Abstract
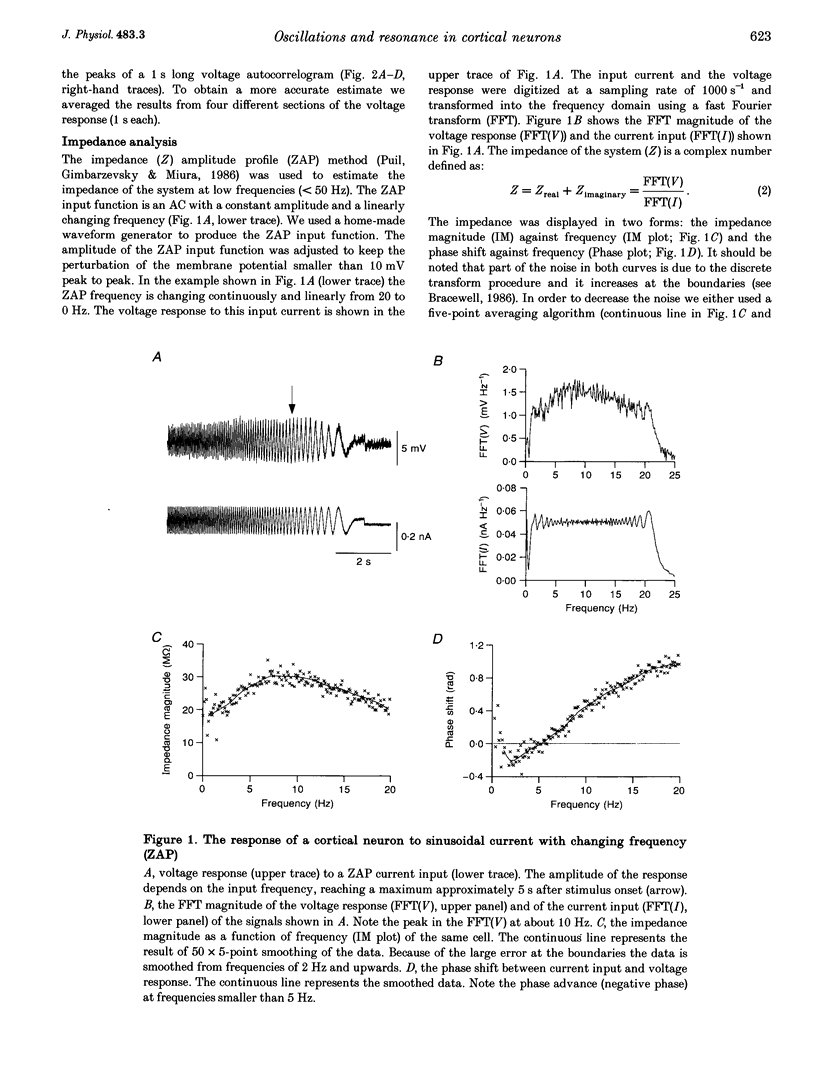
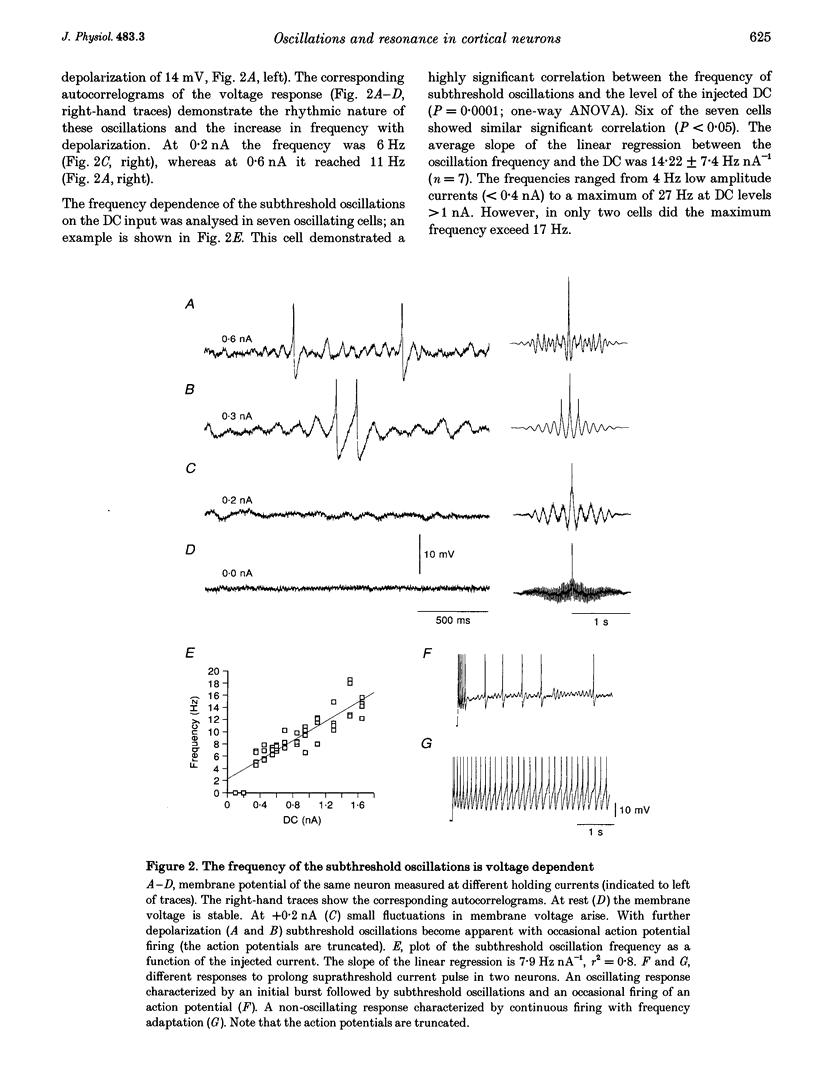
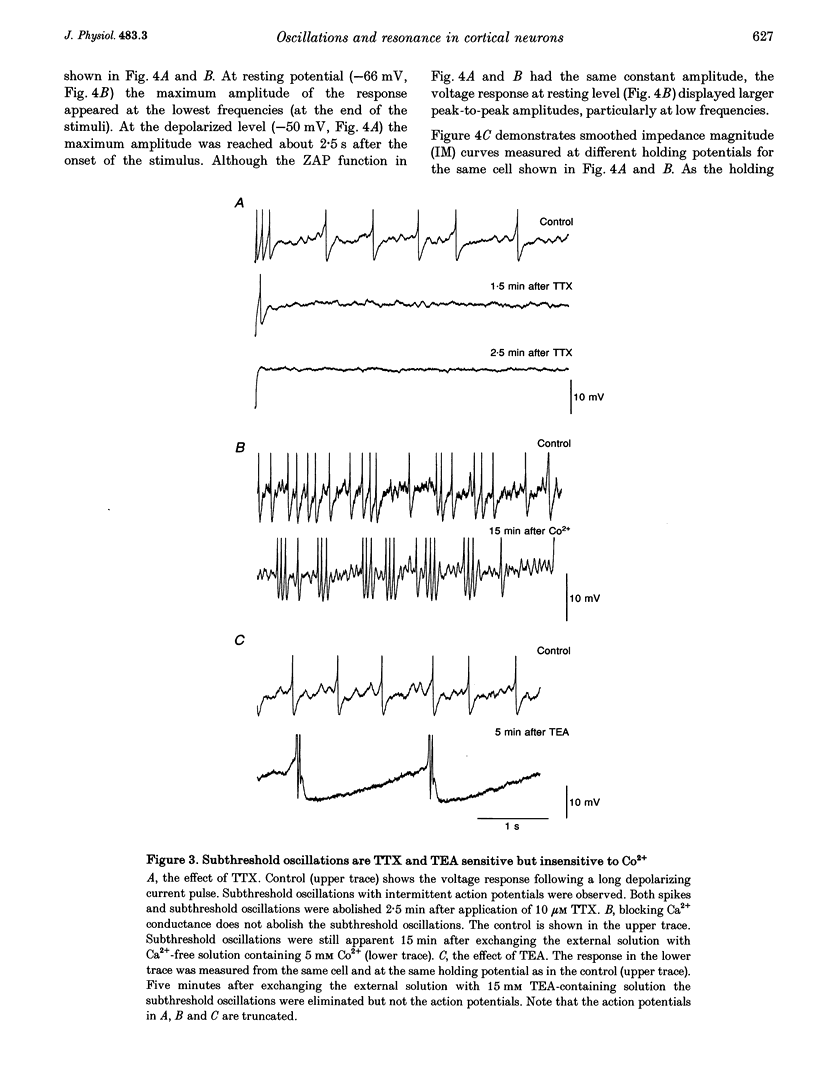
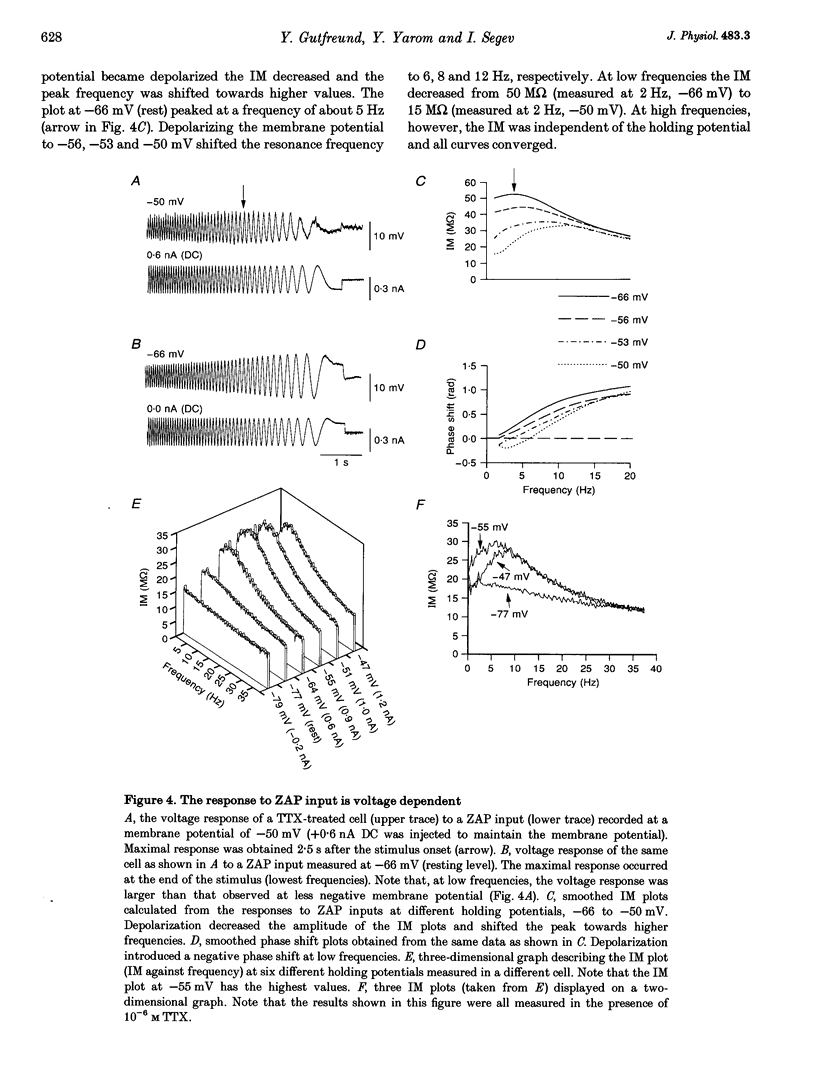
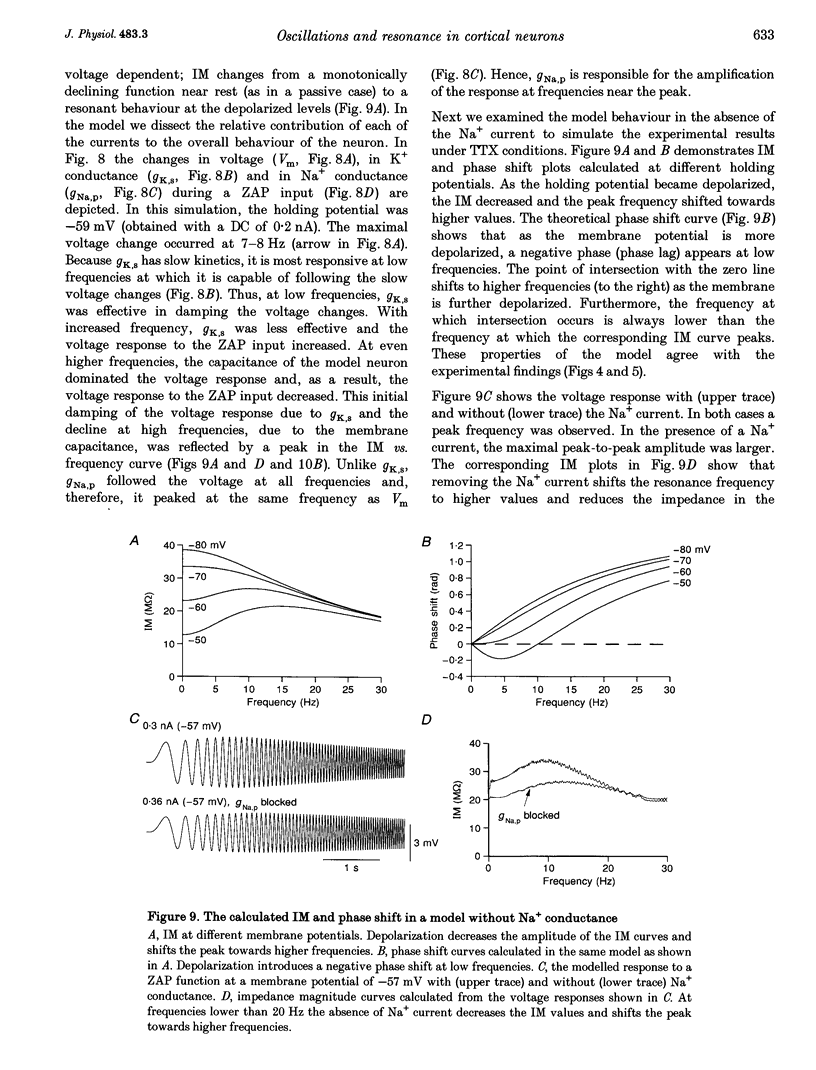
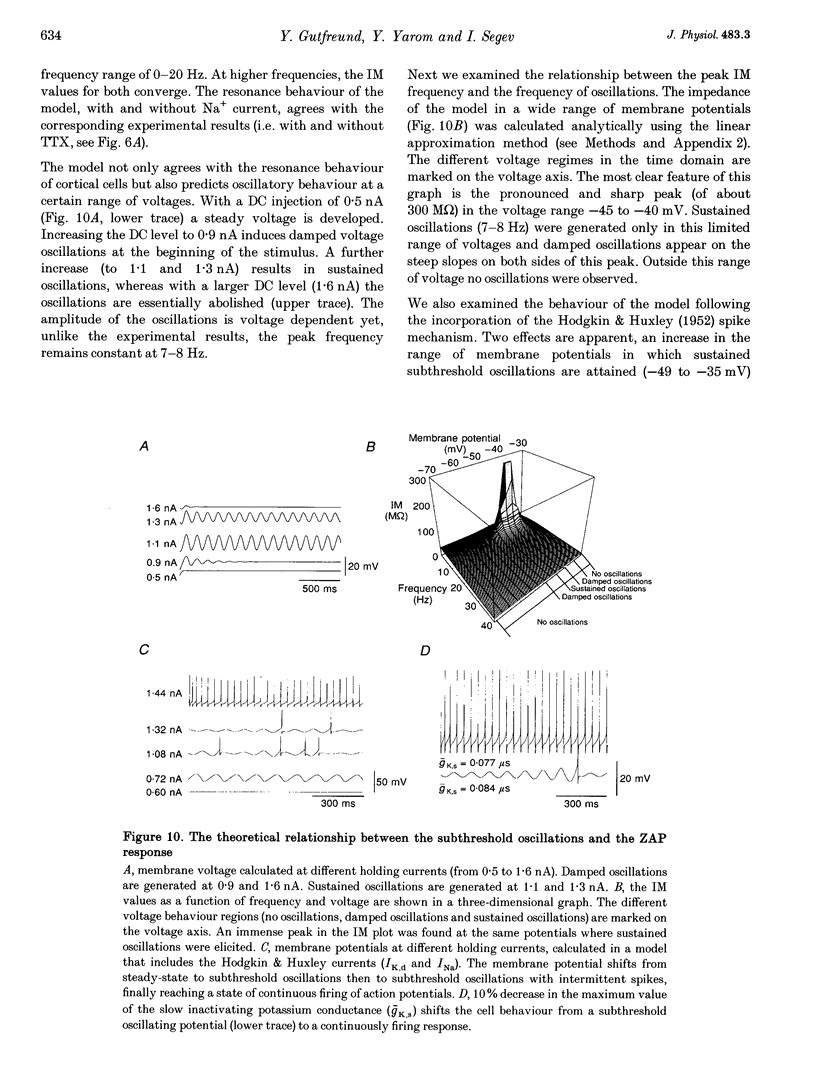
1. Intracellular recordings were made from neurons in slices from guinea-pig frontal cortex. In 50% of the cells, sustained subthreshold voltage oscillations were evoked by long (> 6 s) depolarizing pulses. The peak-to-peak amplitude of these oscillations was less than 5 mV and the frequency was voltage dependent, increasing with depolarization from 4 (near rest) to 20 Hz (at 30 mV depolarization). 2. The impedance-frequency relationship of both oscillating and non-oscillating cells was studied by intracellular injection of sinusoidal current with linearly changing frequency. In most cells, a peak in the impedance magnitude (resonant behaviour) was observed at depolarized levels. The frequency of the peak impedance (peak frequency) increased with depolarization from 3 (near rest) to 15 Hz (at 30 mV depolarization). 3. Application of TTX (10(-6) M) significantly decreased the impedance magnitude near the peak frequency. The subthreshold oscillations, however, as well as the action potentials, were fully blocked by TTX. On the other hand, TEA (15 mM) and Cs+ (5 mM) abolished both the subthreshold oscillations and the resonant behaviour. Replacing Ca2+ with Co2+ (5 mM) or Ni2+ (1 mM) did not abolish the subthreshold oscillations. The peak in the frequency-response curve was only slightly reduced. 4. An isopotential membrane model, consisting of a leak current, a fast persistent sodium current, a slow non-inactivating potassium current (with the kinetics of the M-current) and membrane capacitance, is sufficient to produce both voltage oscillations and resonant behaviour. The kinetics of the K+ current by itself is sufficient to produce resonance behaviour. The Na+ current amplifies the peak impedance magnitude and is essential for the generation of subthreshold oscillation. The model correctly predicted the behaviour of the frequency response before and after TTX and TEA application, as well as the relation between the expected passive impedance and the experimental impedance. 5. We speculate that the tendency of the neurons to generate voltage signals at a certain frequency (as a result of the subthreshold oscillations) and to preferentially respond to inputs arriving at the same frequency (the resonance behaviour) promotes population activity at that preferred frequency.
Full text
PDF









Images in this article
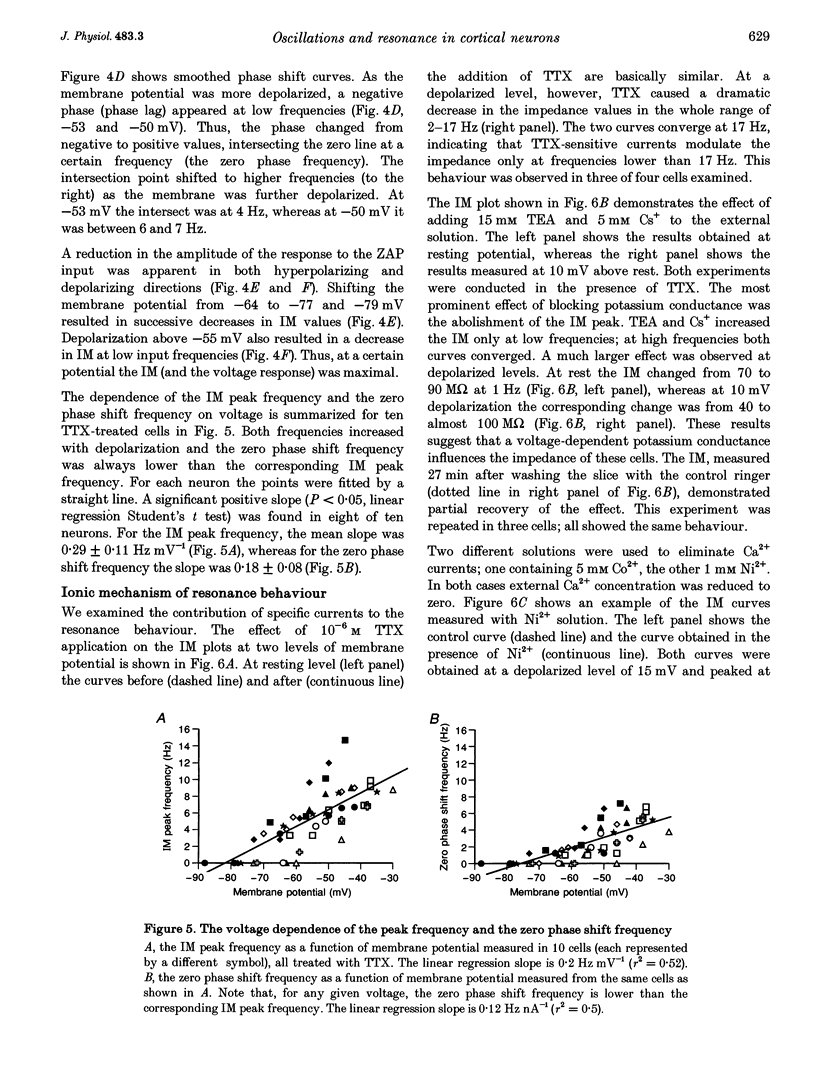
Selected References
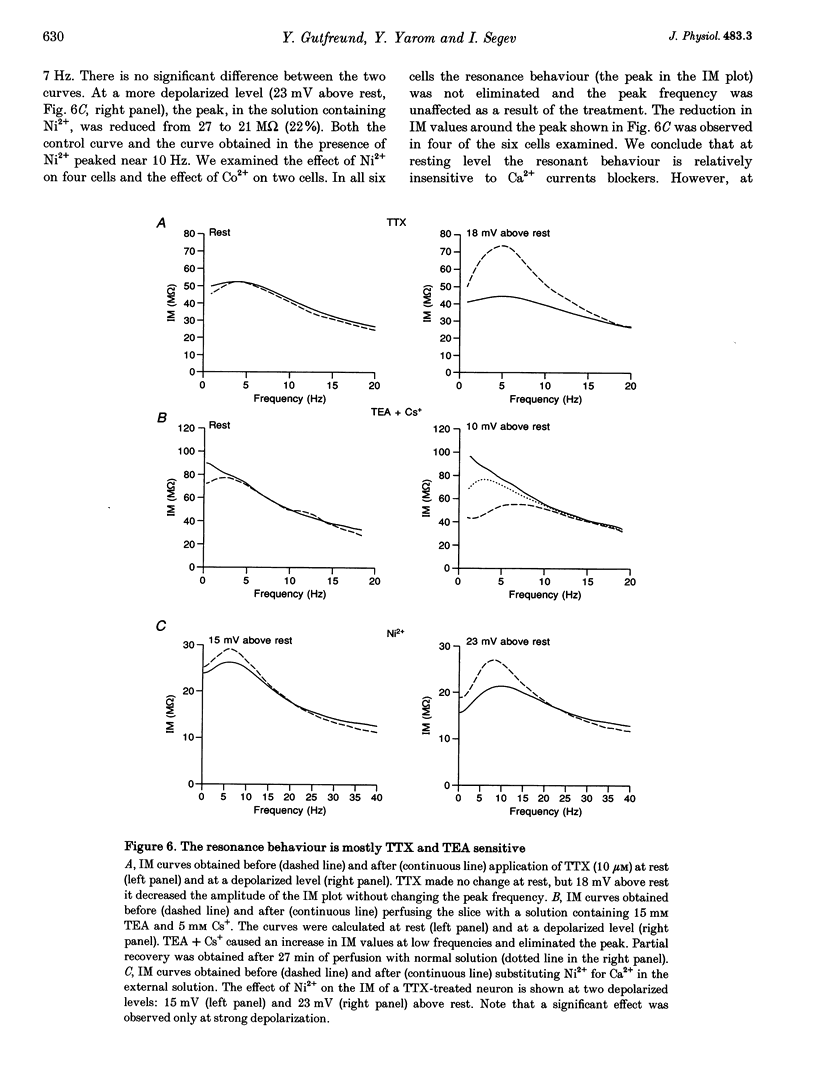
These references are in PubMed. This may not be the complete list of references from this article.
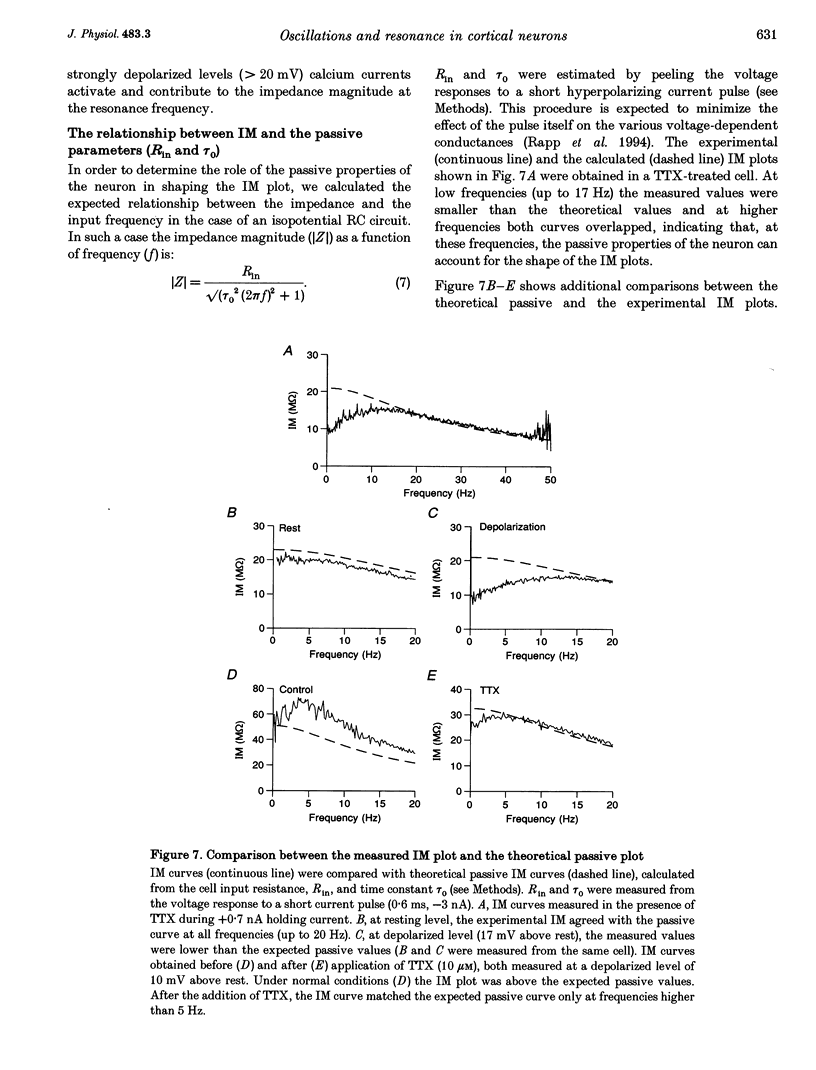
- Bernander O., Douglas R. J., Martin K. A., Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
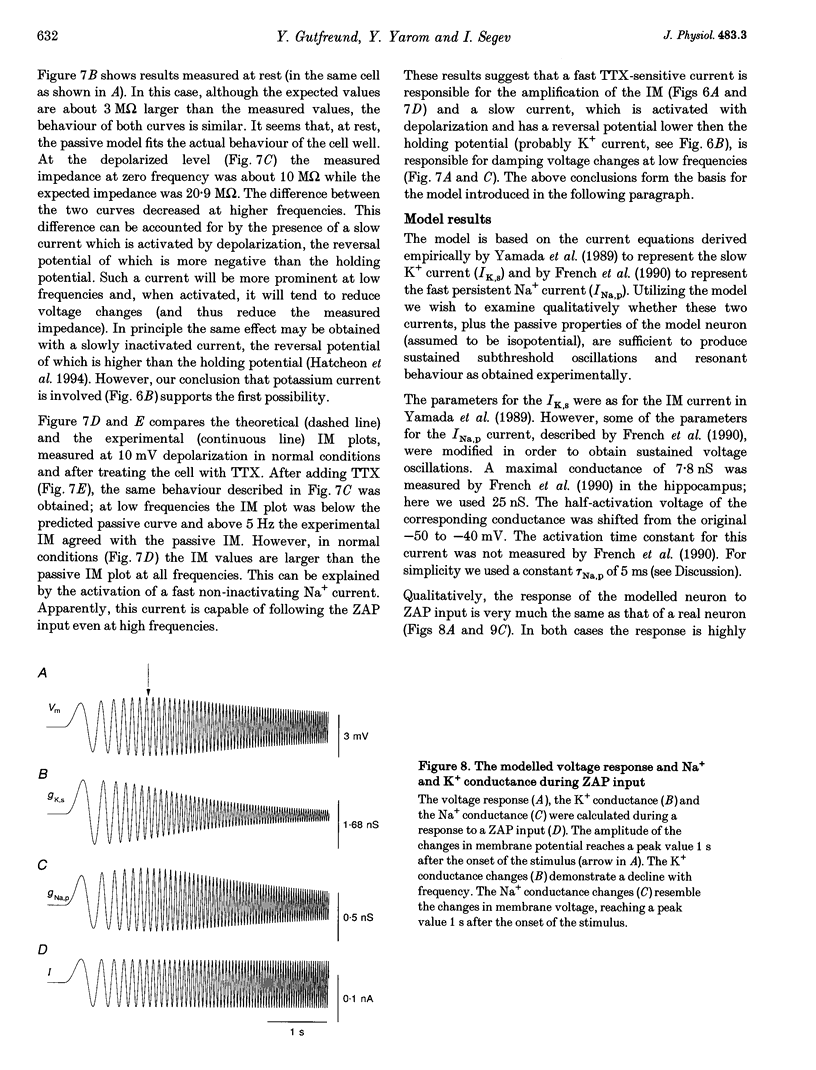
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Correia M. J., Christensen B. N., Moore L. E., Lang D. G. Studies of solitary semicircular canal hair cells in the adult pigeon. I. Frequency- and time-domain analysis of active and passive membrane properties. J Neurophysiol. 1989 Oct;62(4):924–934. doi: 10.1152/jn.1989.62.4.924. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol. 1981 Mar;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D., Carlen P. Electrotonic parameters of neurons following chronic ethanol consumption. J Neurophysiol. 1985 Oct;54(4):807–817. doi: 10.1152/jn.1985.54.4.807. [DOI] [PubMed] [Google Scholar]
- French C. R., Sah P., Buckett K. J., Gage P. W. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J Gen Physiol. 1990 Jun;95(6):1139–1157. doi: 10.1085/jgp.95.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbarzevsky B., Miura R. M., Puil E. Impedance profiles of peripheral and central neurons. Can J Physiol Pharmacol. 1984 Apr;62(4):460–462. doi: 10.1139/y84-074. [DOI] [PubMed] [Google Scholar]
- Gray C. M., König P., Engel A. K., Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989 Mar 23;338(6213):334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon B., Miura R. M., Yarom Y., Puil E. Low-threshold calcium current and resonance in thalamic neurons: a model of frequency preference. J Neurophysiol. 1994 Feb;71(2):583–594. doi: 10.1152/jn.1994.71.2.583. [DOI] [PubMed] [Google Scholar]
- Klink R., Alonso A. Ionic mechanisms for the subthreshold oscillations and differential electroresponsiveness of medial entorhinal cortex layer II neurons. J Neurophysiol. 1993 Jul;70(1):144–157. doi: 10.1152/jn.1993.70.1.144. [DOI] [PubMed] [Google Scholar]
- Klink R., Alonso A. Ionic mechanisms for the subthreshold oscillations and differential electroresponsiveness of medial entorhinal cortex layer II neurons. J Neurophysiol. 1993 Jul;70(1):144–157. doi: 10.1152/jn.1993.70.1.144. [DOI] [PubMed] [Google Scholar]
- Koch C. Cable theory in neurons with active, linearized membranes. Biol Cybern. 1984;50(1):15–33. doi: 10.1007/BF00317936. [DOI] [PubMed] [Google Scholar]
- Lampl I., Yarom Y. Subthreshold oscillations of the membrane potential: a functional synchronizing and timing device. J Neurophysiol. 1993 Nov;70(5):2181–2186. doi: 10.1152/jn.1993.70.5.2181. [DOI] [PubMed] [Google Scholar]
- Llinás R. R., Grace A. A., Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986 Jul;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton W. W., Sejnowski T. J. Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. J Neurophysiol. 1991 Sep;66(3):1059–1079. doi: 10.1152/jn.1991.66.3.1059. [DOI] [PubMed] [Google Scholar]
- Mauro A., Conti F., Dodge F., Schor R. Subthreshold behavior and phenomenological impedance of the squid giant axon. J Gen Physiol. 1970 Apr;55(4):497–523. doi: 10.1085/jgp.55.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Wang Z., Huguenard J. Neurotransmitter control of neocortical neuronal activity and excitability. Cereb Cortex. 1993 Sep-Oct;3(5):387–398. doi: 10.1093/cercor/3.5.387. [DOI] [PubMed] [Google Scholar]
- Meyer J. H., Zakon H. H. Androgens alter the tuning of electroreceptors. Science. 1982 Aug 13;217(4560):635–637. doi: 10.1126/science.217.4560.635. [DOI] [PubMed] [Google Scholar]
- Puil E., Gimbarzevsky B., Miura R. M. Quantification of membrane properties of trigeminal root ganglion neurons in guinea pigs. J Neurophysiol. 1986 May;55(5):995–1016. doi: 10.1152/jn.1986.55.5.995. [DOI] [PubMed] [Google Scholar]
- Puil E., Meiri H., Yarom Y. Resonant behavior and frequency preferences of thalamic neurons. J Neurophysiol. 1994 Feb;71(2):575–582. doi: 10.1152/jn.1994.71.2.575. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969 Dec;9(12):1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp M., Segev I., Yarom Y. Physiology, morphology and detailed passive models of guinea-pig cerebellar Purkinje cells. J Physiol. 1994 Jan 1;474(1):101–118. doi: 10.1113/jphysiol.1994.sp020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva L. R., Amitai Y., Connors B. W. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991 Jan 25;251(4992):432–435. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Steriade M., McCormick D. A., Sejnowski T. J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993 Oct 29;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Wang X. J. Ionic basis for intrinsic 40 Hz neuronal oscillations. Neuroreport. 1993 Dec 13;5(3):221–224. doi: 10.1097/00001756-199312000-00008. [DOI] [PubMed] [Google Scholar]
- Wilcox K. S., Gutnick M. J., Christoph G. R. Electrophysiological properties of neurons in the lateral habenula nucleus: an in vitro study. J Neurophysiol. 1988 Jan;59(1):212–225. doi: 10.1152/jn.1988.59.1.212. [DOI] [PubMed] [Google Scholar]



