Abstract
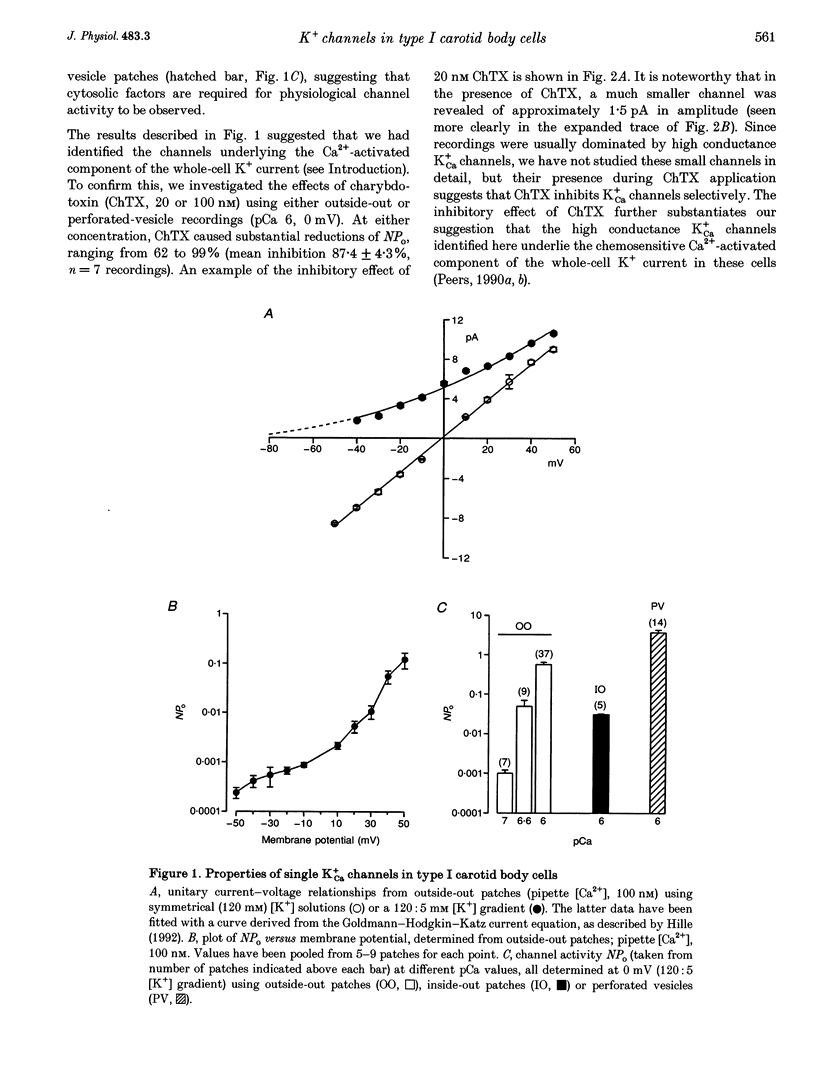
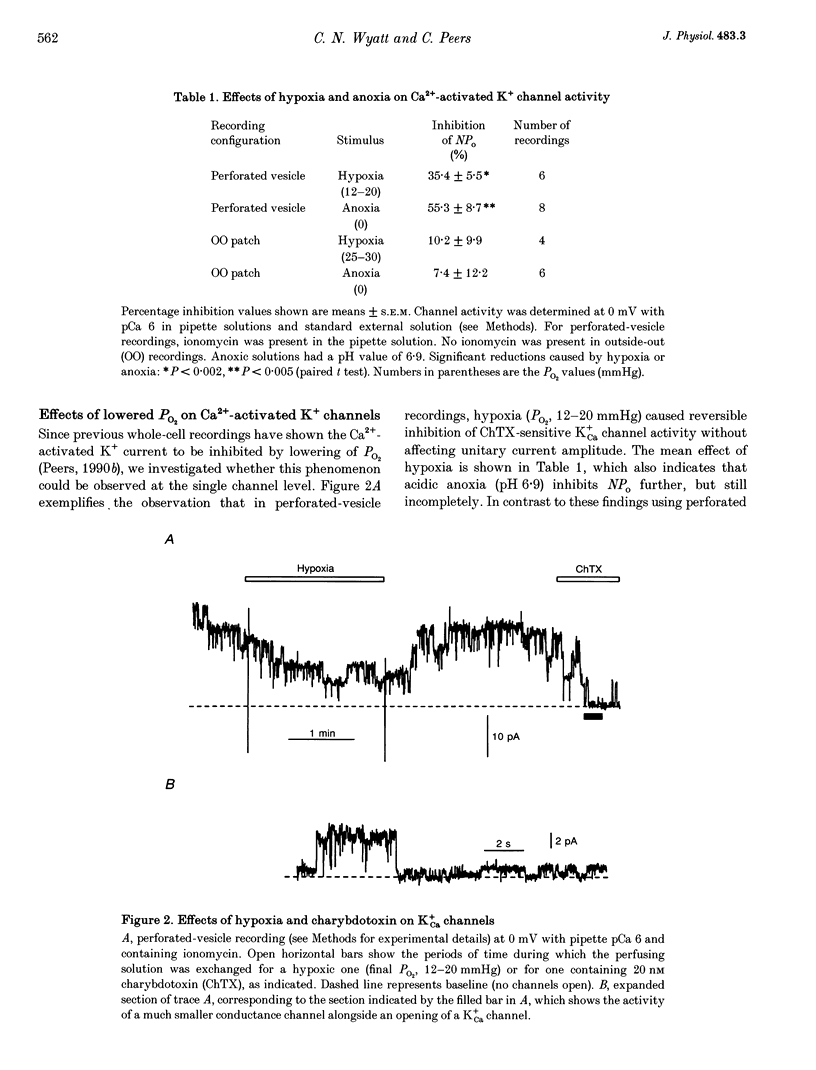
1. Ca(2+)-activated K+ (K+Ca) channels in neonatal rat type I carotid body cells were studied using single channel patch clamp techniques. In outside-out patches, using symmetrical 120 mM [K+] solutions, channels were observed with a slope conductance of 190 pS and a reversal potential of 0 mV. Reducing [K+]o to 5 mM shifted the reversal potential as expected for a K(+)-selective channel. 2. With 100 nM Ca2+ bathing the cytosolic aspect of patches, channel activity (number of active channels in a patch x open probability, NPo) increased with depolarization. NPo also increased with increasing 'cytosolic' [Ca2+] at a fixed membrane potential (0 mV). Using outside-out patches, bath application of 20 or 100 nM charybdotoxin reduced NPo by > 85%. These data indicate the presence of K+Ca channels in type I cells. 3. At 0 mV, using solutions of identical composition (1 microM Ca2+ bathing the cytosolic aspect of the channels), NPo was higher in outside-out patches than in inside-out patches. NPo was greatest in recordings using the perforated-vesicle technique. 4. Hypoxia and anoxia were without effect on K+Ca channels in outside-out patches, but caused significant, reversible reductions of NPo in channels recorded in perforated vesicles. 5. The whole-cell perforated-patch technique was used to record membrane potential at 35-37 degrees C. Hypoxia, anoxia and charybdotoxin all depolarized type I cells. 6. Our results suggest an important role for K+Ca channels in type I carotid body cells, and their activity in relation to a model for chemotransduction is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker H., Bölling B., Delpiano M. A., Dufau E., Görlach A., Holtermann G. The meaning of H2O2 generation in carotid body cells for PO2 chemoreception. J Auton Nerv Syst. 1992 Nov;41(1-2):41–51. doi: 10.1016/0165-1838(92)90125-z. [DOI] [PubMed] [Google Scholar]
- Benot A. R., López-Barneo J. Feedback Inhibition of Ca2+ Currents by Dopamine in Glomus Cells of the Carotid Body. Eur J Neurosci. 1990;2(9):809–812. doi: 10.1111/j.1460-9568.1990.tb00473.x. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Duchen M. R. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol. 1990 Sep;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994 Jul 1;478(Pt 1):157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. R., Henderson L., Jones O. T., Delpiano M. A., Hentschel J., Acker H. Involvement of an NAD(P)H oxidase as a pO2 sensor protein in the rat carotid body. Biochem J. 1990 Dec 15;272(3):743–747. doi: 10.1042/bj2720743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C., Monti-Bloch L., Woodbury J. W. Effects of Putative Neurotransmitters of the Carotid Body on its Own Glomus Cells. Eur J Neurosci. 1990 Jan;2(1):77–88. doi: 10.1111/j.1460-9568.1990.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Fieber L. A., McCleskey E. W. L-type calcium channels in type I cells of the rat carotid body. J Neurophysiol. 1993 Oct;70(4):1378–1384. doi: 10.1152/jn.1993.70.4.1378. [DOI] [PubMed] [Google Scholar]
- Fishman M. C., Greene W. L., Platika D. Oxygen chemoreception by carotid body cells in culture. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1448–1450. doi: 10.1073/pnas.82.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina M. D., López-Barneo J. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2927–2930. doi: 10.1073/pnas.88.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González C., Almaraz L., Obeso A., Rigual R. Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci. 1992 Apr;15(4):146–153. doi: 10.1016/0166-2236(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Kramer R. H. Neuropeptide modulation of single calcium and potassium channels detected with a new patch clamp configuration. Nature. 1990 Dec 6;348(6301):545–547. doi: 10.1038/348545a0. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso A., Rocher A., Fidone S., Gonzalez C. The role of dihydropyridine-sensitive Ca2+ channels in stimulus-evoked catecholamine release from chemoreceptor cells of the carotid body. Neuroscience. 1992;47(2):463–472. doi: 10.1016/0306-4522(92)90260-9. [DOI] [PubMed] [Google Scholar]
- Peers C. Effect of lowered extracellular pH on Ca2(+)-dependent K+ currents in type I cells from the neonatal rat carotid body. J Physiol. 1990 Mar;422:381–395. doi: 10.1113/jphysiol.1990.sp017990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C., Green F. K. Inhibition of Ca(2+)-activated K+ currents by intracellular acidosis in isolated type I cells of the neonatal rat carotid body. J Physiol. 1991 Jun;437:589–602. doi: 10.1113/jphysiol.1991.sp018613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci Lett. 1990 Nov 13;119(2):253–256. doi: 10.1016/0304-3940(90)90846-2. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Stea A., Nurse C. A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 1991 Mar;418(1-2):93–101. doi: 10.1007/BF00370457. [DOI] [PubMed] [Google Scholar]
- Wang Z. Z., Stensaas L. J., Bredt D. S., Dinger B., Fidone S. J. Localization and actions of nitric oxide in the cat carotid body. Neuroscience. 1994 May;60(1):275–286. doi: 10.1016/0306-4522(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Wyatt C. N., Peers C. Nicotinic acetylcholine receptors in isolated type I cells of the neonatal rat carotid body. Neuroscience. 1993 May;54(1):275–281. doi: 10.1016/0306-4522(93)90399-z. [DOI] [PubMed] [Google Scholar]


