Abstract
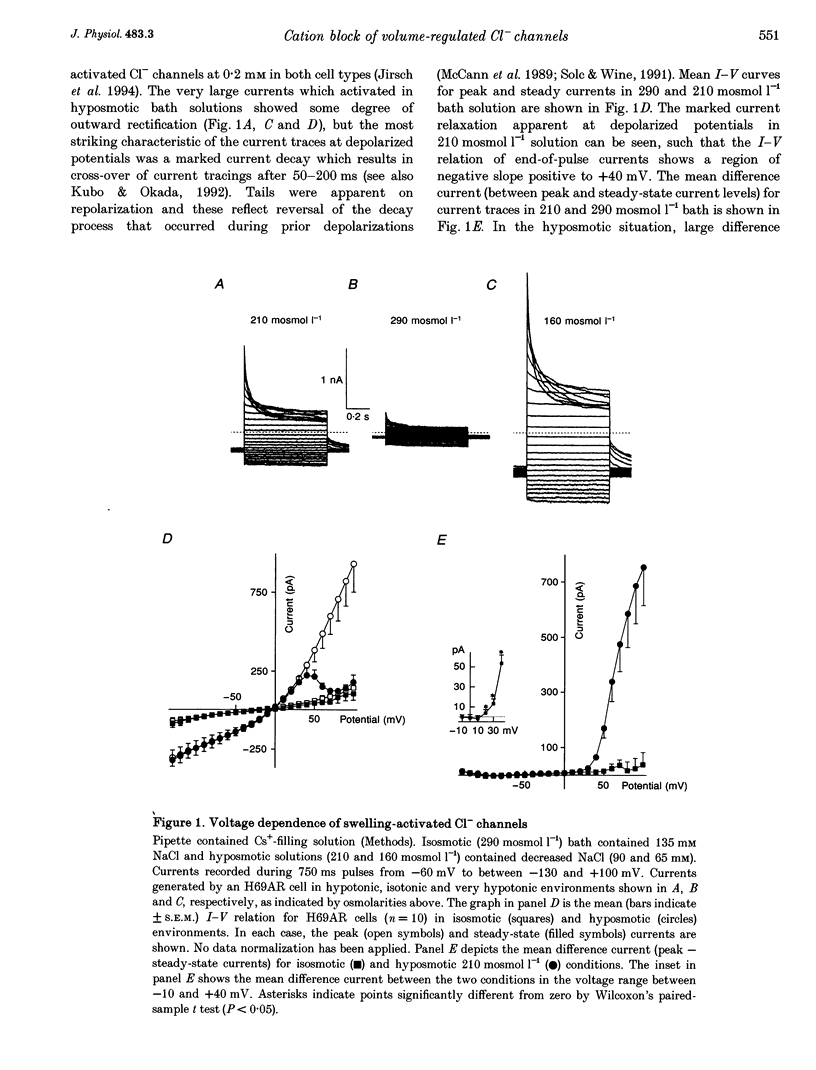
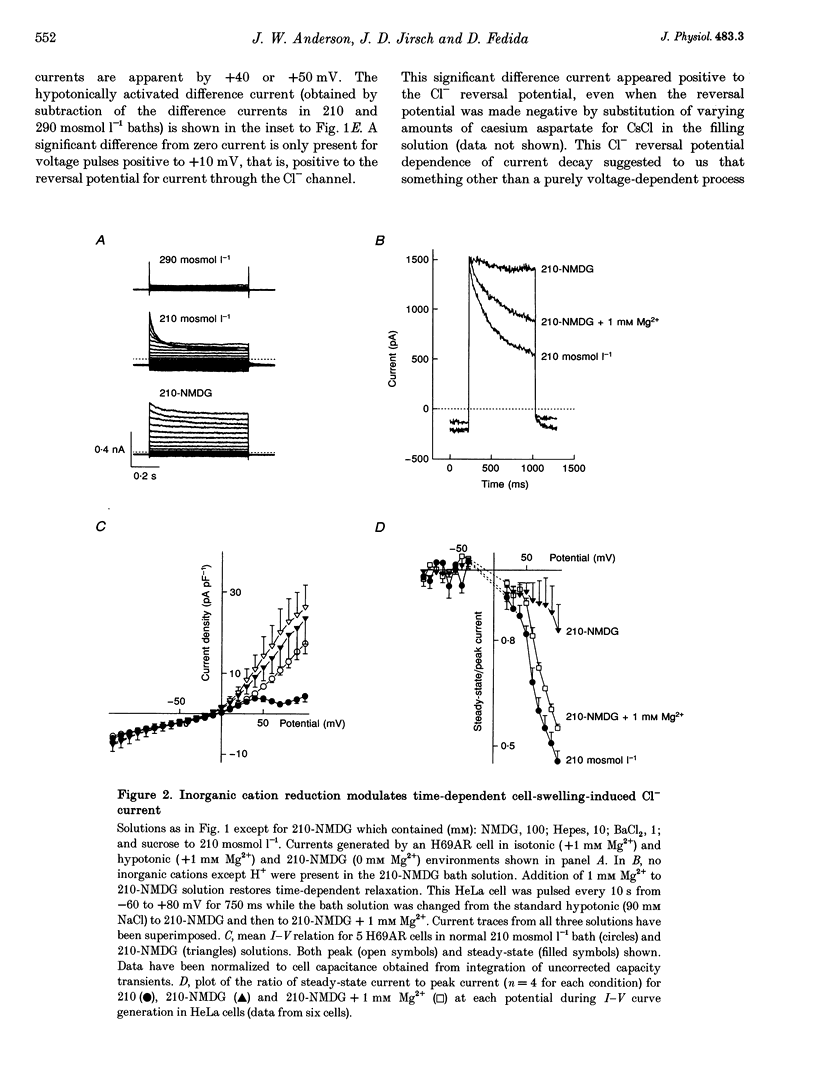
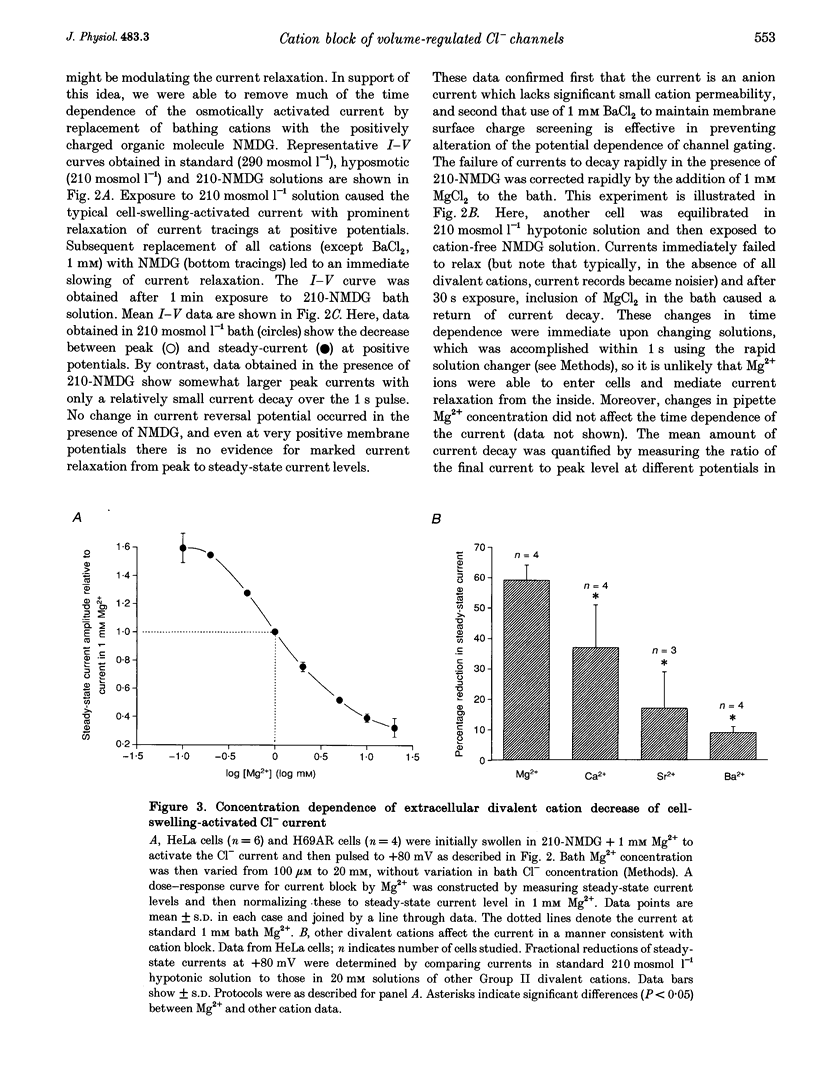
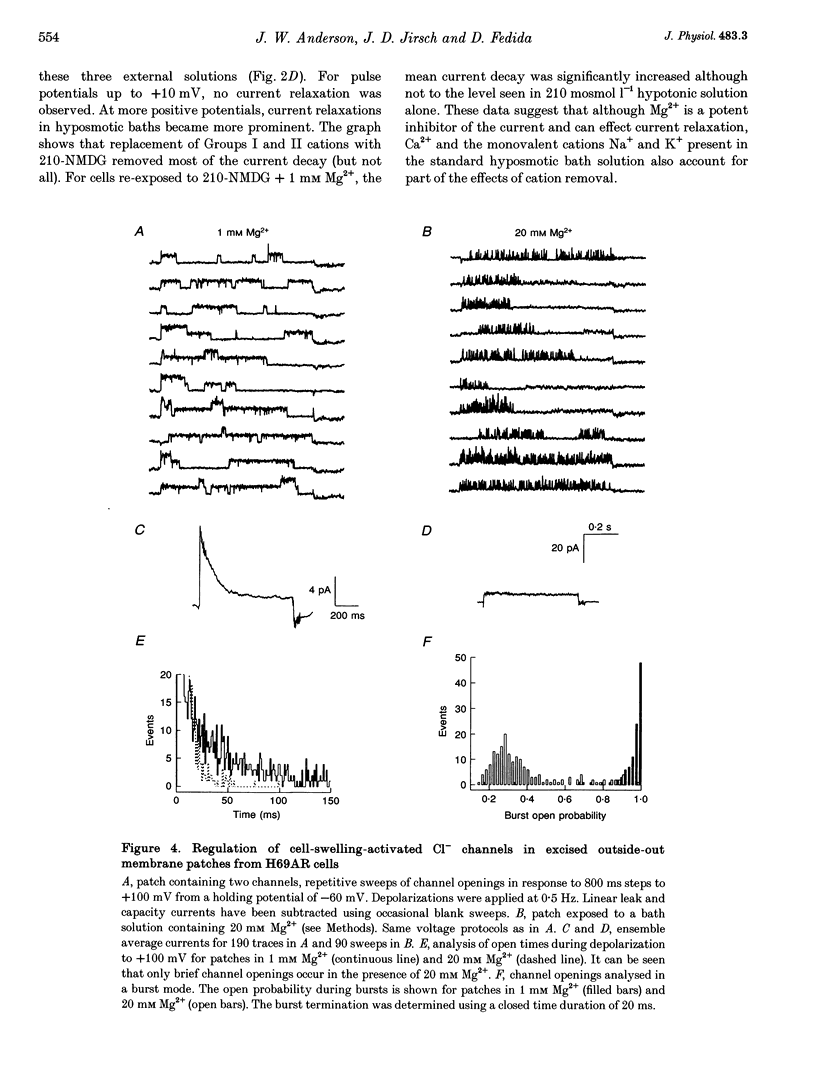
1. In epithelial cells, hyposmotic stress induces visible cell swelling and large Cl- currents, which deactivate on return to isotonic solutions and are abolished by 0.1-0.5 mM DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid). During depolarizing voltage clamp pulses, the currents activate rapidly and show time-dependent relaxation with associated tail currents on return to negative potentials. 2. We used whole-cell and outside-out patch recording to study volume activation of Cl- currents in the epithelial cancer cell lines H69AR and HeLa S5. In a 210 or 160 mosmol l-1 hyposmotic bathing solution containing 90 mM NaCl, 1 mM Ca2+ and 1 mM Mg2+, current relaxation was rapid, occurred positive to the Cl- reversal potential and reduced current to < 30% of its peak level at +100 mV. 3. Replacement of most bath inorganic cations by N-methyl-D-glucamine (NMDG) at constant Cl- concentration and osmolarity eliminated most of the current relaxation and caused an increase in steady-state current levels. Steady-state current was 85 +/- 6% of peak current at +100 mV in NMDG-Cl bath solution. This ratio fell to 55 +/- 2% (n = 5) when 1 mM Mg2+ was re-added to the bath. 4. Re-addition of Mg2+ or other Group II metals (Ca2+, Sr2+, Ba2+) induced immediate changes in current relaxation in a dose- and species-dependent manner. Concentrations of Mg2+ as low as 0.1 mM were effective in causing Cl- current relaxation. The IC50 for steady-state current block by external Mg2+ was 1.75 mM.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman M. J., Wickman K. D., Clapham D. E. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D., Nattel S. Properties of single outwardly rectifying Cl- channels in heart. Circ Res. 1994 Oct;75(4):789–795. doi: 10.1161/01.res.75.4.789. [DOI] [PubMed] [Google Scholar]
- French R. J., Wells J. B. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. J Gen Physiol. 1977 Dec;70(6):707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Jirsch J. D., Loe D. W., Cole S. P., Deeley R. G., Fedida D. ATP is not required for anion current activated by cell swelling in multidrug-resistant lung cancer cells. Am J Physiol. 1994 Sep;267(3 Pt 1):C688–C699. doi: 10.1152/ajpcell.1994.267.3.C688. [DOI] [PubMed] [Google Scholar]
- Krouse M. E., Haws C. M., Xia Y., Fang R. H., Wine J. J. Dissociation of depolarization-activated and swelling-activated Cl- channels. Am J Physiol. 1994 Aug;267(2 Pt 1):C642–C649. doi: 10.1152/ajpcell.1994.267.2.C642. [DOI] [PubMed] [Google Scholar]
- Kubo M., Okada Y. Volume-regulatory Cl- channel currents in cultured human epithelial cells. J Physiol. 1992 Oct;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., Erdmann A., Adragna N. C. K-Cl cotransport, pH, and role of Mg in volume-clamped low-K sheep erythrocytes: three equilibrium states. Am J Physiol. 1994 Jan;266(1 Pt 1):C95–103. doi: 10.1152/ajpcell.1994.266.1.C95. [DOI] [PubMed] [Google Scholar]
- McCann J. D., Li M., Welsh M. J. Identification and regulation of whole-cell chloride currents in airway epithelium. J Gen Physiol. 1989 Dec;94(6):1015–1036. doi: 10.1085/jgp.94.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirski S. E., Gerlach J. H., Cole S. P. Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 1987 May 15;47(10):2594–2598. [PubMed] [Google Scholar]
- Oliet S. H., Bourque C. W. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature. 1993 Jul 22;364(6435):341–343. doi: 10.1038/364341a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker R. L., Frizzell R. A., Dwyer T. M., Farley J. M. Single chloride channel currents from canine tracheal epithelial cells. Biochim Biophys Acta. 1986 Jun 26;858(2):235–242. doi: 10.1016/0005-2736(86)90328-7. [DOI] [PubMed] [Google Scholar]
- Solc C. K., Wine J. J. Swelling-induced and depolarization-induced C1-channels in normal and cystic fibrosis epithelial cells. Am J Physiol. 1991 Oct;261(4 Pt 1):C658–C674. doi: 10.1152/ajpcell.1991.261.4.C658. [DOI] [PubMed] [Google Scholar]
- Spalding B. C., Taber P., Swift J. G., Horowicz P. Zinc inhibition of chloride efflux from skeletal muscle of Rana pipiens and its modification by external pH and chloride activity. J Membr Biol. 1990 Jul;116(3):195–214. doi: 10.1007/BF01868460. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The differential effects of tetraethylammonium and zinc ions on the resting conductance of frog skeletal muscle. J Physiol. 1970 Jul;209(1):231–256. doi: 10.1113/jphysiol.1970.sp009164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J. S., Steinbach J. H., Simchowitz L. Whole cell Cl- currents in human neutrophils induced by cell swelling. Am J Physiol. 1993 Jul;265(1 Pt 1):C156–C165. doi: 10.1152/ajpcell.1993.265.1.C156. [DOI] [PubMed] [Google Scholar]
- Tseng G. N. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. Am J Physiol. 1992 Apr;262(4 Pt 1):C1056–C1068. doi: 10.1152/ajpcell.1992.262.4.C1056. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]


