Abstract
Background:
Cancer incidence is higher in men than women at most shared anatomic sites, for as yet unknown reasons. We quantified the extent to which behaviors (smoking and alcohol use); anthropometrics (body mass index and height); lifestyles (physical activity, diet, medications); and medical history collectively explain the male predominance of risk at 21 shared cancer types.
Methods:
Prospective cohort analyses (n=171,274 male and n=122,826 female participants aged 50–71 years) in the NIH-AARP Diet and Health Study (1995–2011). Cancer-specific Cox regression models were used to estimate male-to-female hazard ratios (HR). The degree to which risk factors explained the observed male-female risk-disparity was quantified using the Peters-Belson method.
Results:
There were 26,693 incident cancers (17,951 in men; 8,742 in women). Incidence was significantly lower in men than women only for thyroid and gallbladder cancers. At most other anatomic sites, risks were higher in men than women (adjusted-HRs range:1.3–10.8), with the strongest increases for: bladder (HR=3.33; 95%CI=2.93–3.79), gastric cardia (HR=3.49; 95%CI=2.26–5.37), larynx (HR=3.53; 95%CI=2.46–5.06), and esophageal adenocarcinoma (HR=10.80; 95%CI=7.33–15.90). Risk factors explained a statistically significant (non-zero) proportion of the observed male excess for esophageal adenocarcinoma and cancers of liver, other biliary tract, bladder, skin, colon, rectum, and lung. Yet only a modest proportion of the male excess was explained by risk factors (ranging from 50% for lung cancer to 11% for esophageal adenocarcinoma).
Conclusions:
Men have a higher risk of cancer than women at most shared anatomic sites. Such male predominance is largely unexplained by risk factors, underscoring a role for sex-related biological factors.
Keywords: sex differences, cancer, incidence, health disparities
Precis:
The male predominance of many non-sex-specific cancers has been explained by differences in exposure prevalence between sexes, but cancer incidence remained significantly higher in men for most sites after a comprehensive adjustment for carcinogenic exposures. These findings suggest a role of sex-related biological mechanisms as the major determinants of sex differences in cancer risk.
Introduction
Cancer is a primary cause of morbidity and mortality in the United States, with an overall cancer incidence rate of 448 per 100,000 and cancer death rate of 156 per 100,000.1 The lifetime probability of developing cancer (including sex-specific cancers) is approximately equal for men and women (40% vs. 39%).2 At shared anatomic sites (excluding sex-specific cancers), the burden of cancer is significantly higher among men than among women, with men having a ≥2-fold higher risk for most cancers than women.3
It is unclear whether the male predominance in cancer incidence at shared sites is related to underlying biological sex differences or behavioral differences between men and women. Differences in smoking, alcohol use, diet, access to and utilization of healthcare, and cancer screening between men and women have been thought to be the putative cause of this sex disparity.4–7 However, most prior studies that have investigated the reasons for male predominance in cancer risk were ecological in design or conducted using cancer registry data without individual-level risk factors.3,8 Very few epidemiological studies have utilized individual-level data to investigate the extent to which sex differences in multiple carcinogenic exposures contribute to higher cancer incidence in men. A growing body of research has focused on height as an explanatory factor for the differences in cancer incidence by sex.9–11
Understanding the reasons underlying sex differences in cancer risk could yield important information regarding cancer etiology. In the current study, we estimated sex differences in the risk of 21 solid tumors at shared anatomic sites, with a particular emphasis on quantifying the degree to which differences in risk behaviors (smoking and alcohol use); anthropometrics (body mass index and height); lifestyle factors (physical activity, diet, medication use); and medical and family history explained higher cancer risk in men.
Methods
The NIH-AARP Diet and Health Study is a large prospective cohort initiated in 1995 when a baseline questionnaire was mailed to 3.5 million members of AARP aged 50–71 years residing in 8 US states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia and Detroit, Michigan).12 A total of 617,119 questionnaires were returned for a 17.6% response rate. The study was approved by the National Cancer Institute Institutional Review Board (IRB #000195). Participant consent was given by the completion and return of the study questionnaires.
The current analysis was restricted to 334,905 individuals who completed both the baseline questionnaire and a follow-up risk factor questionnaire administered between 1996 – 1997 that contained more detailed information on diet and other lifestyle factors. We excluded participants with cancer diagnosis or death before completion of the second questionnaire (n= 21,266), proxy respondents to the baseline questionnaire (n=6,959) or second questionnaire (n=3,424), self-reported poor health (n=4,382), extremely high/low caloric intake (n=2,425), conflicting gender information (n= 2,322), and individuals with missing follow up time (n=27). Therefore, the final cohort included 294,100 individuals (171,274 men and 122,826 women).
Vital status was ascertained by annual linkage to the Social Security Administration Death Master File. Addresses of participants were updated annually using data from the National Change of Address database maintained by the US Postal Service, along with responses to mailings by the study participants. Loss to follow-up was minimal (< 5%). Incident cancers occurring between the date of completion of the risk factor questionnaire to 2011 were identified through linkage with 11 state cancer registries (8 states from baseline together with 3 most common states of relocation: Arizona, Nevada, and Texas). Cancers were defined using the International Classification of Disease for Oncology (3rd edition) (see Supplemental Methods).
The baseline questionnaire was used to gather information by self-report on participant sex, age, race/ethnicity, marital status, health status, height, body mass index, history of diabetes, family history of cancer, physical activity, tobacco use, cigar or pipe use, and alcohol use. This baseline survey also included a food frequency questionnaire to assess intake of grains, fruits, vegetables, discretionary fat, red and white meat, coffee and tea consumption, and white potato consumption. Use of non-calcium containing antacids and nonsteroidal anti-inflammatory drugs were collected from the follow-up risk factor questionnaire.
Statistical Analysis
Analyses included 21 solid cancer types with at least 50 incident cancers in each sex. Follow-up time began at completion of the follow-up risk factor questionnaire and ended at the earliest of cancer diagnosis, death, loss to follow-up, or end of 2011.
Our primary analytic goals were to estimate the crude and covariate adjusted male-to-female risk ratios of cancer incidence as well as to quantify the degree to which covariates/risk factors explained the male-to-female disparity in cancer risk. We assessed differences in cancer risk for each of 21 cancer sites by sex initially using an age-adjusted Poisson regression model. Next, Cox proportional hazards regression models with follow-up time as the time scale were used to estimate the adjusted effect of sex on cancer incidence. Risk factors for adjustment were initially selected for inclusion in a full regression model based on their univariate association (p<0.10) with each cancer site of interest. Covariates were then removed using backward stepwise selection to create a final parsimonious model. A p-value of <0.05 by the Wald test was considered significant for inclusion in this final model. Age and sex were retained in all models and to accommodate non-linear effects, age and height (where applicable) were modeled as quadratic terms (age + age2 and height + height2). We additionally conducted analyses where race/ethnicity and education were forced into the models for all sites and for cancer sites where dietary predictors were selected into the models, we also assessed the impact of further adjustment for caloric intake. These race/ethnicity, education, and caloric adjustments did not materially affect the results (data not shown). The risk factors included in the final covariate-adjusted models for each cancer site are listed in eExcel Tables 1–21. The proportional hazards assumption of the covariate-adjusted model was checked by the inclusion of an interaction term of sex and follow-up time. Area under the receiver operating characteristic curve (AUC) was estimated to quantify the predictive ability of the final model for each cancer outcome.
We quantified the degree to which risk factors explained the observed male-to-female disparity in cancer incidence using the Peters-Belson method for time-to-event survival outcomes.13 Through 10 years of follow-up, we initially estimated the observed survival curve (or 1-survival= cancer incidence, per 100,000) for males (Minc) and for females (Finc). We then developed a cancer site-specific Cox model in males (using the same set of covariates for each cancer site as the final selected models noted herein) and applied this model to females to predict their counterfactual survival if they had been males (Cinc)). The Peters-Belson estimate for the male-to-female disparity in cancer risk that is explained by differences in multivariable covariate distributions between males and females was calculated as (Minc – Cinc)/(Minc – Finc). The interpretation of positive Peters-Belson estimates is that the said percentage of male-to-female differences in cancer risk is explained by differences in covariate/risk factor distributions between men and women, while the interpretation of negative Peters-Belson estimates is that male-to-female disparities would actually have been greater if men had the covariate distribution of women.14 Bootstrap methods (200 resamples) were utilized to construct 95% CIs around the Peters-Belson estimates.
We conducted a series of sensitivity analyses to evaluate the robustness of our results. First, we conducted stratified analyses to evaluate the interaction between sex and a set of a priori cancer risk factors— alcohol use, smoking, body mass index, and age. Multiplicative statistical interactions were assessed by the inclusion of a product term of sex with the risk factor in the Cox models; a Bonferroni correction was used to account for multiple comparisons for interactions (P<0.002). Second, to account for unmeasured confounding from oncogenic infections (Helicobacter pylori for non-cardia gastric cancer, high-risk human papillomavirus (HPV) for oropharyngeal cancer, and hepatitis B and hepatitis C viruses for liver cancer),15 we performed sensitivity analyses using the method described by Steenland and Greenland.16 Lastly, to evaluate the potential impact of cancer screening behaviors, we evaluated the male-to-female HRs for stage at diagnosis (regional/distant vs. local) of colorectal cancer, which were similar across stages (results not shown).
All analyses were conducted using SAS v9.4 (The SAS Institute, Cary, NC) or Stata/SE 15.1 (StataCorp, College Station, TX) with all P-values reported as two-sided.
Results
The frequency and distribution of demographic, lifestyle and dietary factors by sex are listed in Table 1. Overall, there were 294,100 individuals included in the analysis (171,274 men and 122,826 women) contributing a total of 3,499,901 person-years of follow-up with a mean of 11.5 (range: 0.01 – 15.2) person-years for men and 12.4 (range: 0.01 – 15.2) person-years for women. Cancer counts, age-adjusted rates, and male-to-female incidence rate ratios (IRR) for the 21 cancer sites are presented in order of lowest to highest IRR in Table 2. The five most common cancers among men were lung, colon, skin, bladder and kidney, while the five most common cancers among women were lung, colon, skin, pancreas, and kidney. Men had a higher age-standardized rate of most cancers. The highest age-adjusted male-to-female IRR were observed for esophageal adenocarcinoma (12.19, 95% confidence interval [CI]: 8.32, 17.86) and gastric cardia (IRR: 4.93, 95% CI: 3.59, 6.77). The only cancers more common in women than men were thyroid (IRR: 0.59, 95% CI: 0.49, 0.70) and gallbladder (IRR: 0.65, 95% CI: 0.44, 0.94) cancers.
Table 1.
Distribution of demographic, lifestyle and dietary factors among participants in the NIH-AARP study by sex (1996–1997)
| Total N= 294,100 | Men N=171,274 | Women N=122,826 | |
|---|---|---|---|
| Demographics | No. (%) | No. (%) | No. (%) |
| Age (years), n (%) | |||
| <55 | 30,322 (10.3) | 16,442 (12.2) | 13,880 (11.3) |
| 55–59 | 62,267 (21.2) | 35,458 (20.7) | 26,809 (21.8) |
| 60–64 | 81,403 (27.7) | 47,056 (27.5) | 34,347 (28.0) |
| 65–69 | 98,666 (33.6) | 59,325 (34.6) | 39,341 (32.0) |
| ≥70 | 21,442 (7.3) | 12,993 (7.6) | 8,449 (6.9) |
| Median (IQR) | 63.5 (8.7) | 63.7 (8.6) | 63.1 (8.8) |
| Height (inches), Median (IQR) | 68.0 (6.0) | 70.0 (4.0) | 64.0 (4.0) |
| Body mass index, n (%) | |||
| <20 kg/m2 | 9,445 (3.2) | 2,663 (1.6) | 6,782 (5.5) |
| 20.0 – 24.9 kg/m2 | 97,717 (33.2) | 49,632 (29.0) | 48,085 (39.2) |
| 25.0 – 29.9 kg/m2 | 121,544 (41.3) | 82,832 (48.4) | 38,712 (31.5) |
| ≥30 kg/m2 | 59,580 (20.3) | 33,628 (19.6) | 25,952 (21.1) |
| Missing | 5,814 (2.0) | 2,519 (1.5) | 3,295 (2.7) |
| Median (IQR) | 26.2 (23.8, 29.2) | 26.6 (24.4, 29.2) | 25.6 (22.7, 29.3) |
| Race/ethnicity, n (%) | |||
| Non-Hispanic White | 272,595 (92.7) | 16,0604 (93.8) | 111,991 (91.2) |
| Non-Hispanic Black | 9,534 (3.2) | 3,742 (2.2) | 5,792 (4.7) |
| Other/Unknown | 11,971 (4.1) | 6,928 (4.0) | 5,043 (4.1) |
| Education, n (%) | |||
| High school or less | 68,187 (23.2) | 32,194 (18.9) | 35,993 (29.3) |
| Some college | 97,262 (33.1) | 53,311 (31.1) | 43,951 (35.8) |
| College/some post-graduate | 121,635 (41.4) | 82,087 (47.9) | 39,548 (32.2) |
| Unknown | 7,016 (2.4) | 3,682 (2.2) | 3,334 (2.7) |
| Marital status, n (%) | |||
| Married | 200,914 (68.3) | 145,920 (85.2) | 54,994 (44.8) |
| Widowed/Divorced/Separated | 76,700 (26.1) | 18,112 (10.6) | 58,588 (47.7) |
| Never Married | 14,802 (5.0) | 6,452 (3.8) | 8,350 (6.8) |
| Missing | 1,684 (0.6) | 790 (0.5) | 894 (0.7) |
| Self-reported health status, n (%) | |||
| Excellent | 54,457 (18.5) | 32,049 (18.7) | 22408 (18.2) |
| Very Good | 108,288 (36.8) | 63,673 (37.2) | 44,615 (36.3) |
| Good | 100,055 (34.0) | 58,201 (34.0) | 41,854 (34.1) |
| Fair | 28,747 (9.8) | 16,114 (9.4) | 12,633 (10.3) |
| Unknown | 2,553 (0.9) | 1,237 (0.7) | 1316 (1.1) |
| First degree relative with cancer, n (%) | |||
| No | 182,895 (62.2) | 108,238 (63.2) | 74,657 (60.8) |
| Yes | 88,586 (30.1) | 49637 (29.0) | 38,949 (31.7) |
| Unknown | 22,619 (7.7) | 13,399 (7.8) | 9,220 (7.5) |
| Self-reported diabetes, n (%) | |||
| No | 270157 (91.9) | 155,259 (90.7) | 114,898 (93.6) |
| Yes | 23943 (8.1) | 16,015 (9.4) | 7,928 (6.4) |
| Lifestyle factors | |||
|
| |||
| Cigarette smoking | |||
| Never | 106,903 (36.4) | 51,782 (30.2) | 55,121 (44.9) |
| Former, ≤20 cigarettes/day | 83,369 (28.4) | 50,091 (29.3) | 33,278 (27.1) |
| Former, >20 cigarettes/day | 63,495 (21.6) | 48,427 (28.3) | 15,068 (12.3) |
| Current, ≤20 cigarettes/day | 20,784 (7.1) | 9,059 (5.3) | 11,725 (9.6) |
| Current, >20 cigarettes/day | 11,140 (3.8) | 6,744 (3.9) | 4,396 (3.6) |
| Unknown | 8,409 (2.9) | 5,171 (3.0) | 3,238 (2.6) |
| Cigar/pipe smoking | |||
| No | 234377 (79.7) | 117,218 (68.4) | 117159 (95.4) |
| Pipe & cigar | 22382 (7.6) | 22,315 (13.0) | 67 (0.1) |
| Pipe only | 18615 (6.3) | 18,511 (10.8) | 104 (0.1) |
| Cigar only | 10678 (3.6) | 10,391 (6.1) | 287 (0.2) |
| Unknown | 8048 (2.7) | 2,839 (1.7) | 5,209 (4.2) |
| Alcoholic drinks/day | |||
| 0 drinks/days | 67,351 (22.9) | 33,283 (19.4) | 34,068 (27.7) |
| 1 drink/day | 158,001 (53.7) | 85,930 (50.2) | 72,071 (58.7) |
| 2–3 drinks/day | 46,537 (15.8) | 33,197 (19.4) | 13,340 (10.9) |
| >3 drinks/day | 22,211 (7.6) | 18,864 (11.0) | 3,347 (2.7) |
| Median (IQR) | 0.15 (0.02, 0.89) | 0.30 (0.04, 1.3) | 0.08 (0, 0.4) |
| Physical activity (moderate to vigorous) in the last 12 months | |||
| Never | 10,593 (3.6) | 4,590 (2.7) | 6,003 (4.9) |
| Rarely | 37,224 (12.7) | 17,988 (10.5) | 19,236 (15.7) |
| 1–3 times/month | 39,136 (13.3) | 21,773 (12.7) | 17,363 (14.1) |
| 1–2 times/week | 63,428 (21.6) | 37,409 (21.8) | 26,019 (21.2) |
| 3–4 times/week | 81,931 (27.9) | 49,941 (29.2) | 31,990 (26.0) |
| ≥5 times/week | 59,508 (20.2) | 38,437 (22.4) | 21,071 (17.2) |
| Unknown | 2280 (0.8) | 1,136 (0.7) | 1,144 (0.9) |
| Medication Use | |||
|
| |||
| Ibuprofen use in the last 12 months, n (%) | |||
| No | 125,563 (42.7) | 77,731 (45.4) | 47,832 (38.9) |
| Yes | 163,983 (55.8) | 91,056 (53.2) | 72,927 (59.4) |
| Missing | 4,554 (1.6) | 2,487 (1.5) | 2,067 (1.7) |
| Aspirin use in the last 12 months, n (%) | |||
| No | 77,264 (26.3) | 36,103 (21.1) | 41,161 (33.5) |
| Yes | 213,280 (72.5) | 133,325 (77.8) | 79,955 (65.1) |
| Missing | 3,556 (1.2) | 1,846 (1.1) | 1,710 (1.4) |
| Antacid use in the last 12 months, n (%) | |||
| None | 199,640 (67.9) | 122,482 (71.5) | 77,158 (62.8) |
| Tums/Tums Extra | 52,791 (18.0) | 23,664 (13.8) | 29,127 (23.7) |
| Rolaids | 18,995 (6.5) | 11,829 (6.9) | 7,166 (5.8) |
| Titralac | 2,409 (0.8) | 1,382 (0.8) | 1,027 (0.8) |
| Mylanta | 6,441 (2.2) | 3,205 (1.9) | 3,236 (2.6) |
| Alk-Mints | 3,481 (1.2) | 1,855 (1.1) | 1,626 (1.3) |
| Other antacids containing calcium | 11,942 (4.1) | 6,700 (3.9) | 5,242 (4.3) |
| Other antacids (calcium unknown) | 21,206 (7.2) | 12,222 (7.1) | 8,984 (7.3) |
| Dietary Factors | |||
|
| |||
| Caloric intake/day, n (%) | |||
| 1st quartile (338.43 – 1,279.99 kcal) | 73,525 (25.0) | 28,297 (16.5) | 45,228 (36.8) |
| 2nd quartile (1,280.00 – 1,681.82 kcal) | 73,526 (25.0) | 38,535 (22.5) | 34,991 (28.5) |
| 3rd quartile (1,681.83 – 2,194.35 kcal) | 73,535 (25.0) | 47,821 (27.9) | 25,704 (20.9) |
| 4th quartile (2194.36 – 5,943.86 kcal) | 73,524 (25.0) | 56,621 (33.1) | 16,903 (13.8) |
| Median (IQR) | 1681.8 (1280.0, 2194.4) | 1,864.9 (1,442.9, 2,398.4) | 1,454.5 (1,123.3, 1,869.7) |
| Grain ounce equivalent/day,a n (%) | |||
| 1st quartile (0.0 – 3.39 oz) | 73,541 (24.9) | 32,071 (18.7) | 41,470 (33.8) |
| 2nd quartile (3.40 – 4.78 oz) | 73,608 (25.1) | 39,840 (23.3) | 33,768 (27.5) |
| 3rd quartile (4.79 – 6.51 oz) | 73,380 (25.0) | 46,103 (26.9) | 27,277 (22.2) |
| 4th quartile (6.52 – 31.67 oz) | 73,571 (25.0) | 53,260 (31.1) | 20,311 (16.5) |
| Median (IQR) | 4.8 (3.4, 6.5) | 5.3 (3.8, 7.1) | 4.2 (3.0, 5.7) |
| Fruit cup equivalent/day,a n (%) | |||
| 1st quartile (0 – 1.00 c) | 74,008 (25.2) | 42,908 (25.1) | 31,100 (25.3) |
| 2nd quartile (1.01 – 1.70 c) | 73,411 (25.0) | 42,196 (24.6) | 31,215 (25.4) |
| 3rd quartile (1.71 – 2.64 c) | 73,161 (24.9) | 42,128 (24.6) | 31,033 (25.3) |
| 4th quartile (2.65 – 31.98 c) | 73,520 (25.0) | 44,042 (25.7) | 29,478 (24.0) |
| Median (IQR) | 1.7 (1.0, 2.6) | 1.7 (1.0, 2.7) | 1.7 (1.0, 2.6) |
| Total dietary fiber intake (grams),b n (%) | |||
| 1st quartile (0.54 – 12.86 g) | 73558 (25.0) | 35234 (20.6) | 38,324 (31.2) |
| 2nd quartile (12.87 – 17.72 g) | 73434 (25.0) | 40876 (23.9) | 32,558 (26.5) |
| 3rd quartile (17.73 – 23.96 g) | 73599 (25.0) | 45449 (26.5) | 28,150 (22.9) |
| 4th quartile (23.97 – 152.88 g) | 73509 (25.0) | 49715 (29.0) | 23,794 (19.4) |
| Median (IQR) | 17.7 (12.9, 24.0) | 18.9 (13.8, 25.3) | 16.2 (11.7, 22.0) |
| Cooked lean red meat/day (ounces),a n (%) | |||
| 1st quartile (0 – 0.82 oz) | 73726 (25.1) | 28845 (16.8) | 44,881 (36.5) |
| 2nd quartile (0.83 – 1.54 oz) | 73275 (24.9) | 37,450 (21.9) | 35,825 (29.2) |
| 3rd quartile (1.55 – 2.63 oz) | 73738 (25.1) | 47,034 (27.5) | 26,704 (21.7) |
| 4th quartile (2.64 – 29.62 oz) | 73361 (24.9) | 57,945 (33.8) | 15,0.83416 (12.6) |
| Median (IQR) | 1.6 (0.8, 2.6) | 1.9 (1.1, 3.1) | 1.1 (0.6, 1.9) |
| Cooked lean white meat/day (ounces),a n (%) | |||
| 1st quartile (0 – 0.42 oz) | 73,174 (24.9) | 38,543 (22.5) | 34,631 (28.2) |
| 2nd quartile (0.43 – 0.82 oz) | 73,068 (24.8) | 42,146 (24.6) | 30,922 (25.2) |
| 3rd quartile (0.83 – 1.49 oz) | 74,411 (25.3) | 44342 (25.9) | 30,069 (24.5) |
| 4th quartile (1.50 – 30.23 oz) | 73,447 (25.0) | 46,243 (27.0) | 27,204 (22.2) |
| Median (IQR) | 0.8 (0.4, 1.5) | 0.9 (0.5, 1.6) | 0.8 (0.4, 1.4) |
| Discretionary solid fat/day (grams),a n (%) | |||
| 1st quartile (0.18 – 17.54 g) | 73,556 (25.0) | 31,585 (18.4) | 41,971 (34.2) |
| 2nd quartile (17.55 – 27.06 g) | 73,520 (25.0) | 40,058 (23.4) | 33,462 (27.2) |
| 3rd quartile (27.07 – 40.71 g) | 73,487 (25.0) | 46,046 (26.9) | 27,441 (22.3) |
| 4th quartile (40.72 – 204.45 g) | 73,537 (25.0) | 53,585 (31.3) | 19,952 (16.2) |
| Median (IQR) | 27.1 (17.5, 40.7) | 30.6 (20.3, 45.2) | 22.6 (14.8, 34.0) |
| Discretionary oil fat/day (grams),a n (%) | |||
| 1st quartile (0.03 – 8.42 g) | 73,545 (25.0) | 35,941 (22.0) | 37,604 (30.6) |
| 2nd quartile (8.43 – 13.60 g) | 73,485 (25.0) | 41,336 (24.1) | 32,149 (26.2) |
| 3rd quartile (13.61 – 21.12 g) | 73,518 (25.0) | 44,928 (26.2) | 28,590 (23.3) |
| 4th quartile (21.13 – 197.21 g) | 73,552 (25.0) | 49,069 (28.7) | 24,483 (19.9) |
| Median (IQR) | 13.6 (8.4, 21.1) | 14.8 (9.3, 22.6) | 12.1 (7.5, 19.0) |
| Number of white potatoes/serving,a n (%) | |||
| 1st quartile (0 – 0.30 servings) | 72,325 (24.6) | 32,975 (19.3) | 39,350 (32.0) |
| 2nd quartile (0.31 – 0.56 servings) | 73,872 (25.1) | 39,434 (23.0) | 34,438 (28.0) |
| 3rd quartile (0.57 – 1.04 servings) | 73,691 (25.1) | 45,977 (26.8) | 27,714 (22.6) |
| 4th quartile (1.05 – 11.88 servings) | 74,212 (25.2) | 52,888 (30.9) | 21,324 (17.4) |
| Median (IQR) | 0.6 (0.3, 1.1) | 0.7 (0.4, 1.2) | 0.5 (0.2, 0.9) |
| Coffee or tea/day in grams,a n (%) | |||
| 1st quartile (0 – 458.56 g) | 73524 (25.0) | 42063 (24.7) | 31461 (25.6) |
| 2nd quartile (458.60 – 982.70 g) | 73486 (25.0) | 40811 (24.8) | 32675 (26.6) |
| 3rd quartile (982.73 – 1,350.26 g) | 73566 (25.0) | 43804 (25.6) | 29762 (24.2) |
| 4th quartile (1,350.30 – 6,318.01 g) | 73524 (25.0) | 44596 (26.0) | 28928 (23.6) |
| Median (IQR) | 982.7 (458.6, 1,350.3) | 986.2 (472.8, 1,374.8) | 967.9 (443.5, 1,324.0) |
| Total vegetable equivalents/day (cups),a n (%) | |||
| 1st quartile (0 – 1.15 c) | 73,277 (24.9) | 39,955 (23.3) | 33,322 (27.1) |
| 2nd quartile (1.16 – 1.71 c) | 73,521 (25.0) | 42,692 (24.9) | 30,829 (25.1) |
| 3rd quartile (1.72 – 2.46 c) | 73,664 (25.1) | 44,080 (25.7) | 29,584 (24.1) |
| 4th quartile (2.47 – 22.78 c) | 73,638 (25.0) | 44,547 (26.0) | 29,091 (23.7) |
| Median (IQR) | 1.7 (1.2, 2.5) | 1.8 (1.2, 2.5) | 1.7 (1.1, 2.4) |
Abbreviations: C, cups; g, grams; IQR, interquartile range; kcal, kilocalories; n, number; and oz, ounces
Assessed based on the MyPyramid Equivalents of intake per day scale.
Assessed using the Nutrition Data System for Research.
Table 2.
Cancer counts, rates (per 100,000 person-years), and age-adjusted incidence rate ratios comparing men and women in the NIH-AARP cohort
| Cancer | Total Count | Men | Women | Age-Adjusted Incidence Rate Ratio (95% CI) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Count | Rate | Count | Rate | |||
| Thyroid | 520 | 222 | 11.3 | 298 | 19.6 | 0.59 (0.49, 0.70) |
| Gallbladder | 110 | 50 | 2.5 | 60 | 3.9 | 0.65 (0.44, 0.94) |
| Anus | 148 | 82 | 4.2 | 66 | 4.3 | 0.97 (0.70, 1.34) |
| Lung | 8,153 | 4,927 | 249.9 | 3,226 | 211.2 | 1.18 (1.13, 1.23) |
| Pancreas | 1,657 | 1,024 | 48.8 | 633 | 41.3 | 1.26 (1.14, 1.39) |
| Small intestine | 222 | 138 | 7.0 | 84 | 5.5 | 1.28 (0.98, 1.68) |
| Colon | 4,091 | 2,563 | 130.0 | 1,528 | 100.2 | 1.29 (1.21, 1.37) |
| Oral cavity | 407 | 269 | 13.6 | 138 | 9.1 | 1.50 (1.22, 1.85) |
| Rectum | 1,377 | 934 | 47.4 | 443 | 29.1 | 1.61 (1.44, 1.80) |
| Esophagus (squamous cell) | 147 | 101 | 5.1 | 46 | 3.0 | 1.67 (1.18, 2.37) |
| Kidney | 1,783 | 1,284 | 65.1 | 499 | 32.7 | 2.00 (1.80, 2.21) |
| Gastric non-cardia | 347 | 252 | 12.8 | 95 | 6.2 | 2.04 (1.61, 2.58) |
| Other head and neck | 277 | 202 | 10.24 | 75 | 4.88 | 2.08 (1.59, 2.71) |
| Other biliary tract | 223 | 162 | 8.2 | 61 | 4.0 | 2.09 (1.56, 2.81) |
| Skin (excluding basal and squamous) | 3,102 | 2,291 | 116.2 | 811 | 53.1 | 2.20 (2.03, 2.38) |
| Liver | 539 | 406 | 20.6 | 133 | 8.7 | 2.38 (1.96, 2.89) |
| Oropharynx | 382 | 300 | 13.5 | 82 | 5.4 | 2.86 (2.24, 3.65) |
| Bladder | 1,992 | 1,668 | 84.6 | 324 | 21.2 | 3.96 (3.52, 4.46) |
| Larynx | 422 | 354 | 18.0 | 68 | 4.5 | 3.99 (3.07, 5.17) |
| Gastric cardia | 325 | 281 | 14.3 | 44 | 2.9 | 4.93 (3.59, 6.77) |
| Esophagus (adenocarcinoma) | 469 | 441 | 22.4 | 28 | 1.8 | 12.19 (8.32, 17.86) |
The demographic, lifestyle and dietary covariates included in the adjusted models for each site with the associated AUC and male-to-female hazard ratios (HRs) are presented in Table 3 (full models in eExcel Tables 1–21). The models for esophageal adenocarcinoma (AUC: 0.78, 95% CI: 0.76, 0.80), larynx cancer (AUC: 0.76, 95% CI: 0.74, 0.78), and gastric cardia (AUC: 0.75, 95% CI: 0.72, 0.77) had the highest AUCs. These three cancers also had the highest male-to-female HR after adjusting for demographic, lifestyle, and dietary covariates—esophageal adenocarcinoma (HR: 10.80, 95% CI: 7.33, 15.90), larynx cancers (HR: 3.53, 95% CI: 2.46, 5.06), and gastric cardia cancers (HR: 3.49, 95% CI: 2.26, 5.37). Conversely, men had a reduced risk of thyroid (HR: 0.55, 95% CI:0.46, 0.66) and gallbladder (HR: 0.33, 95% CI: 0.18, 0.58) cancers as compared to women. The increased relative risk among men was retained for 11 cancers after adjustment for covariates. By contrast, for 7 cancer sites, adjustment for lifestyle and dietary risk factors rendered the male effect statistically insignificant: lung (HR: 0.99, 95% CI: 0.92, 1.06), pancreas (HR: 1.06, 95% CI: 0.91, 1.23), small intestine (HR: 1.21, 95% CI: 0.91–1.61), colon (HR: 0.99, 95% CI: 0.90, 1.09), oral cavity (1.38, 95% CI: 0.99, 1.91), esophagus squamous cell carcinoma (HR:1.46, 95%CI: 0.98, 2.18), and other head and neck (HR: 1.33, 95% CI: 0.90–1.97).
Table 3.
Covariates selected for inclusion into final multivariable models with the area under the curve statistics and adjusted hazard ratios for each cancer site
| Cancer site | Covariates included in final model | Model with sex AUC (95% CI) | Male-to-Female HR (95% CI) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Demographics | General health/Medication | Diet/Lifestyle | Risk behaviors | |||
| Thyroid | Age, age2, BMI | White meat consumption | 0.587 (0.563, 0.611) | 0.55 (0.46, 0.66) | ||
| Gallbladder | Age, age2, race, BMI | Height, height2 | Red meat consumption | 0.653 (0.600, 0.705) | 0.33 (0.18, 0.58) | |
| Anus | Age, age2, marital status | 0.596 (0.548, 0.644) | 1.17 (0.82, 1.69) | |||
| Lung | Age, age2, BMI, education, race | 1st degree relative w/cancer, height, height2, self-reported health status | Coffee/tea consumption, fruit consumption, grain consumption, red meat consumption, white meat consumption | Alcohol use, cigar/pipe smoking, cigarette smoking | 0.739 (0.734, 0.744) | 0.99 (0.92, 1.06) |
| Pancreas | Age, age2, education | Diabetes, self-reported health status | White potato consumption | Cigarette smoking | 0.622 (0.609, 0.635) | 1.06 (0.91, 1.23) |
| Small intestine | Age, age2 | Self-reported health status | Caloric intake | 0.603 (0.567, 0.639) | 1.21 (0.91, 1.61) | |
| Colon | Age, age2, BMI | Diabetes, height, height2, Ibuprofen use, self-reported health status | Fiber consumption, oil consumption, physical activity, red meat consumption, white potato consumption | Alcohol use, cigarette smoking | 0.632 (0.624, 0.640) | 0.99 (0.90, 1.09) |
| Oral cavity | Age, age2, marital status | Height, height2 | Fiber consumption | Alcohol use, cigarette smoking | 0.648 (0.620, 0.675) | 1.38 (0.99, 1.91) |
| Rectum | Age, age2, education | Antacid use, diabetes, Ibuprofen use | Red meat consumption | Alcohol use, cigarette smoking | 0.634 (0.620, 0.649) | 1.36 (1.20, 1.54) |
| Esophagus – squamous cell | Age, age2, BMI, race | Self-reported health status | Caloric intake | Alcohol use, cigarette smoking | 0.739 (0.695, 0.783) | 1.46 (0.98, 2.18) |
| Kidney | Age, age2, BMI | Diabetes, height, height2, self-reported health status | Fiber consumption, grain consumption | Alcohol use, cigarette smoking | 0.638 (0.626, 0.651) | 1.57 (1.34, 1.83) |
| Gastric (Cardia) | Age, age2, education | Antacid use, diabetes, height, height2 | Coffee/tea consumption, vegetable consumption | Cigarette smoking | 0.748 (0.723, 0.772) | 3.49 (2.26, 5.37) |
| Other head & neck | Age, age2 | Aspirin use, height, height2 | Alcohol use, cigarette smoking | 0.653 (0.600, 0.705) | 1.33 (0.90–1.97) | |
| Other biliary tract | Age, age2 | Cigarette smoking | 0.662 (0.628, 0.695) | 1.99 (1.47, 2.69) | ||
| Skin | Age, age2, BMI education, race | Height, height2 | Physical activity, vegetable consumption, white meat consumption | Alcohol use, cigarette smoking | 0.650 (0.641, 0.660) | 1.42 (1.26, 1.60) |
| Liver | Age, age2, BMI, marital status, race | Aspirin use, diabetes, self-reported health status | Red meat consumption, white meat consumption | Alcohol use, cigarette smoking | 0.726 (0.705, 0.748) | 2.60 (2.06, 3.28) |
| Oropharynx | Age, age2, race | Coffee/tea consumption, grain consumption | Alcohol use, cigarette smoking | 0.687 (0.662, 0.713) | 2.57 (1.99, 3.33) | |
| Bladder | Age, age2, race | Antacid use, diabetes, 1st degree relative w/cancer, self-reported health status | Coffee/tea consumption, fiber consumption | Cigar/pipe smoking, cigarette smoking | 0.728 (0.717, 0.738) | 3.33 (2.93, 3.79) |
| Larynx | Age, age2, race | 1st degree relative w/cancer, height, height2, self-reported health status | Fiber consumption | Alcohol use, cigarette smoking | 0.756 (0.735, 0.777) | 3.53 (2.46, 5.06) |
| Gastric (Non-cardia) | Age, age2, race | Ibuprofen use | Physical activity | Alcohol use, cigarette smoking, coffee/tea consumption | 0.708 (0.680, 0.735) | 2.21 (1.72–2.84) |
| Esophagus – adenocarcinoma | Age, age2, BMI, race | Antacid use, 1st degree relative w/cancer | Cigarette smoking | 0.781 (0.764, 0.798) | 10.80 (7.33, 15.90) | |
Abbreviations: AUC, area under the curve statistic; BMI, body mass index; CI, confidence interval; and HR, hazard ratio
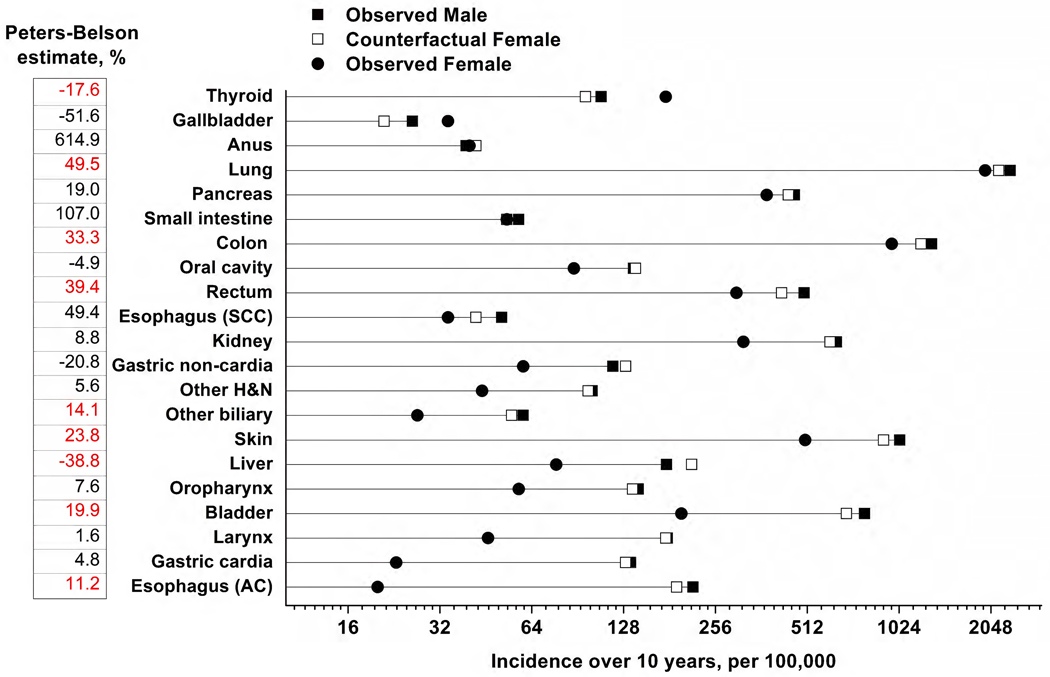
The results of the Cox proportional hazard regression modeling with the Peters-Belson method are shown in Figure 1. For seven cancers (lung, colon, rectum, other biliary tract, skin, bladder, and esophagus-adenocarcinoma), the distribution of the covariates among women explained at least some of the observed difference in cancer incidence between men and women. The estimated Peters-Belson explained percent ranged from 11.2% for esophagus-adenocarcinoma to 49.5% for lung cancer. For 12 cancers (gallbladder, anus, pancreas, small intestine, oral cavity, esophagus-squamous cell carcinoma, kidney, gastric non-cardia, other head and neck, oropharynx, larynx, and gastric cardia) the Peters-Belson estimates were not significantly different from zero, indicating that differences in covariate distributions did not account for male-female differences in cancer incidence. Liver cancer was the only site where men would have experienced an even greater incidence if they had the same exposure distributions of women (−38.8%, 95% CI −73.0%, −4.6%). For thyroid cancer, men would have even lower incidence if they had the exposure distribution of women (−17.6%, 95% CI: −32.2, −2.9).
Figure 1.
Observed ten-year cancer incidence rates per 100,000 in males and females, with Peters-Belson estimates expressed as a percentage
Abbreviations: AC, adenocarcinoma; H&N, head and neck; and SCC, squamous cell carcinoma.
The observed survival curves for males (Minc) and females (Finc) were estimated (10-year incidence per 100,000) and a cancer site-specific Cox model in males was applied to females to predict their counterfactual survival if they had been males (Cinc). The Peters-Belson estimate for the male-to-female disparity in cancer risk that is explained by differences in multivariable covariate distributions between males and females was calculated as (Minc – Cinc)/(Minc – Finc), expressed as a percentage. A positive Peters-Belson estimate indicates that the male-to-female difference in cancer risk is explained by differences in covariate/risk factor distributions between men and women, while a negative estimate indicates the male-to-female disparities would be greater if men had the same covariate distribution as women. Peters-Belson estimates are shown on the left; red font indicates the estimate is statistically significant.
To assess effect modification, we stratified our covariate-adjusted models by four risk factors: alcohol use, smoking status, body mass index, and age group (eFigures 1 – 4). None of the interactions were significant at the Bonferroni-corrected P<0.002 for any of the cancer sites. We performed indirect adjustment of the male-to-female risk ratio for head and neck, liver, and non-cardia gastric cancers in order to control for the potential confounding effects of differences in exposure to the infectious agents associated with cancer development (eTable 1). These analyses indicate that, under plausible assumptions, men remained at an increased risk of liver cancer and non-cardia gastric incidence. By contrast, the observed increased risk of oropharyngeal cancer in men could be explained by the increased prevalence of high-risk oral HPV among men.
Conclusions
While the lifetime risk of cancer is similar among men and women, a male predominance is observed at most shared anatomic sites. Although, such male excess is well recognized, it remains largely unexplained. Through a comprehensive analysis of sex differences in the risk of 21 cancer at anatomic sites within a large US cohort, we provide two key observations. First, with the exception of thyroid and gallbladder cancers, men had a higher risk of cancer than women at most shared anatomic sites, including rectum, kidney, gastric cardia, biliary tract, skin, liver, oropharynx, bladder, larynx, gastric non-cardia, and esophagus adenocarcinoma. Second, such male predominance remained even after adjustment for a wide range of risk behaviors and carcinogenic exposures. Indeed, differences in risk behaviors and carcinogenic exposures between sexes only accounted for a modest proportion (ranging from 11.2% to 49.4% across cancer sites) of the observed male predominance of most cancers. Collectively, our results point to the potential role of sex-related biological mechanisms, rather than differences in carcinogenic exposures, as the major determinants of male-female differences in risk of cancer at most shared anatomic sites.
The higher cancer susceptibility at shared anatomic sites in men is hypothesized to arise from several interrelated biological differences between sexes, including physiological, immunological, genetic, epigenetic, and genomic mechanisms.17,18 Physiologically, differences in sex-steroid hormones, such as progesterone and estrogen, are believed to mediate lower risk of some cancers in women.17,19 Alternatively, higher testosterone levels may promote cell growth20,21 and have been associated with increased risk of skin (malignant melanoma), prostate, and liver cancers in men, and breast and endometrial cancers in women.22,23 Immunologically, stronger innate and adaptive (particularly Th2) immune responses in women could reduce susceptibility to cancer. For example, women mount more robust immune responses to oncogenic infections, such as hepatitis B and hepatitis C viruses and human papillomavirus, which in turn could mediate lower risk of liver and oropharyngeal cancers, respectively.24 Lastly, sex differences in cancer risk could also arise from genetic and epigenetic mechanisms, such as the presence of several immune-related and tumor suppressor genes on the X-chromosome,17 frequent escape of such genes from epigenetic X-chromosome inactivation,19,25 as well as age-related mosaic loss of Y chromosome in men.26,27 Indeed, emerging genomic data indicate key sex differences in mutation burden and mutational signatures across several cancer sites.28,29
Cook et al.3 used the Surveillance, Epidemiology and End Results (SEER) program data to show elevated risks in men for cancers of the lip, larynx, hypopharynx, urinary bladder, esophagus, tonsil, oropharynx, and other urinary organs, as well as elevated risks in women for cancers of the anus, gallbladder, and thyroid. Similarly, combined data from the National Program of Cancer Registries and SEER show higher incidence and worse survival for men at many cancer sites.30 We confirm and extend these observations by showing that the extent of the male predominance was retained, though slightly attenuated, after adjustment for a wide range of risk factors. Recent work from several longitudinal studies9–11 suggests height as a key mediator of the male predominance of cancer across several anatomic sites, contributing to as much as 35% of the male excess of cancer.11 Further, a prior study within NIH-AARP identified height as a risk factor for several cancer sites in sex-stratified analyses (cancers of the lung and prostate cancers in men; breast and endometrium in women; and colorectum, kidney, melanoma, and non-Hodgkin’s lymphoma in both sexes).10 While our results confirm the association of height across a range of cancer sites, we found that height in addition to other carcinogenic exposures did not collectively account for a high explained proportion of the male predominance of cancer at shared sites.
Our results need to be interpreted within the context of some key limitations, primarily residual or unmeasured confounding. Although we adjusted for a wide range of carcinogenic exposures in our regression models, some of our models had low to modest discrimination. The covariates included in our models were collected at only one time point and relied on self-report; given recognized sex differences in accuracy of self-reported data some of our results may have been impacted by differential misclassification.31–33 Also, our models did not include occupation (which accounts for a relatively small fraction of cancers) or carcinogenic infections (which account for larger attributable fractions at select anatomic sites).34 Thus, we conducted indirect adjustment to account for potential sex differences in carcinogenic infection prevalence. However, there are likely other risk factors we did not measure, for example, exposure to ultraviolet light for skin cancer and exposure to fine particulate matter for lung cancer; consequently, we cannot entirely rule out residual/unmeasured confounding due to environmental factors. Despite NIH-AARP being one of the largest US cohort studies, we had low statistical power for uncommon cancers. Finally, the NIH-AARP study is comprised mostly of non-Hispanic white individuals of middle to upper socioeconomic class. As a result, the degree of sex disparities in cancer risk we found may differ among other races/ethnicities or socioeconomic groups. Notable strengths of our study include the use of a large prospective US cohort, comprehensive evaluation of sex differences in risk of cancer at 21 shared anatomic sites, and quantification of the contribution of differences in carcinogenic exposures towards the observed male-female differences in cancer risk.
In summary, in addition to known carcinogenic exposures, our analyses point to sex, and as a corollary, sex-related biological factors as major determinants of cancer incidence in the US. Understanding the sex-related biological mechanisms that lead to the male predominance of cancer at shared anatomic sites could have important implications for etiology and prevention. Notably, the physiological, immunological, and genetic/genomic hypotheses referenced herein have largely been investigated in prior studies that have only included one sex and/or for select carcinogenic exposures and cancer sites. Global evaluations of these mechanisms across a wide range of cancer sites are needed to characterize their contribution towards the greater burden of cancer in men.
Supplementary Material
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute. Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer incidence data from California were collected by the California Cancer Registry, California Department of Public Health’s Cancer Surveillance and Research Branch, Sacramento, California. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (Miami, Florida) under contract with the Florida Department of Health, Tallahassee, Florida. The views expressed herein are solely those of the authors and do not necessarily reflect those of the FCDC or FDOH. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, Louisiana. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, The Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Division of Public and Behavioral Health, State of Nevada Department of Health and Human Services, Carson City, Nevada.
We are indebted to the participants in the NIH-AARP Diet and Health Study for their outstanding cooperation. We also thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management, Ann Taylor at Information Management Services for data support and analysis, and Stephanie Kovalick for statistical support on an earlier draft of this manuscript. All authors contributed to the study design, statistical analysis, and drafting of the manuscript.
Funding
Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI).
Role of the Funding Source
The funding source had no role in the study design, data analysis, interpretation of the results, or the decision to publish this manuscript.
Role of the funders:
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Financial support:
This work was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute
Disclosures:
Morgan Marks, received funding from Merck & Co. Inc for research outside the submitted work. The other authors made no disclosures.
Footnotes
Conflict of interest statement
None declared.
Conflict of interest: The authors declare no potential conflicts of interest
REFERENCES
- 1.Islami F, Ward EM, Sung H, et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J Natl Cancer Inst 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2009;18:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCann J. Gender Differences in Cancer That Don’t Make Sense—Or Do They? JNCI: Journal of the National Cancer Institute 2000;92:1560–2. [DOI] [PubMed] [Google Scholar]
- 5.Zahm SH, Fraumeni JF, Jr. Racial, ethnic, and gender variations in cancer risk: considerations for future epidemiologic research. Environ Health Perspect 1995;103 Suppl 8:283–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet 2012;3:268-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCartney G, Mahmood L, Leyland AH, Batty GD, Hunt K. Contribution of smoking-related and alcohol-related deaths to the gender gap in mortality: evidence from 30 European countries. Tob Control 2011;20:166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgren G, Liang L, Adami HO, Chang ET. Enigmatic sex disparities in cancer incidence. Eur J Epidemiol 2012;27:187–96. [DOI] [PubMed] [Google Scholar]
- 9.Walter RB, Brasky TM, Buckley SA, Potter JD, White E. Height as an explanatory factor for sex differences in human cancer. Journal of the National Cancer Institute 2013;105:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabat GC, Kim MY, Hollenbeck AR, Rohan TE. Attained height, sex, and risk of cancer at different anatomic sites in the NIH-AARP Diet and Health Study. Cancer Causes & Control 2014;25:1697–706. [DOI] [PubMed] [Google Scholar]
- 11.Fu BC, Song M, Li X, et al. Height as a mediator of sex differences in cancer risk. Annals of Oncology 2020;31:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–25. [DOI] [PubMed] [Google Scholar]
- 13.Eberly LE, Hodges JS, Savik K, Gurvich O, Bliss DZ, Mueller C. Extending the Peters-Belson approach for assessing disparities to right censored time-to-event outcomes. Stat Med 2013;32:4006–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Graubard BI, Huang P, Gastwirth JL. Extension of the Peters-Belson method to estimate health disparities among multiple groups using logistic regression with survey data. Statistics in medicine 2015;34:595–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. The Lancet Global health 2019. [DOI] [PubMed] [Google Scholar]
- 16.Steenland K, Greenland S. Monte Carlo sensitivity analysis and Bayesian analysis of smoking as an unmeasured confounder in a study of silica and lung cancer. Am J Epidemiol 2004;160:384–92. [DOI] [PubMed] [Google Scholar]
- 17.Clocchiatti A, Cora E, Zhang Y, Dotto GP. Sexual dimorphism in cancer. Nature reviews Cancer 2016;16:330–9. [DOI] [PubMed] [Google Scholar]
- 18.Haupt S, Caramia F, Klein SL, Rubin JB, Haupt Y. Sex disparities matter in cancer development and therapy. Nature reviews Cancer 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature reviews Immunology 2008;8:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maasberg M, Rotsch M, Jaques G, Enderle-Schmidt U, Weehle R, Havemann K. Androgen receptors, androgen-dependent proliferation, and 5 alpha-reductase activity of small-cell lung cancer cell lines. International journal of cancer 1989;43:685–91. [DOI] [PubMed] [Google Scholar]
- 21.Tutton PJ, Barkla DH. The influence of androgens, anti-androgens, and castration on cell proliferation in the jejunal and colonic crypt epithelia, and in dimethylhydrazine-induced adenocarcinoma of rat colon. Virchows Arch B Cell Pathol Incl Mol Pathol 1982;38:351–5. [DOI] [PubMed] [Google Scholar]
- 22.Hyde Z, Flicker L, McCaul KA, et al. Associations between Testosterone Levels and Incident Prostate, Lung, and Colorectal Cancer. A Population-Based Study. Cancer Epidemiology Biomarkers & Prevention 2012;21:1319–29. [DOI] [PubMed] [Google Scholar]
- 23.Watts EL, Perez-Cornago A, Knuppel A, Tsilidis KK, Key TJ, Travis RC. Prospective analyses of testosterone and sex hormone-binding globulin with the risk of 19 types of cancer in men and postmenopausal women in UK Biobank. International journal of cancer 2021;149:573–84. [DOI] [PubMed] [Google Scholar]
- 24.Klein SL, Flanagan KL. Sex differences in immune responses. Nature Reviews Immunology 2016;16:626–38. [DOI] [PubMed] [Google Scholar]
- 25.Dunford A, Weinstock DM, Savova V, et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat Genet 2017;49:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loftfield E, Zhou W, Yeager M, Chanock SJ, Freedman ND, Machiela MJ. Mosaic Y Loss Is Moderately Associated with Solid Tumor Risk. Cancer Res 2019;79:461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsberg LA, Rasi C, Malmqvist N, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nature Genetics 2014;46:624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li CH, Prokopec SD, Sun RX, et al. Sex differences in oncogenic mutational processes. Nature Communications 2020;11:4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li CH, Haider S, Shiah YJ, Thai K, Boutros PC. Sex Differences in Cancer Driver Genes and Biomarkers. Cancer Res 2018;78:5527–37. [DOI] [PubMed] [Google Scholar]
- 30.Dong M, Cioffi G, Wang J, et al. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2020;29:1389–97. [DOI] [PubMed] [Google Scholar]
- 31.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obesity Reviews 2007;8:307–26. [DOI] [PubMed] [Google Scholar]
- 32.McKenzie BL, Coyle DH, Santos JA, et al. Investigating sex differences in the accuracy of dietary assessment methods to measure energy intake in adults: a systematic review and meta-analysis. The American journal of clinical nutrition 2021;113:1241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert JR, Ma Y, Clemow L, et al. Gender Differences in Social Desirability and Social Approval Bias in Dietary Self-report. American Journal of Epidemiology 1997;146:1046–55. [DOI] [PubMed] [Google Scholar]
- 34.Schottenfeld D, Beebe-Dimmer JL, Buffler PA, Omenn GS. Current Perspective on the Global and United States Cancer Burden Attributable to Lifestyle and Environmental Risk Factors. Annual review of public health 2013;34:97–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.