Abstract
Purpose
In thyroid eye disease (TED), activation and proliferation of orbital fibroblasts (OFs) promotes remodeling and causes an increase in the volume of orbital tissue. Platelet-derived growth factors (PDGFs) are elevated in TED and promote OF activation. The aryl hydrocarbon receptor (AHR), a ligand activated nuclear receptor, is important in regulating OF activation. AHR ligands have been evaluated as therapeutic agents for inflammatory diseases. Here, we hypothesize that AHR ligands will block PDGF-induced signaling in TED OFs.
Methods
OFs from both non-TED and TED patients were treated with PDGFβ, with or without the AHR ligands 6-Formylindolo[3,2-b]carbazole (FICZ) or tapinarof. Cell viability was measured by the Alamar Blue assay. Cell proliferation was quantified using the BrdU assay. Cell lysates were collected and analyzed by Western blotting and real-time quantitative PCR (RT-qPCR) to measure PDGF and AHR signaling. Scratch assays were used to measure OF migration.
Results
PDGFβ induced proliferation in TED OFs significantly more than in non-TED OFs. Additionally, PDGFβ increased phosphorylation of AKT and expression of thymidylate synthase (TYMS). PDGFβ dependent proliferation and downstream signaling were attenuated by FICZ or tapinarof. TYMS and other PDGF target genes were upregulated by PDGFβ and reduced by AHR activation. PDGFβ induced TED OF migration while both FICZ and tapinarof diminished this effect.
Conclusions
PDGF signaling led to increased proliferation and activation of TED OFs. Treatment of TED OFs with the AHR ligands, FICZ and tapinarof, mitigated PDGF induced effects. These studies support the concept that AHR and PDGF signaling could form the basis for new TED therapeutics.
Keywords: thyroid eye disease (TED), platelet derived growth factor (PDGF), tapinarof, fibroblast, aryl hydrocarbon receptor (AHR), therapeutic, eye disease, thymidylate synthase, AKT, scratch assay, cell migration
Thyroid eye disease (TED) is an autoimmune condition resulting in increased volume of orbital tissue, inflammation, and scarring.1,2 It affects 25% to 50% of patients with Graves’ disease, an autoimmune disorder causing hyperthyroidism due to stimulatory autoantibodies against the thyroid-stimulating hormone receptor (TSHR).3,4 Although more prevalent in women, TED paradoxically presents with greater severity in men. Symptoms include ocular irritation, periorbital swelling, lid retraction, exposure keratopathy, optic neuropathy, diplopia, and vision loss.5,6 Current treatments have limited efficacy, primarily focusing on managing symptoms rather than cure or prevention of TED. Therefore, further research into TED’s underlying mechanisms is crucial for developing more effective, targeted therapies.
Orbital fibroblasts (OFs) play a vital role in TED pathology. These cells, located in orbital connective tissue, produce extracellular matrix components such as collagen and hyaluronan.7 In TED, OFs become overactivated, leading to excessive extracellular matrix production, and differentiating into either scar-forming myofibroblasts or lipid-containing adipocytes.2,8 Activation is driven by contact with infiltrating immune cells and/or secreted factors, such as cytokines and lipid mediators.7,9–11 Multiple pathways contribute to orbital fibroblast activation, including TED autoantibodies, insulin-like growth factor-1 (IGF-1), interleukins, transforming growth factor beta (TGFβ), and platelet-derived growth factor (PDGF).12–14
Teprotumumab, the first US Food and Drug Administration (FDA)-approved drug specifically for TED, was introduced in 2020.15 As a monoclonal blocking antibody targeting the insulin-like growth factor 1 receptor (IGF1R), Teprotumumab has significantly improved TED treatment outcomes. However, the treatment has limitations, including high cost, limited availability, variable efficacy, side effects such as hearing loss, muscle spasms, nausea, and hyperglycemia, as well as the potential for disease recurrence post-treatment.16–18 These broad side effects of Teprotumumab stem from the ubiquitous expression of IGF1R, highlighting the need for more targeted therapeutic approaches.
This study focuses on PDGF signaling in OF activation. The five different PDGF cytokines (α, β, α/β, C, and D), constitute a family of dimeric proteins that are potent mitogens, promoting proliferation, cell migration, extracellular matrix production, and increased cell viability. Previous research has shown elevated levels of PDGFα and PDGFβ in orbital tissue of patients with TED, including OFs.7,19 PDGF receptors α and β, like IGF1R, are receptor tyrosine kinases that induce cellular signaling in part through phosphatidylinositol-3 kinase (PI3K)/AKT cascades. Interestingly, PDGF receptors show a more selective expression pattern than IGF1R. TED OFs express PDGF receptors and respond to PDGF signaling by inducing IL-6 production and proliferation, suggesting PDGF signaling is a potential therapeutic target.7
The aryl hydrocarbon receptor (AHR) is a ligand-regulated transcription factor that binds to endogenous and xenobiotic hydrocarbon ligands.20 The receptor is expressed in various immune cells, stem cells, and fibroblasts. AHR is elevated in TED OFs compared to non-TED OFs.20,21 Upon ligand binding, AHR translocates to the nucleus, where it forms a complex with the aryl hydrocarbon receptor nuclear translocator (ARNT), and regulates gene expression.21 Although the role of AHR in TED remains unclear, previous studies have demonstrated that the AHR regulates the immune response and limits TGFβ activity.21,22 We previously found that the endogenous AHR ligand, 6-formylindolo[3,2-b]carbazole (FICZ) reduces OF expansion and collagen production in TED.20
Tapinarof, a recently identified AHR ligand and novel therapeutic agent, has shown promising results in treating inflammatory skin conditions, such as plaque psoriasis.23,24 Its potential in managing TED, particularly through its effects on OFs, has not been studied and warrants investigation. Moreover, there is a lack of research investigating crosstalk through PDGF and AHR signaling. We hypothesize that activating AHR in TED could be beneficial by limiting PDGF signaling and OF proliferation. In this study, we activated PDGF signaling using PDGFβ, which is a potent mitogen that binds all PDGF receptor complexes. We also evaluated the activation of AHR with its ligands, FICZ and tapinarof, in the presence or absence of PDGF, to determine whether AHR activation can mitigate PDGF-mediated fibroblast activation.
Methods
Subjects
Primary orbital fibroblasts from both non-TED and TED patients undergoing orbital decompression surgery at the Flaum Eye Institute were used for this study. Tissue procurement procedures followed the tenets of the Declaration of Helsinki and were approved by the University of Rochester Medical School Research Subjects Review Board. Informed, written consent was obtained from all patients before surgeries. The clinical characteristics of the patients used in this study are listed in the Table.
Table.
Clinical Characteristics of Patients With TED From This Study
| Age (Mean/Range) | 55.5 (34–74) |
| Gender, M/F | 5/7 |
| Smoking, (Y/N | 6/6 |
| Steroid use, Y/N | 5/7 |
| Radiation, Y/N | 1/11 |
Cell Culture
Orbital fibroblasts were cultured in Dulbecco's modified Eagle's Medium/Nutrient Mixture F-12 (DMEM/F12) supplemented with 10% fetal bovine serum (FBS) and antibiotics. All media and supplements were purchased from Gibco (Carlsbad, CA, USA) or Corning Life Sciences (Corning, NY, USA). FBS was from Sigma (St. Louis, MO, USA). The OFs were placed in 0.1% FBS-DMEM/F12 for 24 to 72 hours before addition of 0.1 to 100 ng/mL PDGFβ (PeproTech, Cranbury, NJ, USA) for an additional 24 to 72 hours to drive proliferation. Imatinib from Selleckchem (Houston, TX, USA) was used at 5 µM to block PDGF receptor (PDGFR) signaling. The AHR ligand FICZ was obtained from Enzo Life Sciences (Farmingdale, NY, USA) and was added at 1 µM to selected samples 1 to 2 hours before the addition of PDGFβ. The AHR ligand tapinarof was obtained from Selleckchem and was added at 1 µM prior to the addition of PDGFβ.
Western Blot Analysis
Cells were homogenized with lysis buffer containing 50 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate and a protease inhibitor cocktail (Cell Signaling Biotechnology, Beverly, MA, USA). Total protein concentration was determined with the detergent compatible (DC) protein detection assay (BioRad, Hercules, CA, USA). Then, 5 to 10 µg of total protein lysate per sample was separated via SDS-PAGE, transferred onto 0.45 µm Immobilon-PVDF membranes (Millipore, Billerica, MA, USA), and blocked with 5% non-fat dry milk and 0.1% Tween 20 (BioRad) in 1× tris-buffered saline (TBS). The following antibodies were from Cell Signaling Biotechnology (Danvers, MA, USA): phospho-AKT (rabbit anti-pAKT (Ser473), cat number: 4060), total AKT (rabbit anti-AKT, cat number: 9272), AHR (rabbit anti-AHR, cat number: 83200), Thymidylate Synthase (TYMS; rabbit anti-TYMS, cat number: 3766), and CYP1B1 (rabbit anti-CYP1B1, cat number: sc-32882) which was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The β-tubulin levels were measured for a control housekeeping protein (anti-tubulin-hFAB RHODAMINE, cat number: 12004165, BioRad, Hercules, CA, USA). Anti-rabbit HRP-conjugated secondary antibody was obtained from Jackson Immunoresearch (West Grove, PA, USA). Protein was visualized using Immobilon Western chemiluminescent horseradish peroxidase substrate (Millipore, Billerica, MA, USA). Chemiluminescent signals were captured using a ChemiDoc MP Imaging System and densitometric analysis was performed with Image Lab analysis software (BioRad). Equal protein loading was confirmed using Mini-Protean TGX stain-free gels (BioRad) visualized on the ChemiDoc MP imaging system.
RNA Extraction
Total cell RNA was extracted using Qiazol lysis reagent and isolated with a RNeasy Mini Kit according to the manufacturer's instructions (Qiagen, Valencia, CA, USA). Total RNA concentrations were determined with a DS-11+ Spectrophotometer (DeNovix, Wilmington, DE, USA).
Real-Time Quantitative PCR
The cDNA was generated using the iScript reverse transcription kit (BioRad) and gene expression quantified via real-time quantitative PCR (RT-qPCR) with gene specific primers, a universal SsoFast Evergreen PCR master mix and CFX Connect Real-Time PCR Detection System, all from BioRad. Gene expression was normalized using the ΔΔCt method with the housekeeping genes: ACTB, 18S rRNA, and TBP as control.25 Gene-specific primers sequences are listed in Supplementary Table S1.
Wound Healing (Scratch) Assay
Cells were grown to 95% confluence in 12-well plates and then serum starved in 0.1% FBS DMEM/F12 for 48 hours. A scratch was applied to each well using a flattened P200 micropipette tip and then washed with 1× PBS to remove any debris. Brightfield images were taken at specific locations at time 0 to serve as a baseline for analysis. Selected wells were pretreated for 1 hour with 1 µM FICZ or 1 µM tapinarof. Cells were then treated with or without PDGFβ as described. Additionally, images at the same locations were taken at 24, 48, and 72 hours. Wound area analysis was conducted using ImageJ software (National Institutes of Health [NIH], Bethesda, MD, USA). Areas at each time point were normalized to the baseline area from the initial time 0 scratch.
Alamar Blue Assay
Cells were plated at a density of 1000 cells per well in a 96-well plate. Cells were serum starved for 48 hours in 0.1% FBS DMEM/F12. Cells were pretreated with vehicle (DMSO), 1 µM FICZ or tapinarof followed by stimulation with PDGFβ for 72 hours as indicated. Puromycin (10 µg/mL) was used as a positive control for cytotoxicity. Then, 10% vol/vol of Alamar Blue Cell Viability Reagent (#2245373; Invitrogen, Carlsbad, CA, USA) was add to each well, as directed by the manufacturer’s instructions. Fluorescence of the oxidized Alamar Blue reagent was measured after 1, 2, 4, and 24 hours (excitation 544 nm and emission 590 nm) and normalized to vehicle-treated cells.
Bromodeoxyuridine Incorporation Assay
Cells were seeded in a 96-well plate at a density of 2500 cells per well and allowed to adhere. The deoxythymidine analog, bromodeoxyuridine (BrdU) was added at the time of treatment to make it available for DNA incorporation during cell proliferation. Treatments were performed in triplicate, and the cells were grown for 72 hours. Cells were then fixed and stained with an anti-BrdU antibody, followed by incubation with a horseradish peroxidase (HRP)-conjugated secondary antibody. After addition of the HRP substrate, 3,3',5,5'-tetramethylbenzidine (TMB), BrdU incorporation was quantified using a microplate reader (BioRad) at 655 nm. Readings from vehicle-treated control cells were normalized to 1.0 to measure relative comparisons between treatments.
Statistical Analysis
All experiments were repeated at least three times using different strains of non-TED and TED orbital fibroblasts (n ≥ 3 of each type) to ensure reproducibility. Data were analyzed using GraphPad Prism, version 10.2 (Boston, MA, USA). For comparisons between the two groups, a Student's t-test was used. For comparisons among multiple groups, 1-way or 2-way analysis of variance (ANOVA) with Dunnett's post hoc test for multiple comparisons was applied as appropriate. A P value of ≤ 0.05 was considered statistically significant.
Results
PDGFβ Induces Greater Proliferation in TED OFs Compared to Non-TED OFs
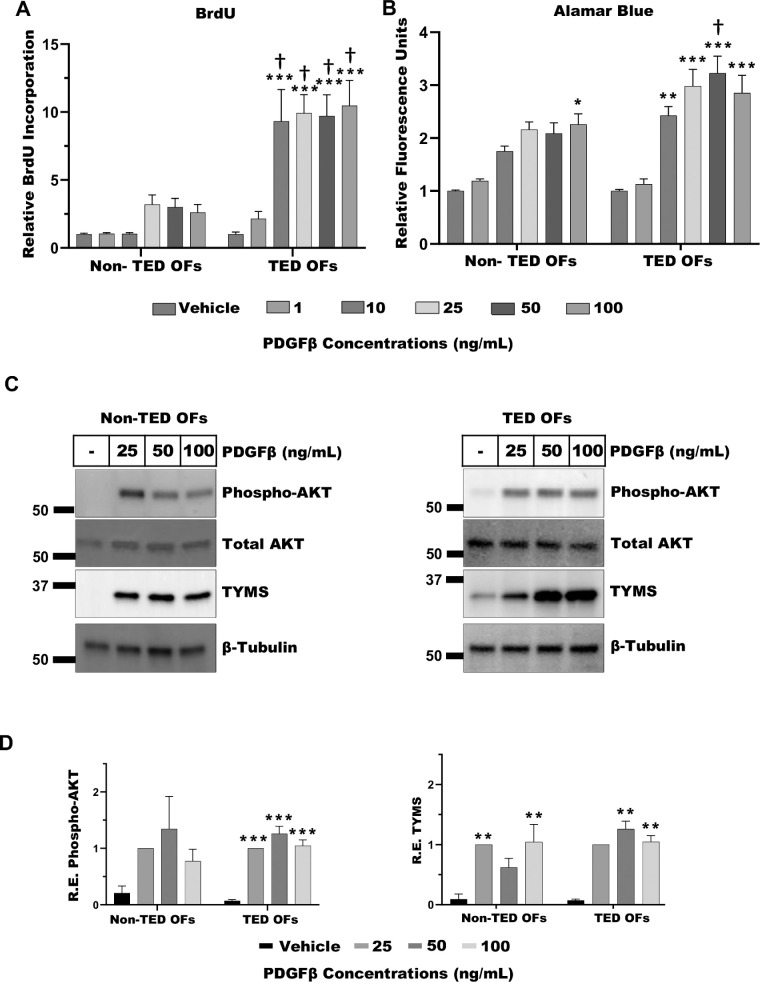
To assess the effects of PDGFβ on the proliferation of TED and non-TED OFs, a BrdU incorporation assay was performed. TED and non-TED OFs were treated with increasing concentrations of PDGFβ (1 to 100 ng/mL) for 72 hours, during which BrdU was added to measure DNA synthesis as an indicator of cell proliferation (Fig. 1A). PDGFβ-dependent BrdU incorporation was significantly elevated in TED OFs compared to non-TED OFs at doses of 10 to 100 ng/mL PDGFβ, indicating enhanced proliferative activity in TED cells. Notably, TED OFs responded significantly to PDGFβ at 10 ng/mL, whereas non-TED OFs showed no detectable response at this dose, suggesting increased sensitivity of TED OFs to PDGFβ.
Figure 1.
PDGFβ increases orbital fibroblast (OF) proliferation, activation, PI3K/AKT signaling, and TYMS expression. (A) Non-TED and TED OFs were treated with vehicle, 1, 10, 25, 50, or 100 ng/mL PDGFβ for 72 hours. The nucleotide analog BrdU was added to measure DNA synthesis. After culture, cells were fixed and the BrdU label was detected by ELISA. PDGFβ treatment resulted in a dose-dependent increase in OF proliferation. TED OFs accumulated significantly more BrdU than non-TED OFs. Results are presented as means ± SEM from triplicate wells. The experiment was performed in four patients with non-TED and four patients with TED OFs. (B) Equal numbers of non-TED or TED OFs were treated with PDGFβ as indicated for 48 to 72 hours. Cells were then treated with Alamar Blue assay reagent for 1 hour. Fluorescent signal showing Alamar Blue reagent reduction was measured. Vehicle (DMSO) treated cells served as control. The experiment was performed in four non-TED and four TED OFs. (C) Non-TED and TED OFs were grown to confluence and treated with PDGFβ for 48 hours. Cells were lysed and analyzed by Western blot for phospho-AKT, total AKT, TYMS, and β-Tubulin (loading control). Phospho-AKT levels were normalized to total AKT levels. TYMS was normalized to β-tubulin levels. (D) Quantification of Western blots for phospho-AKT and TYMS. The experiments were repeated in at least three non-TED and three TED OF strains with representative results shown. One-way ANOVA with Dunnett's multiple comparisons was used to analyze the data. * P ≤ 0.05, ** P = 0.005, *** P < 0.0008, and non-TED versus TED: † P ≤ 0.05.
Building on these findings, we next assessed the impact of PDGFβ on overall cellular metabolic activity. Non-TED and TED OFs were treated with PDGFβ (1 to 100 ng/mL) for 72 hours, followed by incubation with the cell permeable Alamar Blue reagent, a fluorescent REDOX-sensitive molecule that indicates cell viability and metabolic activation (Fig. 1B). Consistent with the BrdU results, Alamar Blue fluorescence was elevated in response to PDGFβ, with significant increases at 10 to 100 ng/mL in TED OFs. Further, Alamar Blue fluorescence in TED OFs was significantly higher than in non-TED OFs at 50 ng/mL PDGFβ dose, indicating elevated metabolic activity in TED OFs.
PDGFβ Increases PI3K/AKT Signaling and Thymidylate Synthase Expression
We next explored downstream signaling mediated by PDGFβ in both TED and non-TED OFs. PDGFβ mediates signaling in part through activation of PI3K/AKT signaling. Here, we measured phosphorylation of AKT at serine 473, (phospho-AKT), which serves as an indicator of PI3K/AKT activation and signaling. We also measured the expression of thymidylate synthase (TYMS), an enzyme required for conversion of deoxyuracil monophosphate into deoxythymidine monophosphate, which is important for cell proliferation and de novo DNA biosynthesis.26 Non-TED and TED OFs were treated with or without 25 to 100 ng/mL PDGFβ for 48 hours before cells were lysed and analyzed by Western blot (Fig. 1C). PDGFβ promoted the phosphorylation of AKT, in both non-TED and TED OFs at 25, 50, and 100 ng/mL PDGFβ (Fig. 1D, left panel). TYMS levels were also induced by PDGFβ in both TED and non-TED OFs, with significant increases obtained at 25 and 100 ng/mL in non-TED OFs and 50 and 100 ng/mL in TED OFs (Fig. 1D, right panel).
PDGFβ Signaling is Attenuated in TED OFs by Imatinib
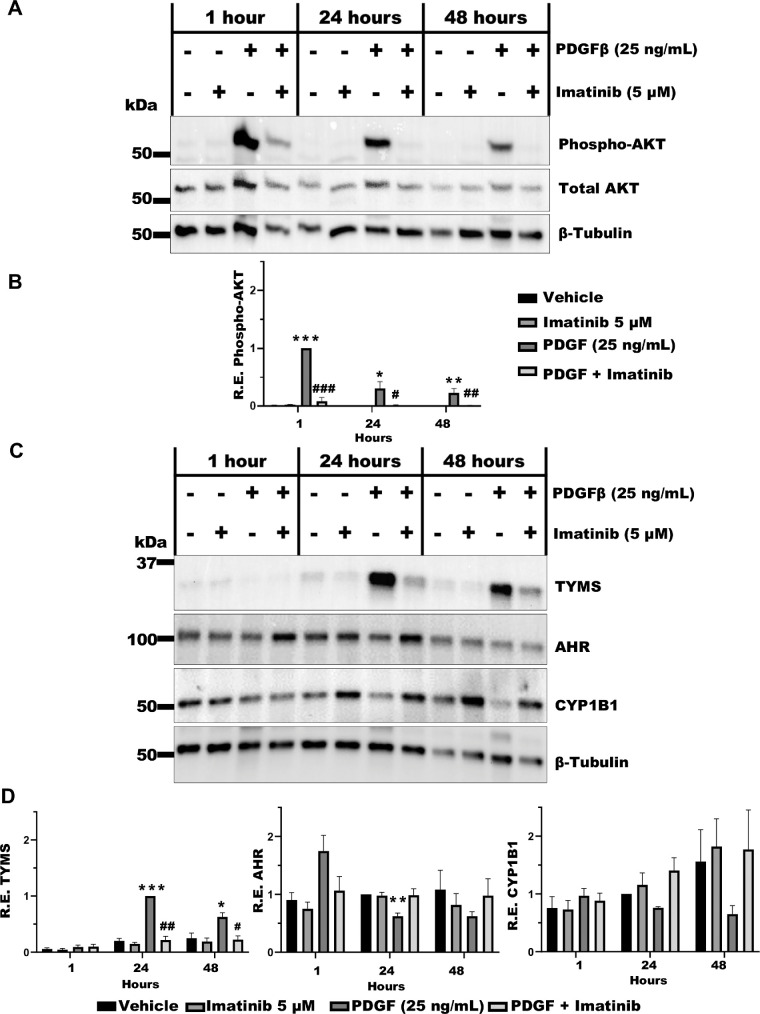
Imatinib, a small molecule tyrosine kinase inhibitor known to block PDGF receptor signaling, was used to further investigate the effects of PDGFβ on TED OFs.27 Here, imatinib (5 µM) was added 1 hour before stimulation with PDGFβ (25 ng/mL) for 1, 24, or 48 hours. Cells were then lysed and analyzed by Western blot for phospho-AKT, total AKT and tubulin expression (Fig. 2A). PDGFβ treatment significantly promoted phosphorylation of AKT at 1, 24, and 48 hours (Fig. 2B). Imatinib treatment was able to block AKT phosphorylation. We also analyzed TYMS, AHR, and the AHR target gene, CYP1B1 expression (Fig. 2C). At the 24- and 48-hour time points, TYMS expression was induced, and this was robustly blocked by imatinib (Fig. 2D). We further observed that AHR levels were significantly reduced at 24 hours post PDGFβ treatment. There was a modest reduction in the level of the AHR target gene, CYP1B1 upon PDGFβ treatment at both 24 and 48 hours, whereas imatinib prevented this reduction (see Fig. 2D).
Figure 2.
PDGFβ signaling is blocked by imatinib. TED OFs were pretreated with imatinib (5 µM) for 1 hour followed by stimulation with PDGFβ (25 ng/mL) for 48 hours. (A) Cells were lysed and analyzed by Western blot for phospho-AKT and total AKT. Phospho-AKT was normalized to total AKT. (B) Quantification of Western blots for phospho-AKT from three TED OF strains. (C) TYMS, AHR, and CYP1B1 were analyzed by Western blot with levels normalized to β-Tubulin. (D) Quantification of Western blots for TYMS, AHR, and CYP1B1. Uncropped blots are presented in the Supplementary Data File. Vehicle (DMSO) treated cells served as control The experiment was repeated in three different TED OF strains with representative results shown. One-way ANOVA with Dunnett's multiple comparisons was used to analyze the data. * P ≤ 0.05, ** P = 0.005, *** P < 0.0008, and PDGF versus treatments: # P ≤ 0.05, ## P ≤ 0.005.
AHR Activation by FICZ or Tapinarof Attenuates PDGFβ Signaling in TED OFs
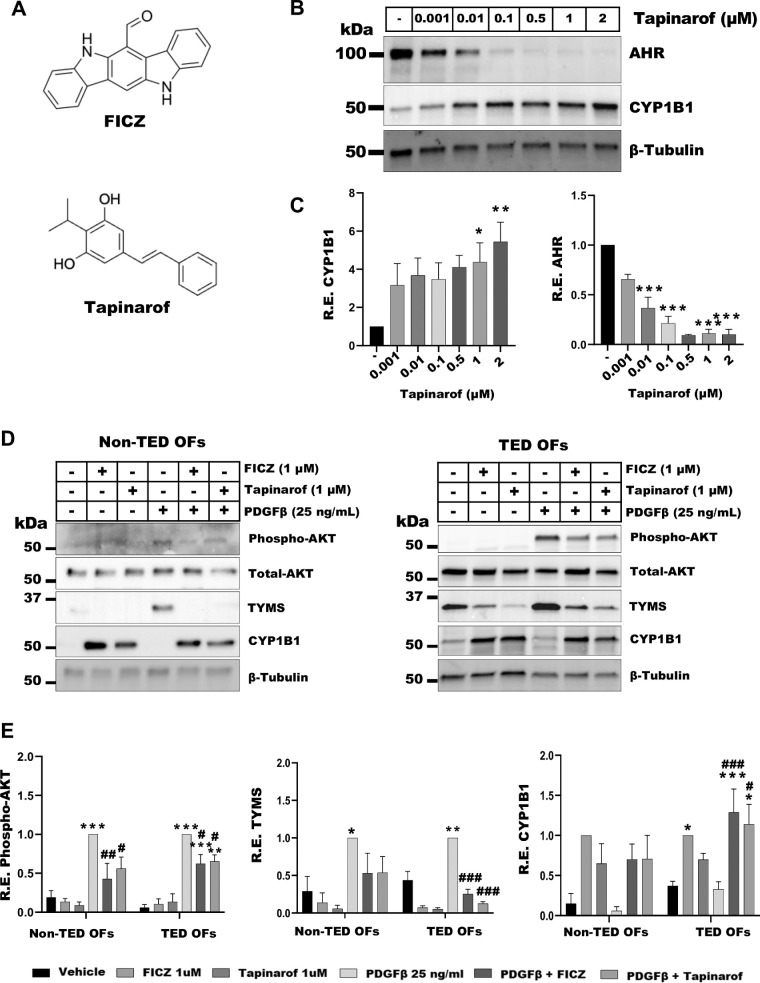
AHR ligands are structurally diverse molecules that can influence AHR activity in ligand-specific ways. For example, FICZ, a short-lived tryptophan derivative is structurally different than tapinarof, a compound that is naturally produced by bacterial symbionts of the nematode28 (Fig. 3A). To understand the role of AHR activation by tapinarof in TED OFs, cells were treated with vehicle (DMSO) or tapinarof (0.001–2 µM), as indicated, for 48 hours. Cells were harvested, lysed, and analyzed by Western blot for AHR, CYP1B1, and tubulin (Fig. 3B). CYP1B1 levels were significantly induced approximately 4-fold by 1 µM and approximately 5-fold by 2 µM tapinarof (Fig. 3C, left panel). Tapinarof treatment also led to a significant reduction of AHR (Fig. 3C, right panel), likely occurring through ligand mediated, proteasome dependent feedback inhibition.29,30
Figure 3.
AHR activation by FICZ or tapinarof attenuates PDGF signaling. (A) The 2D structures of FICZ and tapinarof. (B) TED OFs were treated with vehicle (DMSO) or tapinarof as indicated for 48 hours. Cells were analyzed by Western blot for AHR, CYP1B1, and β-tubulin (loading control). Tapinarof led to a dose dependent reduction in AHR levels and a dose dependent induction of CYP1B1 expression. (C) Quantification of Western blots for AHR and CYP1B1. (D) Non-TED and TED OFs were pretreated for 1 hour with FICZ (1 µM) or tapinarof (1 µM) followed by 48-hour stimulation with PDGFβ (25 ng/mL). Cells were harvested and analyzed by Western blot for phospho-AKT, total AKT, TYMS, CYP1B1, and β-tubulin (loading control). Phospho-AKT was normalized to total AKT, whereas other protein levels were normalized to β-tubulin. FICZ and tapinarof induced CYP1B1 levels. PDGFβ treatment induced phospho-AKT and TYMS, whereas FICZ and tapinarof mitigated these effects. Uncropped blots are presented in the Supplementary Data File. (E) Quantification of Western blots for phospho-AKT, TYMS, and CYP1B1. Vehicle (DMSO) treated cells served as control. The experiment was repeated in three non-TED and three TED OF strains with representative results shown. One-way ANOVA with Dunnett's multiple comparisons was used to analyze the data. * P ≤ 0.05, ** P = 0.005, *** P < 0.0008, and PDGF versus treatments: # P ≤ 0.05, ## P ≤ 0.005, ### P ≤ 0.0005.
To determine if AHR activation could mitigate PDGFβ signaling, non-TED and TED OFs were pretreated for 1 hour with FICZ (1 µM) or tapinarof (1 µM) followed by 48-hour stimulation with PDGFβ (25 ng/mL). Cells were harvested, lysed, and analyzed by Western blot for phospho-AKT, total AKT, TYMS, CYP1B1, and β-tubulin. PDGFβ-induced phospho-AKT levels were reduced with AHR ligand treatment in both TED and non-TED OFs (Figs. 3D, 3E, left panel). FICZ and tapinarof reduced baseline and PDGFβ-induced TYMS expression in TED and non-TED OFs (Figs. 3D, 3E, center panel). In TED OFs, FICZ and tapinarof induced CYP1B1 levels with or without PDGFβ treatments (Fig. 3D). The induction of CYP1B1 was less pronounced in non-TED OFs, suggesting a heightened response to AHR activation in TED.
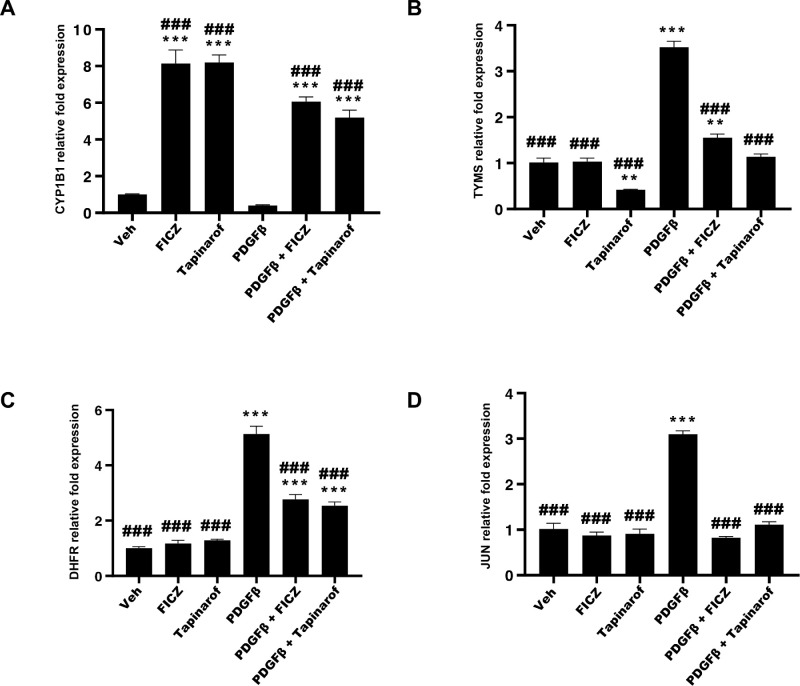
To further investigate the roles of AHR and PDGFβ signaling pathways in TED OF activation, we isolated total RNA from similarly treated TED OFs and analyzed mRNA levels using RT-qPCR. Target genes analyzed were: CYP1B1 (Fig. 4A), TYMS (Fig. 4B), dihydrofolate reductase (DHFR), another enzyme involved in de novo DNA precursor synthesis (Fig. 4C), and the proliferation inducing transcription factor, JUN (Fig. 4D). Basal CYP1B1 levels were reduced by PDGFβ treatment whereas FICZ and tapinarof robustly induced CYP1B1 mRNA levels in both untreated and PDGFβ treated samples. PDGFβ induced TYMS, DHFR, and JUN mRNA levels, which were mitigated by both FICZ and tapinarof treatment (see Fig. 4).
Figure 4.
AHR activation by FICZ and tapinarof attenuates PDGFβ dependent gene expression in TED OFs. TED OFs were pretreated for 1 hour with FICZ (1 µM) or tapinarof (1 µM) followed by 48-hour stimulation with PDGFβ (25 ng/mL). Total RNA was isolated and analyzed by RT-qPCR. (A) CYP1B1, (B) thymidylate synthase (TYMS), (C) dihydrofolate reductase (DHFR), and (D) JUN mRNAs were analyzed. Levels were normalized using the ΔΔCT method to the average CT value of the housekeeping genes, 18S rRNA, TATA binding protein (TBP), and β-actin (ACTB). FICZ and tapinarof increase expression of the AHR dependent gene, CYP1B1. Further, PDGFβ induces TYMS, DHFR, and JUN, whereas FICZ and tapinarof block their expression. There were three replicates per treatment. The experiment was repeated in three different TED OF strains with representative results shown. One-way ANOVA with Dunnett's multiple comparisons of vehicle versus treatments: * P ≤ 0.05, ** P ≤ 0.005, *** P ≤ 0.0001 and PDGF versus treatments: ### P ≤ 0.0005.
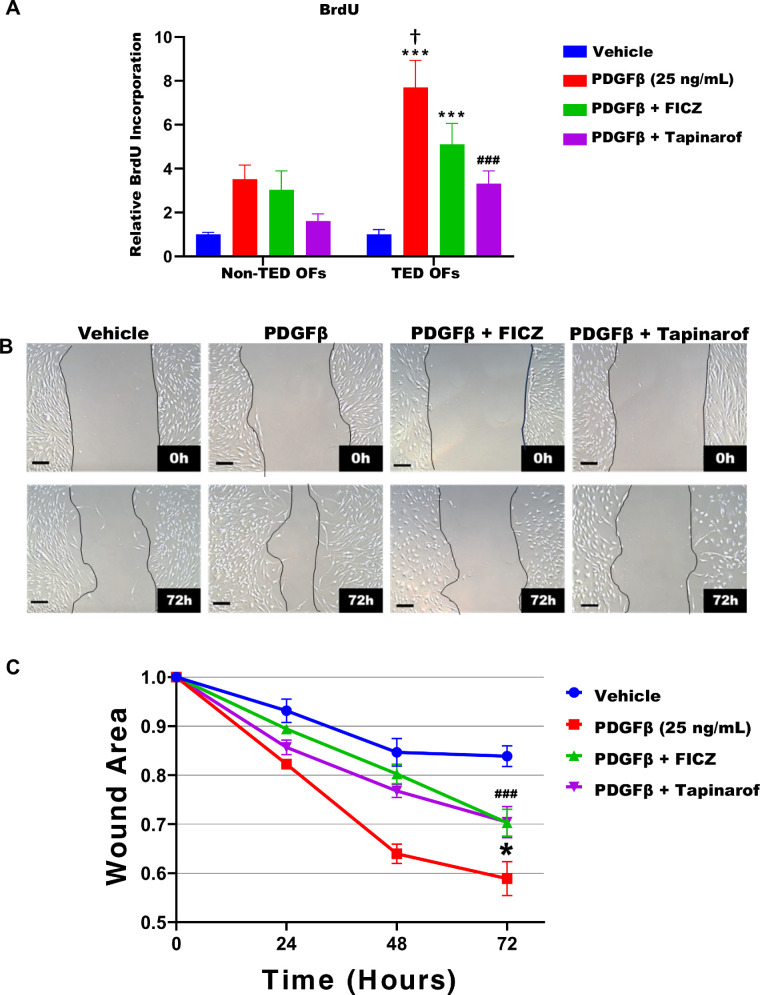
FICZ and Tapinarof Attenuate PDGFβ Induced Cell Proliferation and Migration
OFs proliferation and activation promotes orbital tissue remodeling in TED.31,32 To determine if FICZ or tapinarof block OF proliferation, we used the BrdU incorporation assay (Fig. 5A). As shown in Figure 1, TED OFs proliferate significantly more than non-TED OFs treated with 25 ng/mL PDGFβ. In TED OFs, FICZ and tapinarof reduce proliferation as shown by a reduction of BrdU incorporation by approximately 35% and approximately 55%, respectively. Another method to quantify fibroblast proliferation and activation is through cell migration using a scratch assay.22 Here, TED OFs were grown to confluence and then a uniform area of cells was removed as described in the Methods. Following the scratch, cells were treated with FICZ or tapinarof for 1 hour before stimulation by 25 ng/mL PDGFβ (Fig. 5B). Images of the cells and open area were taken at 0, 24, 48, and 72 hours. Wound areas were quantified using ImageJ software (Fig. 5C). PDGFβ significantly promoted TED OF migration compared to the vehicle group. Both FICZ and tapinarof blunted PDGFβ-induced cell migration. These data suggest that AHR ligands can inhibit TED OF activation and proliferation, potentially offering a therapeutic approach for TED.
Figure 5.
AHR ligands inhibit PDGF-induced cell migration in TED OFs. Non-TED and TED OFs were treated with vehicle or 25 ng/mL PDGFβ with or without 1 uM FICZ or tapinarof for 72 hours. The nucleotide analog BrdU was added to measure DNA synthesis. After culture, cells were fixed and the BrdU label was detected by ELISA as described in the Methods section. (A) PDGFβ treatment increased proliferation, whereas FICZ and tapinarof blocked PDGF induced proliferation in both TED and non-TED OFs. (B) Next, confluent TED OFs were subjected to a wound assay and pretreated with FICZ (1 µM) or tapinarof (1 µM) for 1 hour. PDGFβ (25 ng/mL) was then added to stimulate cell migration. Images were captured at 0, 24, 48, and 72 hours. (C) The area of the scratch was quantified using ImageJ, with the initial wound area normalized to 1.0. Scale bar = 200 µm. Data represent mean ± standard error of the mean from four technical replicates. The experiment was repeated in three different OF strains with representative results shown. One-way ANOVA with Dunnett's multiple comparisons test was used to assess statistical significance vehicle versus treatments: * P ≤ 0.05, ** P ≤ 0.005, *** P ≤ 0.0008 and PDGF versus treatments: ### P ≤ 0.0005, and non-TED versus TED: † P ≤ 0.05.
Discussion
TED is a complex autoimmune condition characterized by excessive growth and remodeling of orbital tissues, leading to potentially severe ocular complications.2,6,20 Our analysis of TED and non-TED OFs reveals important differences in their response to PDGFβ stimulation. Notably, TED orbital fibroblasts demonstrated enhanced proliferative capacity, as evidenced by significantly elevated BrdU incorporation compared to non-TED controls. This heightened responsiveness was particularly evident at lower PDGFβ concentrations (10 ng/mL), where TED fibroblasts showed significant activation while non-TED cells remained largely unresponsive. These findings suggest that TED orbital fibroblasts possess an intrinsically altered state that makes them more susceptible to PDGF-mediated activation, aligning with and extending previous studies showing elevated PDGF signaling in TED.19,27,33–35 The disease-specific nature of these responses provides new insight into why certain individuals develop orbital manifestations while others do not and suggests that targeting PDGF signaling could be effective in patients with TED.
These findings are particularly relevant in the context of current TED treatments. Although Teprotumumab, an IGF1R antibody, is effective, it is associated with significant side effects, such as hearing loss, likely due to the ubiquitous expression of IGF1R in tissues. In contrast, PDGFRs are expressed in a more specific subset of cells including TED OFs but are not detected on immune cells like B and T cells.35,36 This increased cell specificity suggests that targeting PDGF signaling could potentially result in fewer systemic side effects, making it a more attractive therapeutic avenue.
To further analyze PDGF/PDGFR signaling in TED OFs we used the known inhibitor, imatinib. Imatinib not only targets PDGFR but also inhibits other tyrosine kinases including c-KIT and ABL.37,38 Because these targets also participate in cell proliferation, survival, and differentiation, we cannot exclude that some effects of imatinib treatment were PDGFR independent. Additionally, imatinib can cause a variety of adverse effects, including gastrointestinal problems, skin reactions, muscle pain, and fluid retention.39 Therefore, whereas imatinib can be beneficial as a tool to study PDGF/PDGFR signaling in TED, the unwanted side effects as a therapeutic would need to be carefully evaluated.
Our studies provide new insight into PDGF signaling in TED OFs. Here, we show that PDGFβ signals by inducing proliferation, AKT phosphorylation, TYMS expression, and cell migration. Our results also suggest a novel crosstalk between PDGF and AHR signaling. Although AHR has been shown to crosstalk with wnt/β-catenin, EGF, TGFβ, and other cell pathways,20,40,41 we believe these results may be the first to demonstrate such crosstalk with the PDGF pathway. We show for the first time that AHR ligands, specifically FICZ and tapinarof, can mitigate PDGFβ-induced proliferation, activation, and signaling in TED OFs.
The role of AHR in TED is complex and not fully understood.20,21,30 As a ligand-activated transcription factor, AHR regulates the expression of genes involved in metabolism, toxin removal, immune responses, and cellular development. Our laboratory has previously shown that AHR is elevated in TED OFs compared with non-TED OFs,21 and that AHR activation by FICZ can block TGFβ-induced myofibroblast formation.21,22 The current study extends these findings by demonstrating that FICZ and tapinarof can mitigate PDGFβ-induced TED OF activation.
Our findings support AHR as a promising targeted therapeutic approach for TED through several key mechanisms. First, our data demonstrate that AHR signaling has TED-specific effects, with FICZ and tapinarof blocking PDGFβ responses in TED OFs. This is evident in our studies between TED and non-TED OFs, where TED OFs responds more sensitively and robustly to PDGFβ than non-TED OFs. Thus, diseased tissue states with heightened PDGF signaling may be poised for unique AHR signaling patterns that could be therapeutically exploited. Second, unlike systemic antibody treatments, AHR ligands present opportunities for targeted delivery to orbital tissue, potentially minimizing systemic exposure and associated side effects. The localized nature of TED manifestations makes this targeting strategy particularly appealing.
The potential therapeutic implications of these findings are significant especially with tapinarof. In general, AHR ligands vary in stability and receptor affinity, however, tapinarof was recently FDA approved for topical application in treating plaque psoriasis due to its anti-inflammatory and antioxidant properties.23,24,42 Our results suggest that AHR ligands could have similar beneficial effects in TED by modulating inflammation and PDGF signaling. Whereas tapinarof is approved for topical application, no oral or ocular use of the drug is described. Novel delivery systems targeting systemic delivery or orbital tissue specifically could be developed and validated. Some potential approaches that merit investigation include local orbital injection or modified systemic delivery systems, such as oral delivery or subcutaneous injection. Moreover, the possibility of combination therapies targeting both IGF1R (through teprotumumab) and AHR pathways (through tapinarof) presents an intriguing avenue for future investigation.
Although these results are exciting, this study has some limitations. Our experiments used orbital fibroblasts, which, while crucial in TED pathogenesis, do not fully represent the complex in vivo environment of the orbit. Future studies should explore these effects in co-culture systems incorporating other relevant cell types, such as T cells, monocytes, and macrophages, that could be sources for cell-cell activation, PDGF production, and sources of other disease-relevant cytokines.2,19,43,44 Additionally, an animal model would be valuable in recapitulating the organism-level interactions and validating our findings in a more complex biological system.45–47
The broader implications of our findings extend beyond TED. The interplay between PDGF signaling and AHR activation may be relevant to other fibrotic or autoimmune disorders, such as systemic sclerosis or rheumatoid arthritis.48,49 Further, understanding these pathways could provide insights into the puzzling discrepancy between the timing of thyroid and orbital disease manifestations in patients with TED as well as the observation that some patients with Graves disease do not show detectable signs of TED.
In conclusion, our study highlights PDGF signaling as a potential therapeutic target for TED and reveals that AHR ligands can mitigate PDGFβ-induced TED OF activation. These findings not only enhance our understanding of TED pathogenesis but also point to new therapeutic strategies. Future research should focus on elucidating the precise mechanisms linking PDGF and AHR pathways, exploring combination therapies, and validating these findings in more complex experimental models. As we continue to unravel the intricacies of TED, the potential of targeting PDGF signaling and leveraging AHR ligands, such as tapinarof, offers hope for more effective and targeted treatments for this challenging condition.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health Grants EY031398 and an unrestricted grant from Research to Prevent Blindness.
Disclosure: C.C. Patrick, None; E. Roztocil, None; F. Husain, None; S.E. Feldon, None; C.F. Woeller, None
References
- 1. Lehmann GM, Feldon SE, Smith TJ, Phipps RP. Immune mechanisms in thyroid eye disease. Thyroid. 2008; 18: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta V, Hammond CL, Roztocil E, Gonzalez MO, Feldon SE, Woeller CF. Thinking inside the box: current insights into targeting orbital tissue remodeling and inflammation in thyroid eye disease. Surv Ophthalmol. 2022; 67: 858–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diana T, Kahaly GJ. Thyroid stimulating hormone receptor antibodies in thyroid eye disease — methodology and clinical applications. Ophthalmic Plast Reconstr Surg. 2018; 34(4S Suppl 1): S13–S19. [DOI] [PubMed] [Google Scholar]
- 4. McLachlan SM, Rapoport B. Thyrotropin-blocking autoantibodies and thyroid-stimulating autoantibodies: potential mechanisms involved in the pendulum swinging from hypothyroidism to hyperthyroidism or vice versa. Thyroid. 2013; 23: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bahn RS. Current insights into the pathogenesis of Graves' ophthalmopathy. Horm Metab Res. 2015; 47: 773–778. [DOI] [PubMed] [Google Scholar]
- 6. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010; 362: 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Virakul S, van Steensel L, Dalm VA, Paridaens D, van Hagen PM, Dik WA. Platelet-derived growth factor: a key factor in the pathogenesis of graves' ophthalmopathy and potential target for treatment. Eur Thyroid J. 2014; 3: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammond CL, Roztocil E, Gonzalez MO, Feldon SE, Woeller CF. MicroRNA-130a is elevated in thyroid eye disease and increases lipid accumulation in fibroblasts through the suppression of AMPK. Invest Ophthalmol Vis Sci. 2021; 62: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weetman AP, Cohen S, Gatter KC, Fells P, Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves' ophthalmopathy. Clin Exp Immunol. 1989; 75: 222–227. [PMC free article] [PubMed] [Google Scholar]
- 10. Pappa A, Calder V, Ajjan R, et al.. Analysis of extraocular muscle-infiltrating T cells in thyroid-associated ophthalmopathy (TAO). Clin Exp Immunol. 1997; 109: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010; 362: 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Draman MS, Zhang L, Dayan C, Ludgate M. Orbital signaling in Graves’ orbitopathy. Front Endocrinol (Lausanne). 2021; 12: 739994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith TJ, Janssen JAMJL. Building the case for insulin-like growth factor receptor-I involvement in thyroid-associated ophthalmopathy. Front Endocrinol (Lausanne). 2017; 7: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woeller CF, Roztocil E, Hammond C, Feldon SE. TSHR signaling stimulates proliferation through PI3K/Akt and induction of miR-146a and miR-155 in thyroid eye disease orbital fibroblasts. Invest Ophthalmol Vis Sci. 2019; 60: 4336–4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Douglas RS, Wang Y, Dailey RA, et al.. Teprotumumab in clinical practice: recommendations and considerations from the OPTIC trial investigators. J Neuroophthalmol. 2021; 41: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah SA, Lu T, Yu M, Hiniker S, Dosiou C, Kossler AL. Comparison of treatment cost and quality-of-life impact of thyroid eye disease therapies. Invest Ophthalmol Vis Sci. 2022; 63: 4002. [Google Scholar]
- 17. Smith TJ, Kahaly GJ, Ezra DG, et al.. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017; 376: 1748–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Douglas RS, Kahaly GJ, Patel A, et al.. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020; 382: 341–352. [DOI] [PubMed] [Google Scholar]
- 19. van Steensel L, Paridaens D, van Meurs M, et al.. Orbit-infiltrating mast cells, monocytes, and macrophages produce PDGF isoforms that orchestrate orbital fibroblast activation in Graves' ophthalmopathy. J Clin Endocrinol Metab. 2012; 97: E400–E408. [DOI] [PubMed] [Google Scholar]
- 20. Hammond CL, Roztocil E, Gupta V, Feldon SE, Woeller CF. More than meets the eye: the aryl hydrocarbon receptor is an environmental sensor, physiological regulator and a therapeutic target in ocular disease. Front Toxicol. 2022; 4: 791082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woeller CF, Roztocil E, Hammond CL, Feldon SE, Phipps RP. The aryl hydrocarbon receptor and its ligands inhibit myofibroblast formation and activation: implications for thyroid eye disease. Am J Pathol. 2016; 186: 3189–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammond CL, Roztocil E, Phipps RP, Feldon SE, Woeller CF. Proton pump inhibitors attenuate myofibroblast formation associated with thyroid eye disease through the aryl hydrocarbon receptor. PLoS One. 2019; 14: e0222779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lebwohl MG, Stein Gold L, Strober B, et al.. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021; 385: 2219–2229. [DOI] [PubMed] [Google Scholar]
- 24. Smith SH, Jayawickreme C, Rickard DJ, et al.. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017; 137: 2110–2119. [DOI] [PubMed] [Google Scholar]
- 25. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 26. Woeller CF, Anderson DD, Szebenyi DM, Stover PJ. Evidence for small ubiquitin-like modifier-dependent nuclear import of the thymidylate biosynthesis pathway. J Biol Chem. 2007; 282: 17623–17631. [DOI] [PubMed] [Google Scholar]
- 27. van Steensel L, Paridaens D, Schrijver B, et al.. Imatinib mesylate and AMN107 inhibit PDGF-signaling in orbital fibroblasts: a potential treatment for Graves' ophthalmopathy. Invest Ophthalmol Vis Sci. 2009; 50: 3091–3098. [DOI] [PubMed] [Google Scholar]
- 28. Joyce SA, Brachmann AO, Glazer I, et al.. Bacterial biosynthesis of a multipotent stilbene. Angew Chem Int Ed Engl. 2008; 47: 1942–1945. [DOI] [PubMed] [Google Scholar]
- 29. Ma Q, Baldwin KT. 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced degradation of aryl hydrocarbon receptor (AhR) by the ubiquitin-proteasome pathway. Role of the transcription activation and DNA binding of AhR. J Biol Chem. 2000; 275: 8432–8438. [DOI] [PubMed] [Google Scholar]
- 30. Roztocil E, Hammond CL, Gonzalez MO, Feldon SE, Woeller CF. The aryl hydrocarbon receptor pathway controls matrix metalloproteinase-1 and collagen levels in human orbital fibroblasts. Sci Rep. 2020; 10: 8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brandau S, Bruderek K, Hestermann K, et al.. Orbital fibroblasts from graves' orbitopathy patients share functional and immunophenotypic properties with mesenchymal stem/stromal cells. Invest Ophthalmol Vis Sci. 2015; 56: 6549–6557. [DOI] [PubMed] [Google Scholar]
- 32. Smith TJ, Tsai CC, Shih MJ, et al.. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thyroid. 2008; 18: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JY, Yun M, Paik JS, Lee SB, Yang SW. PDGF-BB enhances the proliferation of cells in human orbital fibroblasts by suppressing PDCD4 expression via up-regulation of microRNA-21. Invest Ophthalmol Vis Sci. 2016; 57: 908–913. [DOI] [PubMed] [Google Scholar]
- 34. van Steensel L, Hooijkaas H, Paridaens D, et al.. PDGF enhances orbital fibroblast responses to TSHR stimulating autoantibodies in Graves’ ophthalmopathy patients. J Clin Endocrinol Metab. 2012; 97: E944–E953. [DOI] [PubMed] [Google Scholar]
- 35. van Steensel L, Dik WA. The orbital fibroblast: a key player and target for therapy in Graves’. Orbit. 2010; 29: 202–206. [DOI] [PubMed] [Google Scholar]
- 36. Rajkumar VS, Shiwen X, Bostrom M, et al.. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol. 2006; 169: 2254–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waller CF. Imatinib mesylate. Recent Results Cancer Res. 2018; 212: 1–27. [DOI] [PubMed] [Google Scholar]
- 38. Waller CF. Imatinib mesylate. Recent Results Cancer Res. 2014; 201: 1–25. [DOI] [PubMed] [Google Scholar]
- 39. Othman T, Koller P, Tsai NC, et al.. Toxicities associated with tyrosine kinase inhibitor maintenance following allogeneic hematopoietic cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia. Am J Hematol. 2024; 99: 1680–1690. [DOI] [PubMed] [Google Scholar]
- 40. Bock KW. Aryl hydrocarbon receptor (AHR): from selected human target genes and crosstalk with transcription factors to multiple AHR functions. Biochem Pharmacol. 2019; 168: 65–70. [DOI] [PubMed] [Google Scholar]
- 41. Mathew LK, Sengupta SS, Ladu J, Andreasen EA, Tanguay RL. Crosstalk between AHR and Wnt signaling through R-Spondin1 impairs tissue regeneration in zebrafish. FASEB J. 2008; 22: 3087–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Silverberg JI, Boguniewicz M, Quintana FJ, et al.. Tapinarof validates the aryl hydrocarbon receptor as a therapeutic target: a clinical review. J Allergy Clin Immunol. 2024; 154: 1–10. [DOI] [PubMed] [Google Scholar]
- 43. Feldon SE, O'Loughlin CW, Ray DM, Landskroner-Eiger S, Seweryniak KE, Phipps RP. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol. 2006; 169: 1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang IH, Rose GE, Ezra DG, Bailly M. Macrophages promote a profibrotic phenotype in orbital fibroblasts through increased hyaluronic acid production and cell contractility. Sci Rep. 2019; 9: 9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Banga JP, Moshkelgosha S, Berchner-Pfannschmidt U, Eckstein A. Modeling Graves’ orbitopathy in experimental Graves’ disease. Horm Metab Res. 2015; 47: 797–803. [DOI] [PubMed] [Google Scholar]
- 46. Schluter A, Flogel U, Diaz-Cano S, et al.. Graves’ orbitopathy occurs sex-independently in an autoimmune hyperthyroid mouse model. Sci Rep. 2018; 8: 13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gulbins A, Horstmann M, Daser A, et al.. Linsitinib, an IGF-1R inhibitor, attenuates disease development and progression in a model of thyroid eye disease. Front Endocrinol (Lausanne). 2023; 14: 1211473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang J, Liu C, Wang T, et al.. Single-cell communication patterns and their intracellular information flow in synovial fibroblastic osteoarthritis and rheumatoid arthritis. Immunol Lett. 2023; 263: 1–13. [DOI] [PubMed] [Google Scholar]
- 49. Laczko R, Csiszar K. Lysyl oxidase (LOX): functional contributions to signaling pathways. Biomolecules. 2020; 10: 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.