pathway. PABPC1 was found to be a potential target of miR-423-3p (Created by Figdraw)
Discussion
Research on the effect of temperature on wound healing in mice has been very limited. Both cold or hot temperatures have adverse effects on the healing of skin wounds. A study conducted in 2017 reported that heat delayed the healing of skin wounds in mice [48]. This suggests that temperatures that are either excessively cold or excessively hot are not conducive to wound healing. Cold and heat exposure present multifaceted physiological challenges that trigger a series of adaptive responses in the body. During the process of creating a cold-exposed mouse model, we were greatly interested in the phenomenon of delayed skin wound healing after skin damage caused by fighting between mice. In this study, we confirmed for the first time that CT-sEVs can inhibit the formation and re-epithelialization of neovascularization in the injured area under cold exposure, delaying collagen regeneration and wound healing. CT-sEVs affect the healing of skin wounds through the miR-423-3p/PABPC1 and ERK/AKT signalling pathways. The inhibition of angiogenesis and tissue regeneration in the injured site by CT-sEVs can serve as a new mechanism to explain the delayed healing of wounds under cold exposure.
The normal healing of skin wounds is a complex and precise process, involving various cells and cytokines [17, 49, 50]. Skin wound healing is a process involving multiple cells, including keratinocytes, fibroblasts, macrophages, endothelial cells, and platelet activation. These cells release growth factors, cytokines, and chemokines to coordinate and maintain the healing of healthy individuals [18, 51–53]. During the proliferation stage, both cell proliferation and migration require the formation of granulation tissue to support epithelialization [54], but these processes are disrupted in chronic wounds [55]. During the matrix remodeling phase, the reconstruction of a normal blood supply provides a favorable microenvironment for the migration and proliferation of epidermal and dermal cells. In turn, this leads to re-epithelialization of the wound and restoration of epidermal integrity. The epithelialization process of all types of chronic wounds was impaired [56]. Fibroblasts proliferate and synthesize extracellular matrix on the wound surface. Previous research by Irene Cohen has shown that exposure to heat and cold can cause skin-specific effects such as vasodilation and contraction [57], as well as systemic effects such as changes in pituitary, adrenal, and thyroid activity [58], which may affect healing. Exposure to both hot and cold environments can also lead to weight loss [59, 60], which may hinder healing. Based on the health development of special occupational groups such as polar workers, travel explorers, and emergency medical rescue personnel, it is necessary to study the metabolic effects of temperature on various organs of the body. Previous studies have reported that long-term chronic cold exposure has different effects on various organs of the body. For example, mice living at 4℃ for an extended period can experience delayed onset and progression of diabetes [61]; Low temperatures can activate the autophagy pathway and have a significant protective effect on vascular calcification in the arterial media [3]; low temperatures are associated with longer lifespan [62]; low temperatures can kill some cancer cells [63]. However, low temperature can also have adverse effects on the body, such as frostbite [64], exacerbation of respiratory infections [8], and increased incidence of cardiovascular adverse events [2]. Here we used the temperature of 4℃ to conduct in-depth research on wound healing [3, 61].
This study used the classic ultra-high speed centrifugation method to extract sEVs from mouse plasma. sEVs can selectively sort and transmit active molecules such as mother cell-derived proteins, mRNAs, and miRNAs to recipient cells, playing a signal transduction role between cells [30, 31]. sEVs can be transported remotely to target tissues through the tissue fluid or blood, protecting these active molecules from degradation and dilution by the external environment. sEVs play an important role in the biological effects of wound healing throughout the entire process [65]. Additionally, specific surface ligands on the exosomal membrane ensure their efficient binding to receptor cells [32]. After interacting with the receptor cell, the active substances in the sEVs are released into the target cell and continue to function in the target cell. In recent years, there have been many reports on the relationship between plasma extracellular vesicles and tissue repair functions. Guo et al. [66] reported that sEVs derived from platelet-rich plasma promote re-epiphilization of synchronic copper waves via activation of YAP in a diabetic rat model. Kim et al. [67] suggested that sEVs from human cord blood plasma accelerate copper wound healing by promoting fiberblast function, angiogenesis, and M2 macrophage differentiation. Their research on the function of plasma-derived sEVs was conducted at room temperature, but there have been almost no studies on the wound healing function of plasma-derived sEVs under cold temperatures.
Extracellular vesicles can carry miRNA, which can affect the function of target cells by altering the level of miRNA in the target cells after uptake [68]. miR-423-3p, previously considered a carcinogenic gene for several cancers, has also identified as a biomarker for lung cancer [69, 70], prostate cancer [71], gastric cancer [72] and colorectal cancer [73]. Previous studies have shown that miR-423-3p has a certain correlation with angiogenic activities that are detrimental to wound healing [74]. Therefore, we focused on exosomal miR-423-3p for further investigation. In this study, we identified that miR-423-3p was enriched in CT-sEVs compared with RT-sEVs and required for the CT-sEVs-induced negative effects on angiogenesis and wound healing of HMEC-1. The in vivo results revealed that the knockdown of miR-423-3p impaired the ability of CT-sEVs to inhibit angiogenesis and wound healing. Genetically engineered CT-sEVs + antagomiR-423-3p facilitate facilitated wound repair by promoting angiogenesis. PABPC1 is an important RNA-binding protein (RBP) for the initiation of protein translation and mRNA decay. Biologically, PABPC1 is necessary for regulating vertebrate oocyte and early embryo translation, as well as modulating the protein synthetic capacity of the mammalian heart [75]. PABPC1 promotes the proliferation, migration, and invasion of oesophageal squamous cell carcinoma cells and inhibits apoptosis, similar findings have been reported in liver cancer and gastric cancer [76, 77]. Another study revealed that PABPC1/IFI27 mediates the expression of miR-21-5p, promotes the packaging of miR-21-5p into extracellular vesicles, increases angiogenesis by transporting miR-21-5p to vascular endothelial cells, and subsequently inhibits the expression of CXCL10 in vascular endothelial cells [45]. Therefore, PABPC1 gene may therefore be an angiogenesis suppressor gene. Currently, there is no literature reporting on the targeted relationship between miR-423-3p and the PABPC1 gene. We confirmed the targeted relationship between miR-423-3p and PABPC1 gene using luciferase reporter gene experiments. Western blotting results showed that overexpression of miR-423-3p in HEMC-1 resulted in downregulation of PABPC1 expression, while downregulation of miR-423-3p resulted in upregulation of PABPC1 expression. We further used siRNA transfection technology to knock down the expression of PABPC1 in HEMC-1. The results showed that silencing the expression of PABPC1 in HEMC-1 led to reduced expression of VEGF. Simultaneously, we detected the main signalling pathways related to angiogenesis at the protein level. The results showed that the phosphorylation of ERK and AKT was inhibited, thereby inhibiting the angiogenesis signalling pathway. By inhibiting these two important pathways [78], vascularization did not significantly improve, rapidly inhibiting the formation of granulation tissue, thereby weakening the blood supply to the wound site. This indicated that miR-423-3p antagonised angiogenesis and wound healing of HMEC-1 by targeting the PABPC1 gene.
Wound healing is a complex and coordinated process influenced by various endogenous and exogenous factors. Intercellular communication is essential for wound cell development and the maintenance of wound microenvironment homeostasis. In recent years, sEVs have received special attention due to their regulatory functions in many biological processes. The collection or composition of extracellular vesicles affects cell communication around the wound, and extracellular vesicles rich in various lipids and proteins mediate wound healing stages such as coagulation, inflammation, and angiogenesis, thereby regulating the body’s specific immune response. We have found that cold environments are not conducive to wound healing and that plasma-derived sEVs play an important role, further suggesting that appropriate temperature is crucial for wound healing.
Conclusion
Our in vitro and in vivo results indicate that long-term chronic cold exposure can lead to the accumulation of a large amount of CT-sEVs in mouse plasma, which are rich in miR-423-3p and target PABPC1. Meanwhile, CT-sEVs can also inhibit the phosphorylation levels of ERK and AKT, thereby delaying the healing of skin wounds. Our results reveal a new mechanism by which plasma-derived sEVs regulate miRNA levels to affect wound healing under cold exposure, and also provided a new idea for the treatment of skin wounds.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank the animal care staff and technicians of the Animal Experimental Center of the Second Xiangya Hospital of Central South University for their care and special treatment of these mice.
Author contributions
Conceptualization: L-Q. Y. and F-X-Z. L. Supervision: F-X-Z. L., J-J. L., L-M. L. and Y-H. L. Investigation: F-X-Z. L., F. X., X. L., R-R. C., M-H. Z., B. G., J-Y. D., S-K. S., K-X. T., C-C. L., Y-L. W., S-Y. H., X. C., Y-Y. W and F. W. Visualization: F-X-Z. L., J-J. L. and L-Q. Y. Resources: F-X-Z. L., J-J. L., Y-C. C. and L-Q. Y. Writing—original draft: F-X-Z. L., J-J. L. and L-Q. Y. Reviewing, editing, and funding acquisition: F. X., X. L. and L-Q. Y. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82370892, 82070910, 82100944, 82200869, and 82100494), National Key Research & Development Program (No. 2021YFC2501701), National Clinical Key Specialties Major Research Projects (Z2023026). The Health Research Project in Hunan Province (No. 20231696, 202103062278 and 202103062278), Health Research Project of Hunan Provincial Health Commission (W20243019), the Natural Science Foundation of Hunan Province (No. 2022JJ40721, 2022JJ30799 and 2022JJ40715), and the Scientific Research Launch Project for new employees of the Second Xiangya Hospital of Central South University (No. 7673).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Consent for publication
All authors agree for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bukowiecki LJ. Energy balance and diabetes. The effects of cold exposure, exercise training, and diet composition on glucose tolerance and glucose metabolism in rat peripheral tissues. Can J Physiol Pharmacol. 1989;67(4):382–93. [DOI] [PubMed] [Google Scholar]
- 2.Dong M, Yang X, Lim S, et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab. 2013;18(1):118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li FX, Liu JJ, Xu F, et al. Cold exposure protects against medial arterial calcification development via autophagy. J Nanobiotechnol. 2023;21(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gostimirovic M, Novakovic R, Rajkovic J, et al. The influence of climate change on human cardiovascular function. Arch Environ Occup Health. 2020;75(7):406–14. [DOI] [PubMed] [Google Scholar]
- 5.Urbański A, Czarniewska E, Baraniak E, et al. Impact of cold on the immune system of burying beetle, Nicrophorus vespilloides (Coleoptera: Silphidae). Insect Sci. 2017;24(3):443–54. [DOI] [PubMed] [Google Scholar]
- 6.Hanssen MJ, Hoeks J, Brans B, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015;21(8):863–5. [DOI] [PubMed] [Google Scholar]
- 7.Ikäheimo TM. Cardiovascular diseases, cold exposure and exercise. Temp (Austin). 2018;5(2):123–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Wu J, Qiao C, et al. Impact of chronic cold exposure on lung inflammation, pyroptosis and oxidative stress in mice. Int Immunopharmacol. 2023;115:109590. [DOI] [PubMed] [Google Scholar]
- 9.Zhou E, Zhang L, He L, et al. Cold exposure, gut microbiota and health implications: a narrative review. Sci Total Environ. 2024;916:170060. [DOI] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. [DOI] [PubMed] [Google Scholar]
- 11.Lee P, Smith S, Linderman J, et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63(11):3686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tipton MJ, Collier N, Massey H, et al. Cold water immersion: kill or cure? Exp Physiol. 2017;102(11):1335–55. [DOI] [PubMed] [Google Scholar]
- 13.Saari MUD, Raiko T. Postprandial oxidative metabolism of Human Brown Fat indicates thermogenesis. Cell Metab. 2018;28(2):207–e163. [DOI] [PubMed] [Google Scholar]
- 14.Mawhinney C, Heinonen I, Low DA, et al. Changes in quadriceps femoris muscle perfusion following different degrees of cold-water immersion. J Appl Physiol (1985). 2020;128(5):1392–401. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowers S, Franco E. Chronic wounds: evaluation and management. Am Fam Physician. 2020;101(3):159–66. [PubMed] [Google Scholar]
- 17.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117(5):1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453(7193):314–21. [DOI] [PubMed] [Google Scholar]
- 19.Qian Y, Zheng Y, Jin J, et al. Immunoregulation in Diabetic Wound Repair with a Photoenhanced Glycyrrhizic Acid Hydrogel Scaffold. Adv Mater. 2022;34(29):e2200521. [DOI] [PubMed] [Google Scholar]
- 20.Hu N, Cai Z, Jiang X, et al. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023;157:175–86. [DOI] [PubMed] [Google Scholar]
- 21.Dekoninck S, Blanpain C. Stem cell dynamics, migration and plasticity during wound healing. Nat Cell Biol. 2019;21(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S, Walimbe T, Chen H, et al. Bioactive extracellular matrix scaffolds engineered with proangiogenic proteoglycan mimetics and loaded with endothelial progenitor cells promote neovascularization and diabetic wound healing. Bioact Mater. 2022;10:460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irisawa T, Matsuyama T, Iwami T, et al. The effect of different target temperatures in targeted temperature management on neurologically favorable outcome after out-of-hospital cardiac arrest: a nationwide multicenter observational study in Japan (the JAAM-OHCA registry). Resuscitation. 2018;133:82–7. [DOI] [PubMed] [Google Scholar]
- 24.Jansen MM, van de Ven AA, van der Valk PG, et al. Measuring sensory and pain thresholds by Semmes-Weinstein monofilaments in patients with leg ulcers: a pilot study. J Wound Care. 2019;28(10):647–55. [DOI] [PubMed] [Google Scholar]
- 25.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliot S, Catanuto P, Pereira-Simon S, et al. Urine-derived exosomes from individuals with IPF carry pro-fibrotic cargo. Elife. 2022;11:e79543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai J, Gong L, Li G, et al. Exosomes in ovarian cancer ascites promote epithelial-mesenchymal transition of ovarian cancer cells by delivery of miR-6780b-5p. Cell Death Dis. 2021;12(2):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Li M, Pan Z, et al. Mir-3184-3p enriched in cerebrospinal fluid exosomes contributes to progression of glioma and promotes M2-like macrophage polarization. Cancer Sci. 2022;113(8):2668–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo ZW, Li FX, Liu YW, et al. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11(43):20884–92. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Li F, Liu B, et al. Adipose-derived mesenchymal stem cell exosomes inhibit transforming growth factor-β1-induced collagen synthesis in oral mucosal fibroblasts. Exp Ther Med. 2021;22(6):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li FX, Lin X, Xu F, et al. The role of mesenchymal stromal cells-derived small extracellular vesicles in diabetes and its chronic complications. Front Endocrinol (Lausanne). 2021;12:780974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li FX, Liu JJ, Xu F, et al. Role of tumor-derived exosomes in bone metastasis. Oncol Lett. 2019;18(4):3935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu YL, Lin ZJ, Li CC, et al. Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study. Signal Transduct Target Ther. 2023;8(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong Y, Chen L, Liu P, et al. All-in-One: multifunctional hydrogel accelerates oxidative Diabetic Wound Healing through timed-release of exosome and fibroblast growth factor. Small. 2022;18(1):e2104229. [DOI] [PubMed] [Google Scholar]
- 35.Yang G, Waheed S, Wang C, et al. Exosomes and their bioengineering strategies in the Cutaneous Wound Healing and related complications: current knowledge and future perspectives. Int J Biol Sci. 2023;19(5):1430–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pegtel DM, Gould SJ, Exosomes. Annu Rev Biochem. 2019;88:487–514. [DOI] [PubMed] [Google Scholar]
- 37.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang ZX, Luo ZW, Li FX, et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat Commun. 2022;13(1):1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin H, Chen CY, Liu YW, et al. Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis. Theranostics. 2019;9(9):2678–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CY, Rao SS, Ren L, et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8(6):1607–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CY, Yin H, Chen X, et al. Ångstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci Adv. 2020;6(43):eaba0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu YY, Shan SK, Lin X, et al. Cellular Crosstalk in the Vascular Wall Microenvironment: the role of exosomes in vascular calcification. Front Cardiovasc Med. 2022;9:912358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao CL, Somero GN. The impact of acute temperature stress on hemocytes of invasive and native mussels (Mytilus galloprovincialis and Mytilus californianus): DNA damage, membrane integrity, apoptosis and signaling pathways. J Exp Biol. 2012;215(Pt 24):4267–77. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Rao SS, Wang ZX, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through mir-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8(1):169–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Chen C, Liu Z, et al. PABPC1-induced stabilization of IFI27 mRNA promotes angiogenesis and malignant progression in esophageal squamous cell carcinoma through exosomal miRNA-21-5p. J Exp Clin Cancer Res. 2022;41(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin M, Hu L, Shen S, et al. Atherosclerosis-related biomarker PABPC1 predicts pan-cancer events. Stroke Vasc Neurol. 2023;9(2):108–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei X, Huo Y, Pi J, et al. METTL3 preferentially enhances non-m(6)a translation of epigenetic factors and promotes tumourigenesis. Nat Cell Biol. 2022;24(8):1278–90. [DOI] [PubMed] [Google Scholar]
- 48.Dos Santos-Silva MA, Trajano ET, Schanuel FS, et al. Heat delays skin wound healing in mice. Exp Biol Med (Maywood). 2017;242(3):258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006;14(5):558–65. [DOI] [PubMed] [Google Scholar]
- 50.Kahlina K, Goren I, Pfeilschifter J, et al. p68 DEAD box RNA helicase expression in keratinocytes. Regulation, nucleolar localization, and functional connection to proliferation and vascular endothelial growth factor gene expression. J Biol Chem. 2004;279(43):44872–82. [DOI] [PubMed] [Google Scholar]
- 51.Le NN, Rose MB, Levinson H, et al. Implant healing in experimental animal models of diabetes. J Diabetes Sci Technol. 2011;5(3):605–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Driskell RR, Lichtenberger BM, Hoste E, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504(7479):277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–43. [DOI] [PubMed] [Google Scholar]
- 54.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25(7):304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nowell CS, Odermatt PD, Azzolin L, et al. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat Cell Biol. 2016;18(2):168–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carvajal-Gonzalez JM, Roman AC, Cerezo-Guisado MI, et al. Loss of dioxin-receptor expression accelerates wound healing in vivo by a mechanism involving TGFbeta. J Cell Sci. 2009;122(Pt 11):1823–33. [DOI] [PubMed] [Google Scholar]
- 57.Solomon GF, Moos RH, Stone GC, et al. peripheral vasoconstriction induced by emotional stress in rats. Angiology. 1964;15:362–5. [DOI] [PubMed]
- 58.Alrich EM, Carter JP, Lehman EP. The effect of ACTH and cortisone on wound healing; an experimental study. Ann Surg. 1951;133(6):783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnett SA. The skin and hair of mice living at a low environmental temperature. Q J Exp Physiol Cogn Med Sci. 1959;44(1):35–42. [DOI] [PubMed] [Google Scholar]
- 60.Riesenfeld A. The effect of extreme temperatures and starvation on the body proportions of the rat. Am J Phys Anthropol. 1973;39(3):427–59. [DOI] [PubMed] [Google Scholar]
- 61.Chevalier C, Stojanović O, Colin DJ, et al. Gut microbiota orchestrates Energy Homeostasis during Cold. Cell. 2015;163(6):1360–74. [DOI] [PubMed] [Google Scholar]
- 62.Lee HJ, Alirzayeva H, Koyuncu S, et al. Cold temperature extends longevity and prevents disease-related protein aggregation through PA28γ-induced proteasomes. Nat Aging. 2023;3(5):546–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seki T, Yang Y, Sun X, et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature. 2022;608(7922):421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sidhar K, Elliott K, Ibrahem M, Heat. Cold, and Environmental emergencies in athletes. Clin Sports Med. 2023;42(3):441–61. [DOI] [PubMed] [Google Scholar]
- 65.Shi R, Jin Y, Hu W, et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318(5):C848–56. [DOI] [PubMed] [Google Scholar]
- 66.Guo SC, Tao SC, Yin WJ, et al. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7(1):81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim S, Kim Y, Hyun YS, et al. Exosomes from human cord blood plasma accelerate cutaneous wound healing by promoting fibroblast function, angiogenesis, and M2 macrophage differentiation. Biomater Sci. 2021;9(8):3028–39. [DOI] [PubMed] [Google Scholar]
- 68.Yu X, Odenthal M, Fries JW. Exosomes as miRNA carriers: formation-function-future. Int J Mol Sci. 2016;17(12):2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Y, Li T, Chen G, et al. Identification of a serum microRNA expression signature for detection of lung cancer, involving miR-23b, miR-221, miR-148b and miR-423-3p. Lung Cancer. 2017;114:6–11. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y, Huang R, Xie D, et al. ZNF674-AS1 antagonizes mir-423-3p to induce G0/G1 cell cycle arrest in non-small cell lung cancer cells. Cell Mol Biol Lett. 2021;26(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo T, Wang Y, Jia J, et al. The identification of plasma exosomal mir-423-3p as a potential predictive biomarker for prostate Cancer castration-resistance development by plasma exosomal miRNA sequencing. Front Cell Dev Biol. 2020;8:602493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kong P, Zhu X, Geng Q, et al. The microRNA-423-3p-Bim Axis promotes Cancer Progression and activates oncogenic autophagy in gastric Cancer. Mol Ther. 2017;25(4):1027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li HT, Zhang H, Chen Y, et al. MiR-423-3p enhances cell growth through inhibition of p21Cip1/Waf1 in colorectal cancer. Cell Physiol Biochem. 2015;37(3):1044–54. [DOI] [PubMed] [Google Scholar]
- 74.de Oliveira A, Castanhole-Nunes MMU, Biselli-Chicote PM, et al. Differential expression of angiogenesis-related miRNAs and VEGFA in cirrhosis and hepatocellular carcinoma. Arch Med Sci. 2020;16(5):1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chorghade S, Seimetz J, Emmons R, et al. Poly(A) tail length regulates PABPC1 expression to tune translation in the heart. Elife. 2017;6:e24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H, Sheng C, Yin Y, et al. PABPC1 interacts with AGO2 and is responsible for the microRNA mediated gene silencing in high grade hepatocellular carcinoma. Cancer Lett. 2015;367(1):49–57. [DOI] [PubMed] [Google Scholar]
- 77.Zhu J, Ding H, Wang X, et al. PABPC1 exerts carcinogenesis in gastric carcinoma by targeting miR-34c. Int J Clin Exp Pathol. 2015;8(4):3794–802. [PMC free article] [PubMed] [Google Scholar]
- 78.Llorián-Salvador M, Byrne EM, Szczepan M, et al. Complement activation contributes to subretinal fibrosis through the induction of epithelial-to-mesenchymal transition (EMT) in retinal pigment epithelial cells. J Neuroinflammation. 2022;19(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.