Abstract
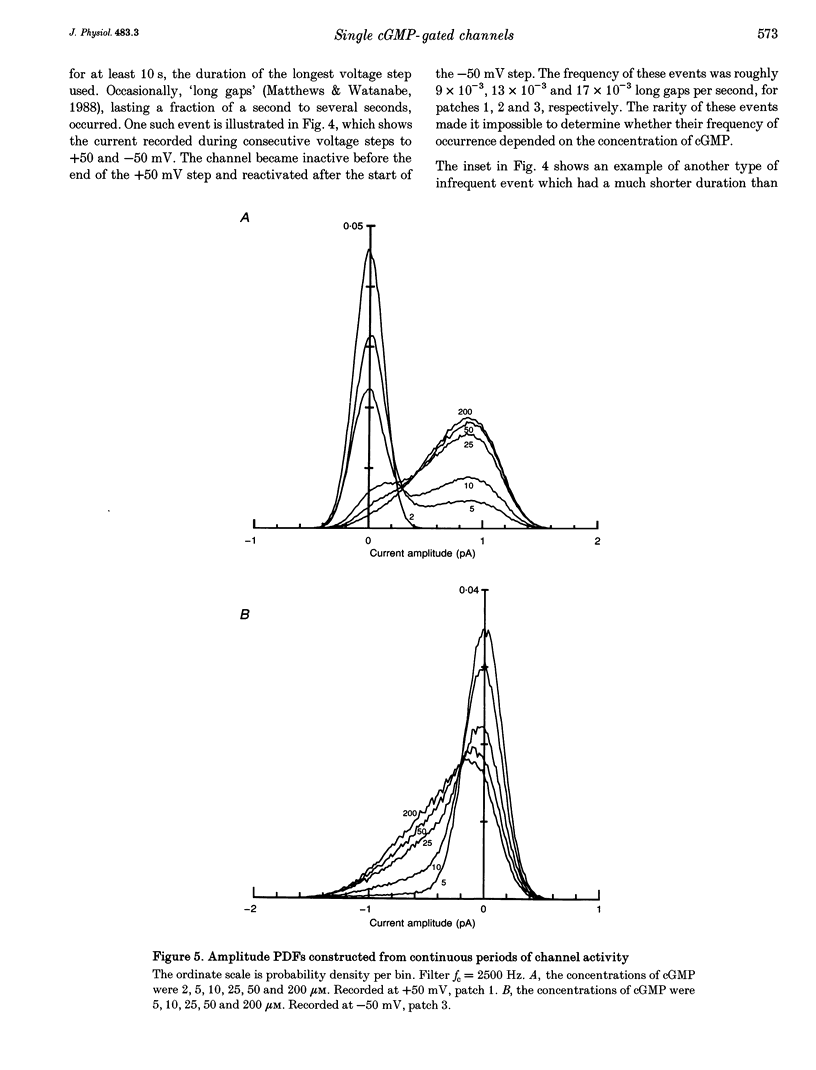
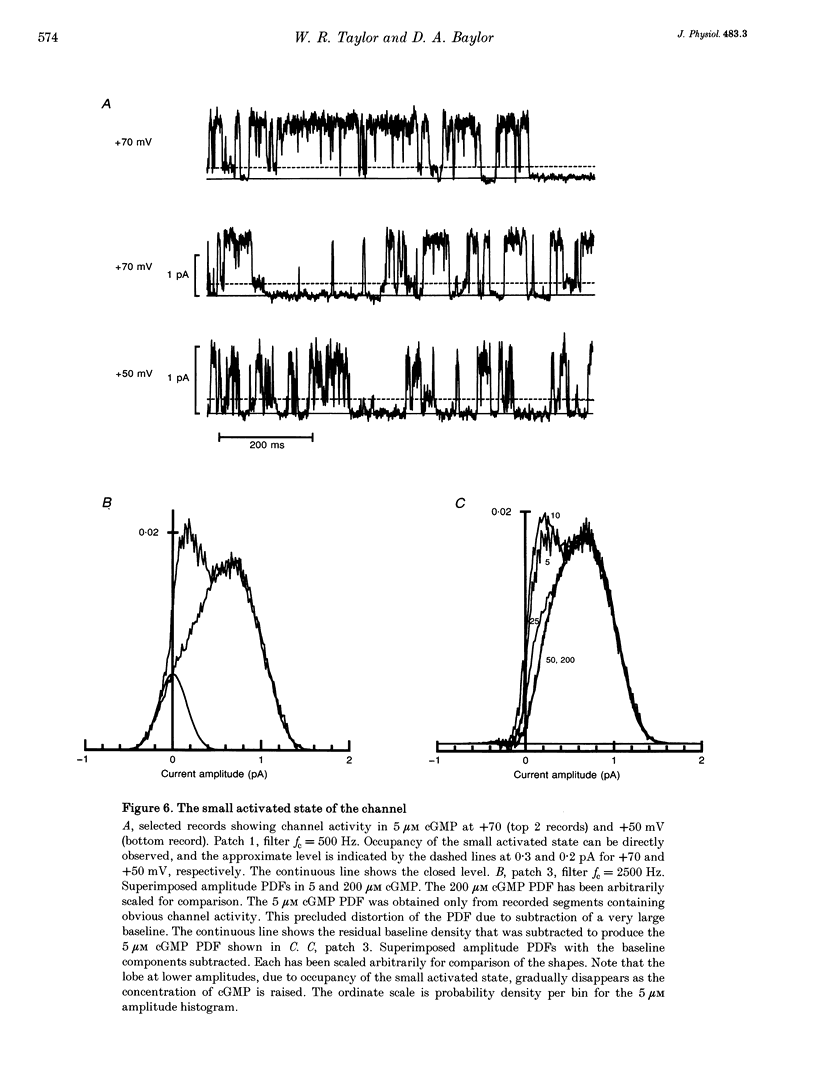
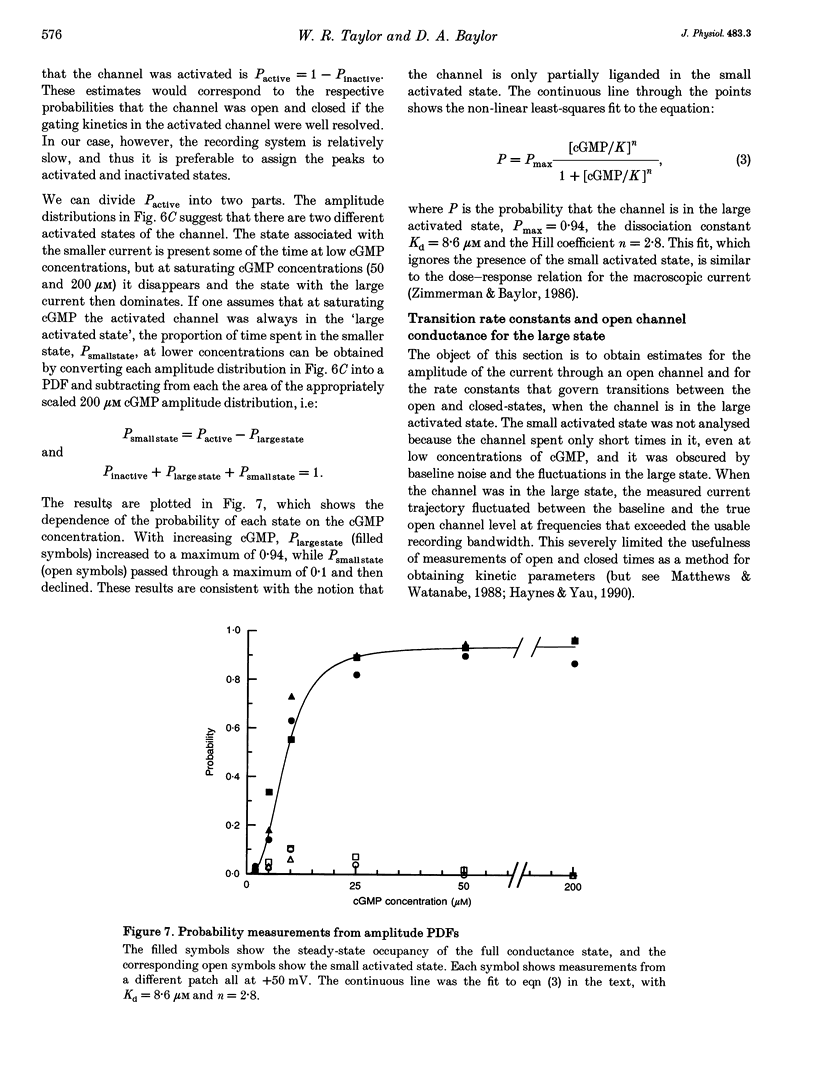
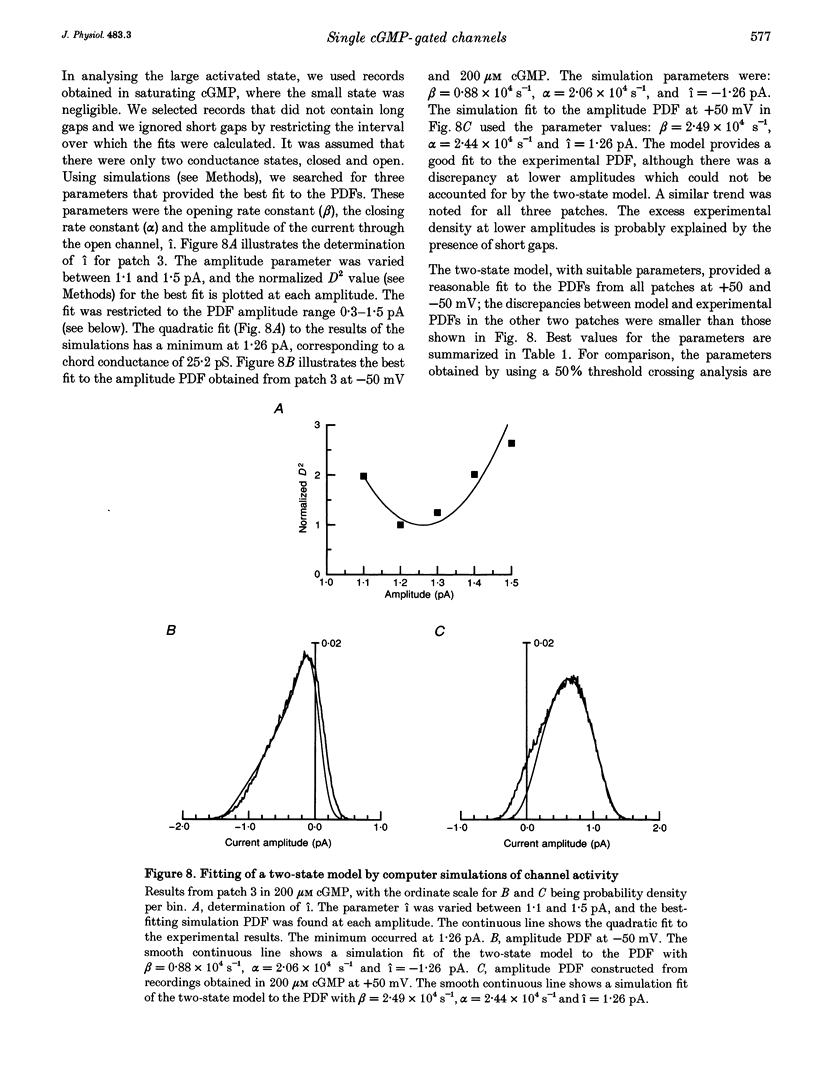
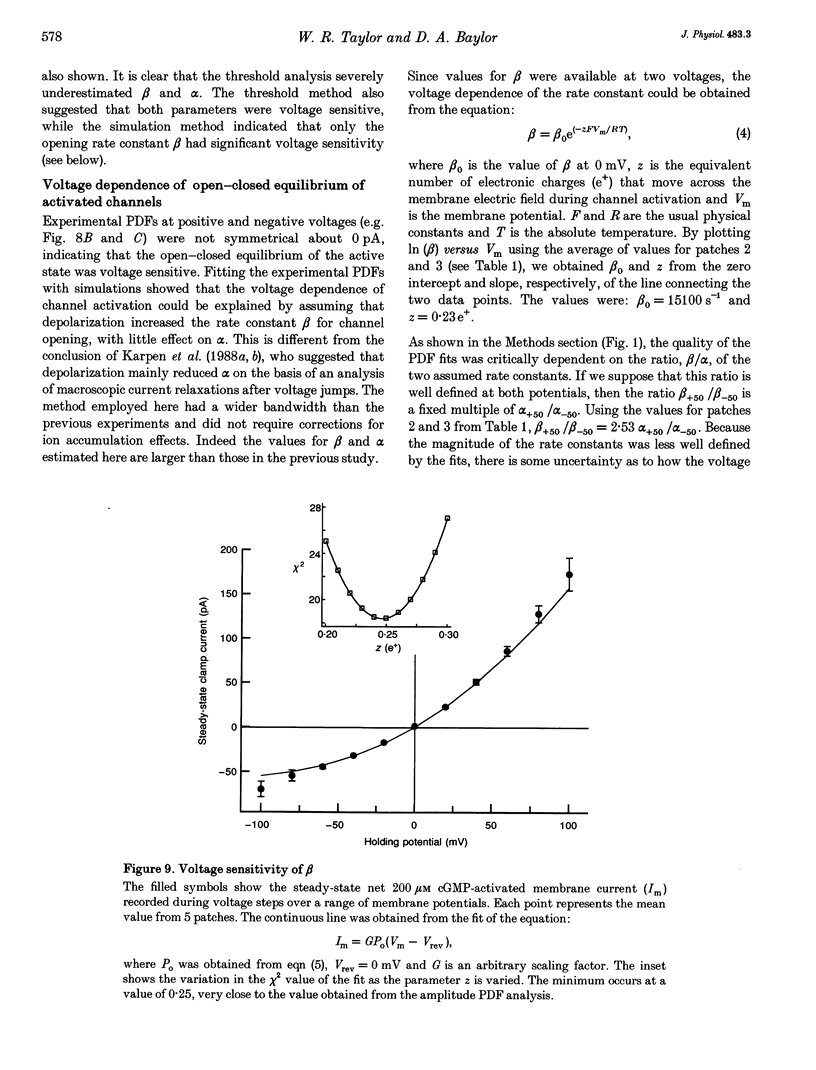
1. The conductance and kinetics of single 3',5'-cyclic guanosine monophosphate (cGMP)-activated channels of retinal rod outer segments were studied in inside-out membrane patches. The size of the single channel currents was increased by using low concentrations of divalent cations. 2. At saturating cGMP concentration, the current flickered at high frequency. Occasionally, the current was interrupted by closures lasting tens or hundreds of milliseconds. At +50 mV the maximum current during an opening was slightly more than 1 pA, but the open channel level was poorly resolved due to the speed of the gating transitions. 3. Amplitude histograms confirmed the presence of a sublevel of current, roughly a quarter the size of the peak current, at low cGMP concentrations. The fraction of time in the sublevel decreased with increasing cGMP concentration, suggesting that the sublevel may be due to opening by the partially liganded channel. 4. Consistent with previous macroscopic current recordings, single channel activation by cGMP had an apparent dissociation constant of 8.6 microM, and a Hill coefficient of 2.8. 5. At saturating cGMP concentrations, the channel was modelled as a two-state system with the following parameters. The open channel conductance was 25 pS. The opening rate constant, beta, was 1.5 x 10(4) s-1 at 0 mV, and had a voltage sensitivity equivalent to the movement of 0.23 electronic charges outward through the membrane electric field. The closing rate constant, alpha, was 2.1 x 10(4) s-1 and was voltage insensitive. Assuming that the open-state chord conductance was voltage independent, the inferred voltage dependence of beta largely accounted for the outward rectification in the steady-state macroscopic current-voltage relation of multichannel patches, at saturating cGMP concentration.
Full text
PDF

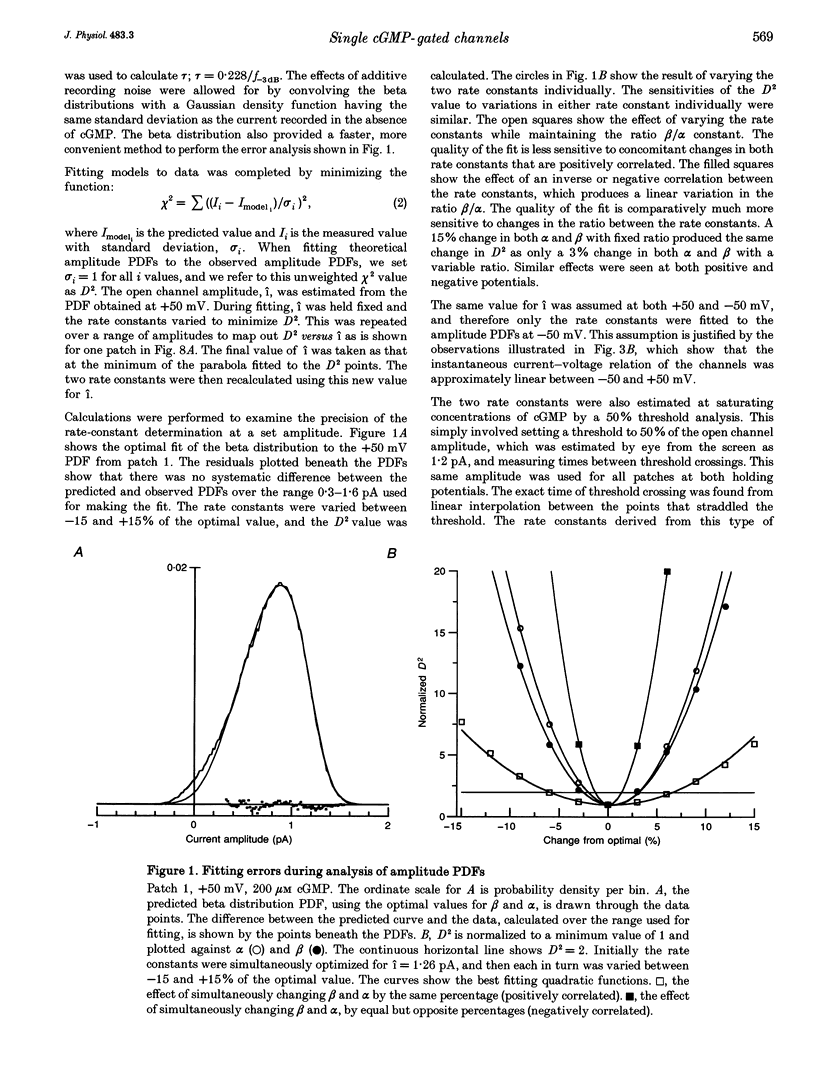





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett N., Clerc A. Activation of cGMP phosphodiesterase in retinal rods: mechanism of interaction with the GTP-binding protein (transducin). Biochemistry. 1989 Sep 5;28(18):7418–7424. doi: 10.1021/bi00444a040. [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Peng Y. W., Dhallan R. S., Ahamed B., Reed R. R., Yau K. W. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993 Apr 22;362(6422):764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Furman R. E., Tanaka J. C. Photoreceptor channel activation: interaction between cAMP and cGMP. Biochemistry. 1989 Apr 4;28(7):2785–2788. doi: 10.1021/bi00433a007. [DOI] [PubMed] [Google Scholar]
- Goulding E. H., Ngai J., Kramer R. H., Colicos S., Axel R., Siegelbaum S. A., Chess A. Molecular cloning and single-channel properties of the cyclic nucleotide-gated channel from catfish olfactory neurons. Neuron. 1992 Jan;8(1):45–58. doi: 10.1016/0896-6273(92)90107-o. [DOI] [PubMed] [Google Scholar]
- Hanke W., Cook N. J., Kaupp U. B. cGMP-dependent channel protein from photoreceptor membranes: single-channel activity of the purified and reconstituted protein. Proc Natl Acad Sci U S A. 1988 Jan;85(1):94–98. doi: 10.1073/pnas.85.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Yau K. W. Single-channel measurement from the cyclic GMP-activated conductance of catfish retinal cones. J Physiol. 1990 Oct;429:451–481. doi: 10.1113/jphysiol.1990.sp018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ildefonse M., Bennett N. Single-channel study of the cGMP-dependent conductance of retinal rods from incorporation of native vesicles into planar lipid bilayers. J Membr Biol. 1991 Aug;123(2):133–147. doi: 10.1007/BF01998084. [DOI] [PubMed] [Google Scholar]
- Karpen J. W., Zimmerman A. L., Stryer L., Baylor D. A. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Matthews G. Single-channel recordings demonstrate that cGMP opens the light-sensitive ion channel of the rod photoreceptor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):299–302. doi: 10.1073/pnas.84.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Watanabe S. Activation of single ion channels from toad retinal rod inner segments by cyclic GMP: concentration dependence. J Physiol. 1988 Sep;403:389–405. doi: 10.1113/jphysiol.1988.sp017255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G., Watanabe S. Properties of ion channels closed by light and opened by guanosine 3',5'-cyclic monophosphate in toad retinal rods. J Physiol. 1987 Aug;389:691–715. doi: 10.1113/jphysiol.1987.sp016678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menini A. Currents carried by monovalent cations through cyclic GMP-activated channels in excised patches from salamander rods. J Physiol. 1990 May;424:167–185. doi: 10.1113/jphysiol.1990.sp018061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Sesti F., Straforini M., Lamb T. D., Torre V. Gating, selectivity and blockage of single channels activated by cyclic GMP in retinal rods of the tiger salamander. J Physiol. 1994 Jan 15;474(2):203–222. doi: 10.1113/jphysiol.1994.sp020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J. C., Eccleston J. F., Furman R. E. Photoreceptor channel activation by nucleotide derivatives. Biochemistry. 1989 Apr 4;28(7):2776–2784. doi: 10.1021/bi00433a006. [DOI] [PubMed] [Google Scholar]
- Tanaka J. C., Furman R. E., Cobbs W. H., Mueller P. Incorporation of a retinal rod cGMP-dependent conductance into planar bilayers. Proc Natl Acad Sci U S A. 1987 Feb;84(3):724–728. doi: 10.1073/pnas.84.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre V., Straforini M., Sesti F., Lamb T. D. Different channel-gating properties of two classes of cyclic GMP-activated channel in vertebrate photoreceptors. Proc Biol Sci. 1992 Dec 22;250(1329):209–215. doi: 10.1098/rspb.1992.0151. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cation interactions within the cyclic GMP-activated channel of retinal rods from the tiger salamander. J Physiol. 1992 Apr;449:759–783. doi: 10.1113/jphysiol.1992.sp019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]


