Abstract
Background:
Treatment delays affect breast cancer survival and constitute poor-quality care. Black patients experience more treatment delay, but the relationship of geography to these disparities is poorly understood.
Methods:
We studied a population-based, retrospective, observational cohort of patients with breast cancer in North Carolina between 2004 and 2017 from the Cancer Information and Population Health Resource, which links cancer registry and sociodemographic data to multipayer insurance claims. We included patients >18 years with Stage I-III breast cancer who received surgery or chemotherapy as their first treatment. Delay was defined as >60 days from diagnosis to first treatment. Counties were aggregated into nine Area Health Education Center regions. Race was dichotomized as Black versus non-Black.
Results:
Among 32,626 patients, 6190 (19.0%) were Black. Black patients were more likely to experience treatment delay >60 days (15.0% of Black vs. 8.0% of non-Black). Using race-stratified modified Poisson regression, age-adjusted relative risk of delay in the highest risk region was approximately twice that in the lowest risk region among Black (relative risk, 2.1; 95% Cl, 1.6–2.6) and non-Black patients (relative risk, 1.9; 95% Cl, 1.5–2.3). Adjustment for clinical and sociodemographic features only slightly attenuated interregion differences. The magnitude of the racial gap in treatment delay varied by region, from 0.0% to 9.4%.
Conclusions:
Geographic region was significantly associated with risk of treatment delays for both Black and non-Black patients. The magnitude of racial disparities in treatment delay varied markedly between regions. Future studies should consider both high-risk geographic regions and high-risk patient groups for intervention to prevent delays.
Keywords: Breast Cancer, Cohort Study, Geographical Distribution, Racial Disparities, Treatment Initiation
INTRODUCTION
Delays in breast cancer diagnosis and treatment compromise survival and constitute poor-quality care. Delays of 30 to 60 days in time to treatment are associated with decrements in disease-specific and overall survival.1–4 Delays in the time between surgery and adjuvant chemotherapy,5–9 and in completion of all therapy,10 have also been linked to higher risk of breast cancer recurrence and worsened survival. The relationship of treatment delays to poor outcomes is consistent across studies from institutional, registry, and clinical trial settings, and has been confirmed in meta-analyses of pooled data.5
Black patients are at disproportionate risk of treatment delays across the breast cancer care delivery spectrum. State-specific studies in Georgia,11 New Jersey,12,13 North Carolina,14,15 and California2 have all demonstrated an elevated risk of surgical delays among Black women, with particular concern for younger women. A study from the National Cancer Database16 and a cohort study across multiple urban areas17 drew similar conclusions. Racial disparities in the timeliness of breast surgery persist even in health systems that theoretically provide uniform access to care, such as the US military health system.18 Racial disparities have also been documented in the timeliness of adjuvant chemotherapy and radiation,19,20 and we have previously reported that Black women are more likely to experience delays in completion of all therapy compared with others with similar treatment plans.15 These treatment delays are a contributory factor to the well-known and persistent disparity in breast cancer survival among Black women.21,22
It is likely that racial and ethnic disparities in timely access to cancer care are influenced by the characteristics of the health system and communities within which individuals seek cancer care. Black and Hispanic patients with breast cancer receive surgical care at different hospitals than non-Hispanic White peers, are less involved with selection of their surgeon and treatment facility, and rely less or reputation when selecting care.23 These differences in site of care are impactful; patients in public hospitals experience more frequent delays in breast surgery compared with private institutions,24 and adjustment for hospital-level and health system-level characteristics partially attenuates the association of race with treatment delay.19,20 However, previous studies relating racial disparities in treatment delay to different geographies and health systems have been limited to single cities or to older patients. We hypothesized that among a large, diverse, and geographically dispersed patient population, the risk of treatment delay would vary depending on geographic subregion and would vary differently for Black versus non-Black patients. Therefore, we designed a large population-based cohort study to examine how the time from diagnosis to treatment initiation varied along the two axes of race and geographic region, using multipayer insurance claims from Black and non-Black women with newly diagnosed breast cancer in a populous state with considerable racial and geographic diversity in North Carolina from 2004 to 2015.
METHODS
Study population
We assembled a retrospective cohort of all Stage I-III breast cancer cases between 2004 and 2015 in the North Carolina Central Cancer Registry. Patient records were linked to the University of North Carolina Lineberger Comprehensive Cancer Center’s Cancer Information and Population Health Resource, which links registry data to multiple-payer insurance claims from private health insurance plans, Medicare fee for service and managed care plans, and Medicaid. Annually, these payers cover approximately 65% of the state’s total population and 72% of the insured population. The Cancer Information and Population Health Resource data resource has been described previously.25 For this study, geographic location of the patient’s home address was estimated from the census tract centroid to protect privacy.
Cohort creation is illustrated in Figure S1. Briefly, we identified patients diagnosed with Stage I-III breast cancer as their first or only primary cancer diagnosis in 2004–2015 and aged 18 years or older at diagnosis. Eligible patients were required to have insurance between 1 month prediagnosis and 12 months postdiagnosis to ensure capture of treatments through insurance claims, and to receive surgery or chemotherapy as their first treatment. We required that patients be enrolled in any included insurance plan during any 10 of the 13 months. This flexible enrollment criterion improved the retention of Black patients and Medicaid beneficiaries without otherwise substantively changing sample characteristics. Patients were excluded if diagnosed by death certificate or autopsy, missing information on county or date of diagnosis, if they had no therapeutic surgery within 12 months, or had radiation before other therapy. The included billing codes are listed in Table S1. This study was considered exempt by the University of North Carolina institutional review board (#20–1242).
Study outcome
Our primary outcome of interest was the risk of treatment delay, defined based on the threshold of treatment >60 days from diagnosis associated with survival decrements in prior studies.1,4 Time to treatment was defined as the duration between the registry-documented diagnosis date and the first treatment date from claims. Here, we refer to treatment delays in terms of risk and percentage points interchangeably. Treatments of interest were identified through International Classification of Diseases, Ninth Revision, Clinical Modification procedure and Current Procedural Terminology/Healthcare Common Procedure Coding System codes broadly categorized as breast-conserving surgery, mastectomy, and chemotherapy (Table S1).
Study exposures
The main exposures of interest were the geographic region of residence at diagnosis and race of the patient. Because North Carolina contains 100 counties, some with low populations and no cancer care facilities, counties were grouped into the nine Area Health Education Center (AHEC) regions of North Carolina. AHEC regions are groups of contiguous counties partitioned within the state to address and support medical education and the delivery of health care,26 and counties within an AHEC region tend to have similar health services characteristics. Here, we refer to the AHEC regions using a numbering system for clarity and ease of interpretation. Patient self-reported race was obtained from the cancer registry and was dichotomized as Black or non-Black.
Other variables
Patient characteristics included the first treatment type (chemotherapy, breast-conserving surgery, or mastectomy), age, Stage (I, II, III), and hormone receptor status (negative, positive). Because HER2 status was only available for the latest years of registry data, it was not included as a clinical covariate. Patient sociodemographic characteristics included insurance type, distance to first treatment, urbanicity, and Social Deprivation Index (SDI) of the patient’s census tract. Distance was measured in miles from the patient ZIP code centroid to the ZIP code centroid for the claim corresponding to first treatment and categorized as (<5, 5-<10, 10-<20, and ≥20 miles). Urbanicity was measured at tract level using Rural Urban Commuting Area code of patient’s residence.27 The SDI is a validated composite measure of neighborhood socioeconomic variation incorporating income, education, employment, housing, household characteristics, and transportation.28–30 The SDI ranges from 0 to 1, with the higher value suggesting greater deprivation.
Statistical analysis:
Patient characteristics were described using the median and interquartile range for continuous variables and frequencies and percentages for categorical variables. Results were tabulated overall and stratified by race. Black-non-Black differences in characteristics were assessed using χ2 and Mann-Whitney U tests.
To evaluate racial differences in treatment delay across regions, we used multivariable modified Poisson regression including race, AHEC region, and their interaction to estimate the risk of delays but sequentially adjusting for (model A) patient age at diagnosis, (model B) clinical factors, and (model C) sociodemographic factors. Following the Institute of Medicine’s guidance on treating race as a social construct inclusive of sociodemographic characteristics,31 we prioritized multivariable model B, which adjusted for age and clinical variables only. However, we also conducted exploratory analyses (model C), adjusting for sociodemographic characteristics likely to vary by race and/or geography, including insurance type, SDI, distance to care, and urbanicity, to assess whether adjustment for these factors attenuated the observed relationships. We then estimated predicted percentage of Black and non-Black patients in each AHEC region with treatment delays from model B, and constructed choropleth maps to illustrate the race-specific geographic risk distribution as well as the racial risk difference across regions. We also estimated risk of treatment delay across clinical factors and across AHEC regions within race groups. The multicollinearity of candidate covariates in multivariable models was assessed using a variance inflation factor threshold of 5, where no variables required removal.
To better understand the relationship of geographic and racial variation in treatment delays, we made a series of cross-region comparisons: (1) within-region percentage point gap in estimated risk of delay between Black and non-Black patients and (2) within-race difference in risk of delay across regions, using the region with the lowest overall risk of delay (region 1) as the reference group. The 25 total comparisons were adjusted for multiple tests using the conservative Bonferroni approach as well as the less conservative false-discovery rate adjustment32; the false-discovery rate adjustments are presented in this manuscript. All analyses were conducted using SAS v9.4 (Cary, North Carolina) and maps were generated using ArcMAP 10.8.1.
RESULTS
Cohort characteristics
Cohort characteristics are summarized in Table 1. Among 32,626 patients, 6190 (19.0%) were Black. The non-Black subcohort included 98% White, 1% American Indian, and 1% Asian/Pacific Islander patients. Compared with non-Black patients, Black patients were younger (64 vs. 68 years), more likely to have chemotherapy as their first treatment (14.8% vs. 7.6%), had Stage III disease (15.2% vs. 9.3%) and hormone receptor-negative tumors (29.3% vs. 15.6%), Medicaid insurance (46.7% vs. 14.9%), and to live within 5 miles of their treatment site (30.6% vs. 25.2%). More than one-half of Black patients (52.0%), compared with 18.6% of non-Black patients, resided in a census tract in the highest category of social deprivation. Black patients were more likely to experience a treatment delay >60 days (15.0% of Black patients vs. 8.0% of non-Blacks), and median time to treatment was also longer (30 vs. 26 days).
TABLE 1.
Cohort characteristics
| Overall |
Non-Black |
Black |
||||||
|---|---|---|---|---|---|---|---|---|
| n = 32,626 | % | n = 26,436 | % | n = 6190 | % | |||
|
| ||||||||
| Time to first treatment, days | Median, IQR | 27 | 15–40 | 26 | 15–38 | 30 | 17–47 | ** |
| Treatment >60 days | n, % | 3027 | 9.3 | 2101 | 8.0 | 926 | 15.0 | ** |
| AHEC region | 1 | 2680 | 8.2 | 1927 | 7.3 | 753 | 12.2 | ** |
| 2 | 3362 | 10.3 | 3269 | 12.4 | 93 | 1.3 | ||
| 3 | 4879 | 15.0 | 3781 | 14.3 | 1098 | 17.7 | ||
| 4 | 1941 | 6.0 | 1623 | 6.1 | 318 | 5.1 | ||
| 5 | 1372 | 4.2 | 797 | 3.0 | 575 | 9.3 | ||
| 6 | 3666 | 11.2 | 2695 | 10.2 | 971 | 15.7 | ||
| 7 | 5263 | 16.1 | 4729 | 17.9 | 534 | 8.6 | ||
| 8 | 3867 | 11.9 | 3141 | 11.9 | 726 | 11.7 | ||
| 9 | 5596 | 17.2 | 4474 | 16.9 | 1122 | 18.1 | ||
| First treatment | Chemotherapy | 2923 | 9.0 | 2006 | 7.6 | 917 | 14.8 | ** |
| Breast-conserving surgery | 20,874 | 64.0 | 17,125 | 64.8 | 3749 | 60.6 | ||
| Mastectomy | 8829 | 27.1 | 7305 | 27.6 | 1524 | 24.6 | ||
| Age at diagnosis, years | Median, IQR | 67 | 56, 74 | 68 | 58, 74 | 64 | 52, 71 | ** |
| 18–49 | 4629 | 14.2 | 3400 | 12.9 | 1229 | 19.9 | ||
| 50–64 | 8409 | 25.8 | 6415 | 24.3 | 1994 | 32.2 | ||
| 65–69 | 6716 | 20.6 | 5592 | 21.2 | 1124 | 18.2 | ||
| 70+ | 12,872 | 39.5 | 11,029 | 41.7 | 1843 | 29.8 | ||
| Stage at diagnosis | I | 17,803 | 54.6 | 15,051 | 56.9 | 2752 | 44.5 | ** |
| II | 11,417 | 35.0 | 8917 | 33.7 | 2500 | 40.4 | ||
| III | 3406 | 10.4 | 2468 | 9.3 | 938 | 15.2 | ||
| Hormone receptor | Negative | 5698 | 18.2 | 3947 | 15.6 | 1751 | 29.3 | ** |
| Positive | 25,602 | 81.8 | 21,379 | 84.4 | 4223 | 70.7 | ||
| Insurance coverage | Any private | 7854 | 24.1 | 6872 | 26.0 | 982 | 15.9 | ** |
| Medicaid only | 6831 | 20.9 | 3940 | 14.9 | 2891 | 46.7 | ||
| Medicare FFS only | 15,527 | 47.6 | 13,649 | 51.6 | 1878 | 30.3 | ||
| Medicare Advantage | 2414 | 7.4 | 1975 | 7.5 | 439 | 7.1 | ||
| Distance to first treatment | <5 miles | 8557 | 26.2 | 6661 | 25.2 | 1896 | 30.6 | ** |
| 5-<10 miles | 7180 | 22.0 | 5742 | 21.7 | 1438 | 23.2 | ||
| 10-<20 miles | 7154 | 21.9 | 6083 | 23.0 | 1071 | 17.3 | ||
| >20 miles | 9733 | 29.8 | 7949 | 30.1 | 1784 | 28.8 | ||
| Social Deprivation Index quarter | 1 (0–25) | 6167 | 18.9 | 5756 | 21.8 | 411 | 6.6 | ** |
| Based on distribution in state | 2 (25–50) | 7448 | 22.9 | 6690 | 25.3 | 758 | 12.3 | |
| 3 (50–75) | 10,848 | 33.3 | 9052 | 34.3 | 1796 | 29.0 | ||
| 4 (75+) | 8136 | 25.0 | 4915 | 18.6 | 3221 | 52.1 | ||
| Urbanicity | Rural | 8324 | 25.5 | 6681 | 25.3 | 1643 | 26.5 | * |
| Urban | 24,302 | 74.5 | 19,755 | 74.7 | 4547 | 73.5 | ||
Column totals for hormone receptor status and social deprivation index may not sum to 100% because of missing data, χ2 and Mann-Whitney U tests for Black-non-Black differences. Urbanity measured from Rural Urban Commuting Area definitions at the census tract level. Quarter is based on the distribution of the State.
Abbreviations: AHEC, Area Health Education Center; FFS, fee for service; IQR, interquartile range.
Significant difference in distribution of variable between Black and non-Black at a threshold of p < .05.
p < .01.
Influence of clinical factors and sociodemographic characteristics on geographic differences in treatment delay
Table 2 displays predicted risks and risk ratios from Poisson regression models A, B, and C for all AHEC regions across both racial subgroups. In models minimally adjusted for age (model A), relative risk of treatment delay in the highest-risk region (region 9) was more than double that of the referent region (region 1) among Black patients (relative risk [RR], 2.1; 95% Cl, 1.6–2.6) and almost doubled among non-Black patients (RR, 1.9; 95% Cl, 1.5–2.3). Adjustment for clinical features (model B) minimally attenuated regional differences in risk of delay for Black patients (RR, 2.0; 95% Cl, 1.5–2.5 for the highest risk compared with the referent region); the risk estimate did not change among non-Black patients. Additional adjustment for sociodemographic characteristics (model C) did not further attenuate regional differences in risk of delay in either group. In exploratory analyses, we further tested the interactions of race by insurance type, and geography by insurance type, in model C to evaluate whether the relationship of these factors to delays differed by insurance type. The interaction of geographic region with insurance was significant; we then stratified model C by insurance type. Generally, insurance-stratified models showed a consistent pattern of racial disparities in the risk of delay across all three insurance categories, and persistently higher risk in regions 7 through 9 across insurance types. There were some insurance-specific variations in relative performance among regions: the elevated risk for Black patients in region 3 was limited to those with Medicare or Medicaid, and region 5 performed among the worst in the state for non-Blacks with private insurance, but not for other groups. However, definitive conclusions from this exploratory analysis are limited by small sample sizes. Stratified tables for model C are included in Table S3 and effect sizes for all covariates are presented in Table S4.
TABLE 2.
Estimated risk ratios across AHEC regions within race groups for models A, B, and C
| Model A: Minimally adjusted for age |
Model B: Adjusted for age and clinical characteristics |
Model C: Adjusted for age, clinical, and access characteristics |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black |
Non-Black |
Black |
Non-Black |
Black |
Non-Black |
|||||||
| AHEC region | % Delayed | RR (95% CL) | % Delayed | RR (95% CL) | % Delayed | RR (95% CL) | % Delayed | RR (95% CL) | % Delayed | RR (95% CL) | % Delayed | RR (95% CL) |
|
| ||||||||||||
| 1 | 9.5 | REF | 5.1 | REF | 9.5 | REF | 4.8 | REF | 8.7 | REF | 4.8 | REF |
| 2 | 6.2 | 0.7 (0.3–1.5) | 6.4 | 1.3 (1.0–1.6) | 5.4 | 0. 6 (0.3–1.2) | 6.1 | 1.3 (1.0–1.6) | 5.0 | 0.6 (0.3, 1.3) | 5.9 | 1.2 (1.0–1.6) |
| 3 | 15.2 | 1.6 (1.3–2.1) | 7.0 | 1.4 (1.1–1.7) | 14.3 | 1.5 (1.2–1.9) | 6.6 | 1.4 (1.1–1.7) | 13.7 | 1.6 (1.2–2.0) | 6.7 | 1.4 (1.1–1.8) |
| 4 | 13.3 | 1.4 (1.0–2.0) | 7.1 | 1.4 (1.1–1.8) | 13.2 | 1.4 (1.0–2.0) | 6.7 | 1.4 (1.1–1.8) | 12.1 | 1.4 (1.0–2.0) | 6.7 | 1.4 (1.1–1.8) |
| 5 | 10.2 | 1.1 (0.8–1.5) | 6.9 | 1.4 (1.0–1.9) | 10.1 | 1.1 (0.8–1.5) | 6.8 | 1.4 (1.0–1.9) | 9.3 | 1.1 (0.8–1.5) | 7.1 | 1.5 (1.1–2.0) |
| 6 | 13.7 | 1.4 (1.1–1.9) | 8.4 | 1.6 (1.3–2.1) | 12.7 | 1.3 (1.0–1.8) | 8.1 | 1.7 (1.3–2.1) | 11.3 | 1.3 (1.0–1.7) | 7.9 | 1.6 (1.3–2.1) |
| 7 | 15.1 | 1.6 (1.2–2.1) | 8.8 | 1.7 (1.4–2.1) | 14.5 | 1.5 (1.1–2.0) | 8.2 | 1.7 (1.4–2.1) | 13.3 | 1.5 (1.1–2.0) | 8.1 | 1. 7 (1.4–2.1) |
| 8 | 15.4 | 1.6 (1.2–2.1) | 9.1 | 1.8 (1.4–2.2) | 14.4 | 1.5 (1.2–2.0) | 8.8 | 1.8 (1. 5–2.3) | 13.0 | 1.5 (1.1–2.0) | 8.8 | 1.8 (1.5–2.3) |
| 9 | 19.4 | 2.1 (1.6–2.6) | 9.6 | 1.9 (1.5–2.3) | 18.5 | 2.0 (1.5–2.5) | 9.1 | 1.9 (1.5–2.3) | 17.0 | 2.0 (1.5–2.5) | 9.1 | 1. 9 (1.5–2.3) |
Model B further adjusts for first treatment type, stage at diagnosis, and hormone receptor status. Model C further adjusts for insurance, distance to first treatment, Social Deprivation index, and urbanicity.
Abbreviations: AHEC, Area Health Education Center; CL, confidence limits; RR, risk ratio.
Race and regional differences
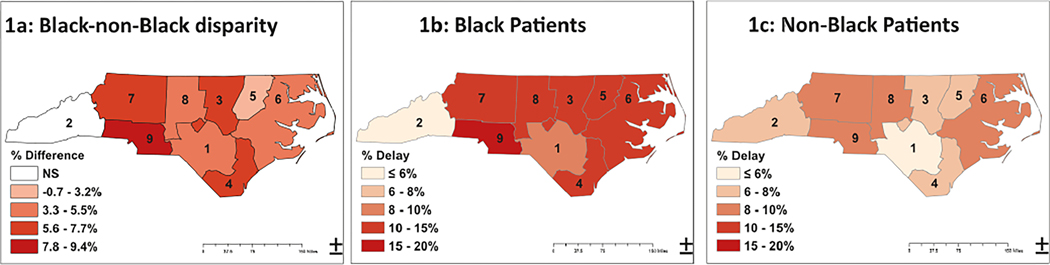
Figure 1 presents choropleth maps comparing the estimated risk of treatment delay adjusted for age and clinical features for Black (Figure IB) and non-Black patients (Figure 1C) across regions, as well as the magnitude of racial risk difference in treatment delays within each region (Figure 1A). The proportion of patients with treatment delays was significantly greater for Black patients in every AHEC region, with the exception of region 2, in which only 2.7% (93/3362) of patients were Black. However, the magnitude of the Black-non-Black gap varied across the state (Figure;1A), ranging from no significant difference in region 2 to a 9.4% in region 9.
FIGURE 1.
Choropleth maps illustrating the magnitude of racial disparity by region (A), and distributions among Black (B) and non-Black (C) patients. Estimates of% delayed are adjusted for age, stage, first treatment type, and hormone receptor status. Darker areas indicate more delays or greater difference in delays. NS indicates nonsignificant Black-non-Black difference after false discovery rate adjustment. All other region differences were statistically significant.
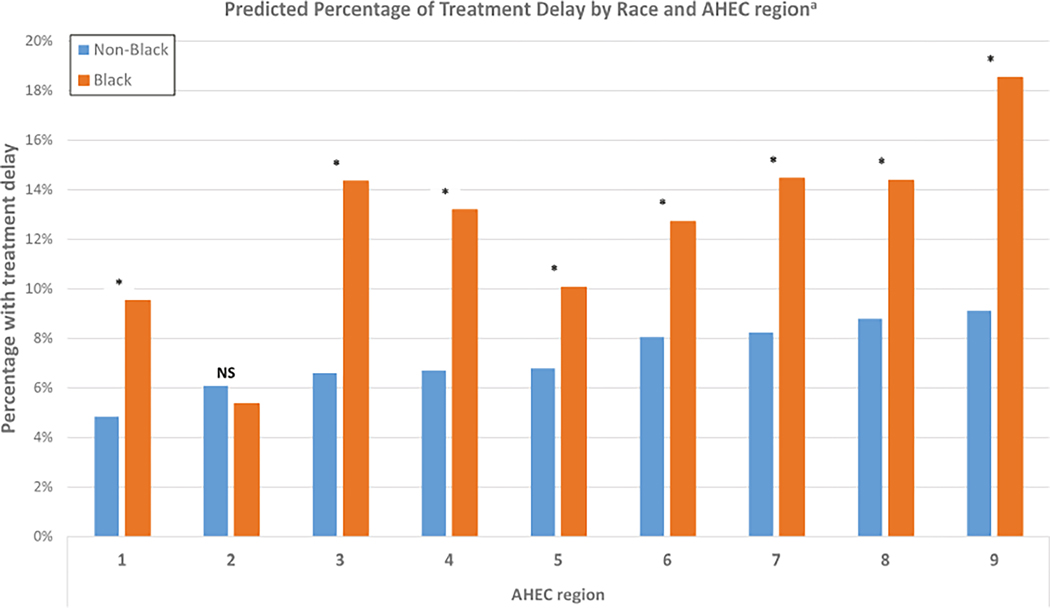
Figure 2 shows paired estimates of treatment delays for Black and non-Black patients by region. In four of the nine AHEC regions, >14% of Black patients experienced delays, and the highest risk region (region 9) had an 18.5% risk of delay after adjustment for age and clinical factors. Table S2 presents the percent difference and adjusted p value for racial and regional comparisons. The absolute difference in risk of delay between the best-performing and worst-performing region was 9.0% for Black patients but only 4.3% for non-Black patients.
FIGURE 2.
Predicted treatment delays among Black and non-Black patients across regions. aEstimates adjusted for age, first treatment type, stage at diagnosis, and hormone receptor status. NS indicates nonsignificant difference in risk by race for AHEC region 2 after false-discovery rate adjustment. *Indicates statistically significant difference. AHEC, Area Health Education Center.
Risk of treatment delay in clinical subgroups
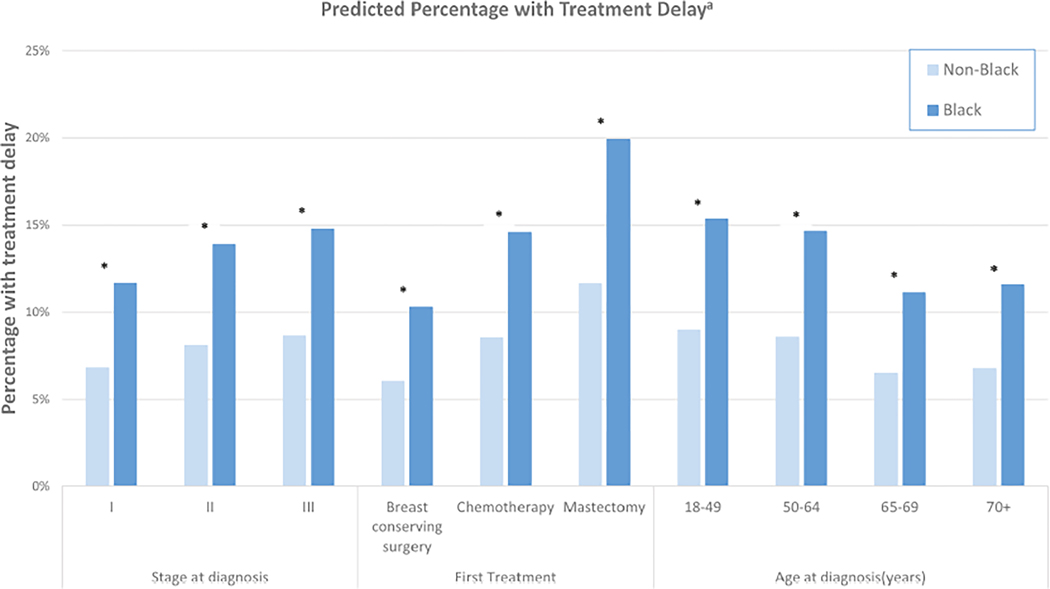
We examined the risk of delays >60 days by clinical subgroups (Figure 3). For both Black and non-Black patients, risk of delay increased with disease stage, and delays were more likely in patients whose first treatment was mastectomy, as well as patients younger than age 50 years. Although associations of clinical features with treatment delay were similar among Black and non-Black women, the absolute risk of treatment delay was higher among Black women in every clinical subgroup.
FIGURE 3.
Predicted treatment delay by race and clinical characteristics. aEstimates adjusted for age, first treatment type, stage at diagnosis, and hormone receptor status. *Indicates statistically significant difference.
DISCUSSION
In a diverse, population-based cohort of breast cancer patients, we found that although racial disparities in treatment delay were widespread, the likelihood of treatment delay, as well as the magnitude of racial disparity, varied considerably across geographic subregions. Previous population-based studies have described geographic disparities in late-stage diagnosis and breast cancer mortality, with patients of color being more likely to live in geographic hotspots of poor outcomes.33,34 However to our knowledge, this is the first large population-based study to describe variation in a key indicator of breast cancer quality by race and geography simultaneously. Although the magnitude of racial disparities in treatment timeliness varied between regions, some degree of racial disparity was observed in every region with a significant Black population and interregion variations in timeliness were larger among Black patients than among non-Black patients.
Disparities in the quality of cancer care, whether by race or geography, are often attributed to the sociodemographic characteristics of marginalized patient populations, and it is common practice to include risk adjustment for sociodemographic characteristics in outcome-based oncology quality measures.35 Individual sociodemographics certainly predict the risk of treatment delays at the patient level.36 However, it is notable that in our study, adjustment for patient-level characteristics did not attenuate the observed geographic differences in treatment timeliness for either racial group. On the contrary, significant interregion differences in timeliness and racial parity persisted after adjustment for sociodemographic factors. These findings suggest that structural features of local health care systems, rather than variation in patient characteristics, may be key explanatory factors for geographic disparities in treatment delays and other cancer outcomes. Ongoing research by our group will explore region-level and health system characteristics predictive of timely and equitable breast cancer care.
We chose a threshold of treatment delay, 60 days, that has been consistently linked to poor outcomes and should represent clearly unacceptable cancer care. Despite this conservative threshold, our findings suggest that treatment delays are alarmingly common in patients at high risk for breast cancer death, including young Black women and patients with Stage III disease. We note that the two regions with the greatest disparities in treatment delay, as well as the highest absolute risk of treatment delay for Black patients, surround large urban centers (regions 3 and 9). Although this requires confirmation, these findings suggest that interventions to facilitate access for Black patients and those with high clinical risk in complex urban health systems, such as patient navigation, should be prioritized to combat treatment disparities and improve outcomes at the state level. Such interventions are challenging to design and sustain and will require adaptation to the local health care system structure as well as to specific patient needs.
As with any large retrospective study of cancer care delivery, our work has limitations. These findings may not be replicable across other states or care settings or across other tumor types. The data presented in this manuscript reflect the care experience of insured patients, and thus the substantive barrier posed by lack of insurance and its relationship to racial disparities in cancer care, is outside the scope of this study. However, we have compared our estimates of median time to treatment and risk of treatment delay by race with treatment data from the state cancer registry, which include uninsured patients, and found the patterns of delay by race and region to be generally similar. Cancer care that was delivered without charge to the patient’s insurer also would not be captured in our analysis. However, sensitivity analyses using cancer registry-ascertained treatment data, which have different limitations but do include uninsured patients, suggest a similar magnitude of racial disparities in treatment delay and similar geographic patterns of delay in the state. No large secondary data source can fully explore the patient experience and the complex reasons for delays in care, and it is vitally important to pair these research methods with community-engaged research to uncover and target root causes of delayed and inequitable care.
In summary, we found that, in a large, population-based cohort of patients with newly diagnosed breast cancer in a large state with racial and geographic diversity, the likelihood of a clinically impactful delay in initiating breast cancer treatment, as well as the magnitude of disparity in delays between Black and non-Black patients, varied significantly by geographic subregion. Importantly, regional differences in timeliness were not explained by the clinical or sociodemographic characteristics patients. The substantial geographic variation we observed in the delivery of timely and equitable breast cancer care suggests that these outcomes are modifiable and should be a clinical and policy priority for intervention in regions of high risk. High priorities for future research include defining problem areas of delay and disparity in other cancer types, further exploration of region-level and local health system characteristics associated with treatment delay, and the development of targeted, effective, and sustainable interventions in high-risk regions and patient groups.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the Susan G. Komen Foundation and the NC State Employees’ Credit Union. Work was also supported by the Cancer Information and Population Health Resource at the UNC Lineberger Comprehensive Cancer Center, with funding provided by the University Cancer Research Fund via the state of North Carolina. The findings and conclusions in this publication are those of the author(s) and do not necessarily represent the views of the North Carolina Department of Health and Human Services, Division of Public Health.
Funding information
Susan G. Komen, Grant/Award Number: None assigned; North Carolina State Employees’ Credit Union Foundation, Grant/Award Number: None assigned; University Cancer Research Fund, UNC Chapel Hill, Grant/Award Number: None Assigned; Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill
Footnotes
CONFLICT OF INTEREST
Katherine E. Reeder-Hayes and Stephanie B. Wheeler received unrelated grant funding paid to their institution by the Pfizer Medical Foundation. All other authors made no disclosures.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.de Melo Gagliato D, Lei X, Giordano SH, et al. Impact of delayed neoadjuvant systemic chemotherapy on overall survival among patients with breast cancer. Oncology. 2020;25(9):749–757. doi: 10.1634/theoncologist.2019-0744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013; 148(6):516–523. doi: 10.1001/jamasurg.2013.1680 [DOI] [PubMed] [Google Scholar]
- 3.Mateo AM, Mazor AM, Obeid E, et al. Time to surgery and the impact of delay in the non-neoadjuvant setting on triple-negative breast cancers and other phenotypes. Ann Surg Oncol. 2020;27(5):1679–1692. doi: 10.1245/sl0434-019-08050-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2(3):330–339. doi: 10.1001/jamaoncol.2015.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raphael MJ, Biagi JJ, Kong W, Mates M, Booth CM, Mackillop WJ. The relationship between time to initiation of adjuvant chemotherapy and survival in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016;160(l):17–28. doi: 10.1007/s10549-016-3960-3 [DOI] [PubMed] [Google Scholar]
- 6.Gagliato Dde M, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;32(8):735–744. doi: 10.1200/jco.2013.49.7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2(3):322–329. doi: 10.1001/jamaoncol.2015.3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okines AFC, Kipps E, Irfan T, et al. Impact of timing of adjuvant chemotherapy for early breast cancer: the Royal Marsden Hospital experience. Br J Cancer. 2021;125(2):299–304. doi: 10.1038/s41416-021-01428-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farolfi A, Scarpi E, Rocca A, et al. Time to initiation of adjuvant chemotherapy in patients with rapidly proliferating early breast cancer. Eur J Cancer. 2015;51(14):1874–1881. doi: 10.1016/j.ejca.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 10.Pratt D, Burneikis T, Tu C, Grobmyer S. Time to completion of breast cancer treatment and survival. Ann Surg Oncol. 2021;28(13):8600–8608. doi: 10.1245/sl0434-021-10116-9 [DOI] [PubMed] [Google Scholar]
- 11.Lund MJ, Brawley OP, Ward KC, Young JL, Gabram SS, Eley JW. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109(3):545–557. doi: 10.1007/sl0549-007-9675-8 [DOI] [PubMed] [Google Scholar]
- 12.George P, Chandwani S, Gabel M, et al. Diagnosis and surgical delays in African American and White women with early-stage breast cancer. J Worn Health. 2015;24(3):209–217. doi: 10.1089/jwh.2014.4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasubramanian BA, Demissie K, Crabtree BF, Strickland PA, Pawlish K, Rhoads GG. Black Medicaid beneficiaries experience breast cancer treatment delays more frequently than Whites. Ethn Dis. 2012;22(3):288–294. [PubMed] [Google Scholar]
- 14.Emerson MA, Golightly YM, Aiello AE, et al. Breast cancer treatment delays by socioeconomic and health care access latent classes in Black and White women. Cancer. 2020;126(22):4957–4966. doi: 10.1002/cncr.33121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeder-Hayes KE, Mayer SE, Olshan AF, et al. Race and delays in breast cancer treatment across the care continuum in the Carolina Breast Cancer Study. Cancer. 2019;125(22):3985–3992. doi: 10.1002/cncr.32378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson DK, Li Y, Eskander MF, et al. Racial disparities in low-value surgical care and time to surgery in high-volume hospitals. J Surg Oncol. 2021;123(2):676–686. doi: 10.1002/jso.26320 [DOI] [PubMed] [Google Scholar]
- 17.Sheppard VB, Oppong BA, Hampton R, et al. Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol. 2015;22(9):2902–2911. doi: 10.1245/sl0434-015-4397-3 [DOI] [PubMed] [Google Scholar]
- 18.Eaglehouse YL, Georg MW, Shriver CD, Zhu K. Racial differences in time to breast cancer surgery and overall survival in the US military health system. JAMA Surg. 2019;154(3):el85113. doi: 10.1001/jamasurg.2018.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman RA, He Y, Winer EP, Keating NL. Racial/ethnic differences in receipt of timely adjuvant therapy for older women with breast cancer: are delays influenced by the hospitals where patients obtain surgical care? Health Serv Res. 2013;48(5):1669–1683. doi: 10.1111/1475-6773.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler SB, Carpenter WR, Peppercorn J, Schenck AP, Weinberger M, Biddle AK. Structural/organizational characteristics of health services partly explain racial variation in timeliness of radiation therapy among elderly breast cancer patients. Breast Cancer Res Treat. 2012;133(l):333–345. doi: 10.1007/sl0549-012-1955-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA A Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412 [DOI] [PubMed] [Google Scholar]
- 22.Miller JW, Smith JL, Ryerson AB, Tucker TC, Allemani C. Disparities in breast cancer survival in the United States (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):5100–5118. doi: 10.1002/cncr.30988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman RA, Kouri EM, West DW, Keating NL. Racial/ethnic differences in patients’ selection of surgeons and hospitals for breast cancer surgery. JAMA Oncol. 2015;l(2):222–230. doi: 10.1001/jamaoncol.2015.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosunjac M, Park J, Strauss A, et al. Time to treatment for patients receiving BCS in a public and a private university hospital in Atlanta, breast J. 2012; 18(2): 163–167. doi: 10.1111/j.l524-4741.2011.01205.x [DOI] [PubMed] [Google Scholar]
- 25.Meyer AM, Olshan AF, Green L, et al. Big data for population-based cancer research: the integrated cancer information and surveillance system. N C Med J. 2014;75(4):265–269. doi: 10.18043/ncm.75.4.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North Carolina Area Health Education Centers. Accessed December 15, 2021. https://www.ncahec.net/
- 27.US Department of Agriculture - Economic Research Service. Rural-Urban Commuting Area Codes. US Department of Agriculture; 2020. [Google Scholar]
- 28.Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48(2 Pt l):539–559. doi: 10.1111/j.l475-6773.2012.01449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Robert Graham Center. Social Deprivation Index (SDI). Accessed December 15,2021. https://www.graham-center.org/maps-data-tools/social-deprivation-index.html
- 30.Glassman B, Poverty Statistics Branch Social, Economic, and Housing Statistics Division, U.S. Census Bureau. The Multidimensional Deprivation Index Using Different Neighborhood Quality Definitions. Western Economic Association Annual Conference; 2020. [Google Scholar]
- 31.Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. In: Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press (US); 2003. [PubMed] [Google Scholar]
- 32.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850–857. doi: 10.1016/j.jclinepi.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 33.Fan Q, Yao XA, Han X. Spatial variation and disparity in female breast cancer relative survival in the United States. Cancer. 2021;127(21):4006–4014. doi: 10.1002/cncr.33801 [DOI] [PubMed] [Google Scholar]
- 34.Mobley LR, Tangka FKL, Berkowitz Z, et al. Geographic disparities in late-stage breast cancer diagnosis rates and their persistence over time. J Worn Health. 2021;30(6):807–815. doi: 10.1089/jwh.2020.8728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kline RM, Muldoon LD, Schumacher HK, et al. Design challenges of an episode-based payment model in oncology: The Centers for Medicare & Medicaid Services Oncology Care Model. J Oncol Pract. 2017;13(7):e632–e645. doi: 10.1200/jop.2016.015834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdone CG, Bayron JA, Chang C, et al. A tool to predict disparities in the timeliness of surgical treatment for breast cancer patients in the USA. Breast Cancer Res Treat. 2022;191(3):513–522. doi: 10.1007/sl0549-021-06460-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.