Abstract
Objectives: To quantify levels of two inflammation-related indexes, namely neutrophil-lymphocyte ratio (NLR) and systemic immune-inflammation index (SII) in systemic scleroderma patients and determine the association with clinical manifestations and features of heart ultrasound. Methods: The study group consisted of 34 patients with diagnosis of systemic scleroderma which were admitted to the hospital during 2015-2019. Patient data included the presence and type of clinical manifestations of systemic scleroderma, chest imaging to screen for lung disease, heart ultrasound reports and the laboratory investigations needed to quantify inflammatory indexes. We analysed the levels of inflammatory indexes and compared results based on the prevalence of systemic manifestations. Results: Higher serum levels of NLR and SII are associated with the presence of joint, lung and pericardial involvement. Statistical significance was observed only for NLR levels with regard to the presence of articular involvement and ILD. Low ejection fraction was also associated with higher levels of both inflammatory indexes, without statistical significance. Conclusion: Inflammatory indexes are cost-effective markers that reflect active disease manifestations of systemic scleroderma and can thus be a useful tool to include in the regular follow-up of patients in order to better inform organ-specific assessments.
Keywords: Systemic scleroderma , inflammation , neutrophils , biomarkers
Introduction
Systemic scleroderma (SSc) is a rare autoimmune condition without a well-defined etiology which commonly features skin thickening which can associate internal organ involvement through inflammatory lesions and fibrosis.
It is more common in women and develops usually in the age interval from 30 to 50 years.
It is classified in diffuse and limited forms depending on the extent of skin involvement.
One common pathologic process which underpins SSc development and organ-specific manifestations is the vascular injury.
Alterations in the function of vascular endothelium and dysregulated angiogenesis leads to microcirculatory disorders in the form of capillary loss and arterial stenosis.
The accumulation of free radicals and products of oxidative stress triggers an inflammatory reaction, infiltration of circulating immune cells in the perivascular ares that will facilitate the activation of interstitial fibroblasts and deposition of collagen fibers in the extracellular matrix [1, 2, 3].
Clinical manifestations of SSc typically include thickening of the skin and Raynaud’s phenomen which can associate various internal organ involvement such as the lungs, gastrointestinal tract, heart, kidneys or musculoskeletal system [4].
Diffuse cutaneous SSc is associated with a poorer prognosis, faster organ involvement progression and a lower 10-year survival rate compared to the limited cutaneous form [4].
Cardiac disease in SSc can be primary or secondary to lung or kidney abnormalities and it is considered one of the main causes of mortality.
Most heart structures can be affected by SSc and this can lead to various heart abnormalities such as coronary heart disease, left ventricle dysfunction (systolic or dyastolic), conduction abnormalities or myocardial fibrosis [5].
Risk factors for cardiac disease in SSc include male sex, onset at older age, rapid progression of skin disease and diagnosis of diffuse cutaneous form [6].
One of the main pulmonary manifestations of SSc is the interstitial lung disease (ILD) with a pattern called nonspecific interstitial pneumonia (NSIP) that features ground glass opacities.
Pulmonary arterial hypertension (PAH) can be a direct result of pre-capillary pulmonary arterioles remodeling or it can arise secondary to ILD of left heart dysfunction [5].
Assessment of disease severity is based on the extent of specific organ involvement which can be underdiagnosed in the early stages when these can be clinically silent.
Novel inflammatory-related serum markers including the neutrophil-to-lymphocyte ratio (NLR) and the systemic immune-inflammation index (SII) have been proven to reflect inflammatory status and prognosis in various clinical setting such as cancer, coronary artery disease and inflammatory diseases [7].
Higher levels of NLR and SII result from an imbalance of the peripheral inflammatory cells.
The formula for calculating SII is platelets×neutrophils÷lymphocytes.
More recent studies in patients with rheumatoid arthritis [8, 9], systemic scleroderma [7] or systemic lupus erythematosus [10, 11] have shown the potential use of these inflammatory indexes for monitoring disease activity.
The aim of our study was to quantify levels of NLR and SII in patients diagnosed with systemic scleroderma and determine associations with clinical manifestations and heart ultrasound features.
Materials and Methods
We conducted a retrospective study on data retrieved from patients diagnosed with SSc which were admitted to the Rheumatology Clinic of the Emergency County Hospital Craiova between 2015-2019. Patients did not have a history of SSc diagnosis or immunosupresive therapy prior to admission.
The study population included 34 patients with SSc either diffuse or limited cutaneous form.
Clinical data collection included demographics, clinical form, type of systemic manifestation which was present at the moment of admission.
During the selection process only patients with extensive cardiopulmonary assessment were included.
This required that each patient had performed electrocardiogram, heart ultrasound, lung computed tomography (CT) and pulmonary function tests.
Patients with preexisting heart or lung disease were excluded.
With regard to therapy, no patient was taken corticotherapy or other immunosupresive drugs such as metotrexate, micofenolat mofetil or cyclophosphamide.
Prior to inclusion in the study, an informed consent form was signed by all patients.
A study approval was granted by the institutional Ethics Committee with registered nr. 206/20.09.2023.
Data from laboratory investigations included complete blood count based on which further calculations were performed in order to determine the levels of NLR and SII.
The obtained data were recorded in Microsoft Excel files, anonymously to preserve the confidentiality of the individuals included in the study, then being processed statistically, in order to analyze the relationships between the clinical, laboratory and imaging features.
Categorical data is summarised as percent prevalence.
Analysis for continuous data included mean and standard deviation for variables which are normally distributed or median and interquantile range for nonparametric variables.
Significant differences between continuous variables were analyzed by performing an Independent Sample t Test or Mann-Whitney test. R version 4.3.2 was used for statistical analysis and data visualization.
Results
The study population consists of 34 patients with a diagnosis of SSc, with a mean age of 54 years, and a ratio of females to males of 32:2.
Almost all patients (94%) displayed clinical features suggestive of skin involvement and Raynaud’s phenomenon.
Other notable clinical manifestations with high prevalence that we’re observed in the study group included articular (arthralgia or arthritis-82%), digestive (47%) and pulmonary involvement (ILD-32%, PAH-68%).
About one third of patients registered a low ejection fraction.
Characteristics of patient population are presented in Table 1.
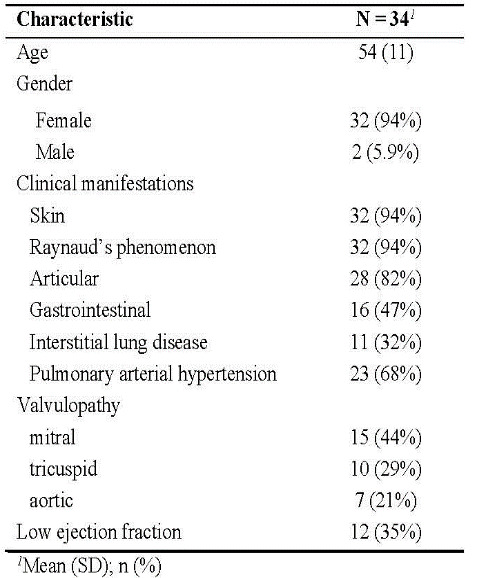
Table 1. Study population characteristics
Heart ultrasound parameters which were recorded included measurements of the ascending aorta, aortic ring, interventricular septum, both atria and ventricles.
The estimated pulmonary arterial systolic pressure ranged from 20 to 35mmHg, with a mean value of 28mmHg.
A summary of heart ultrasound assessments with mean values and range of measurements registered is displayed in Table 2.
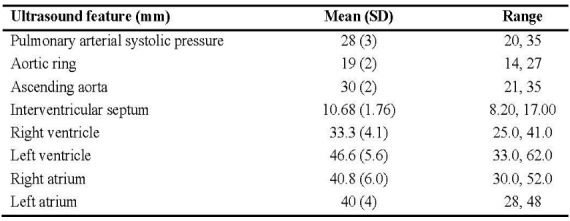
Table 2. Summary of ultrasound measurements
The analysis of inflammatory indexes NLR and SII with regard to the presence of clinical manifestations revealed some association.
In general, higher levels of both inflammatory indexes were recorded in the presence of all clinical features analyzed, with the exception of SII levels in the presence of GI tract involvement.
Importantly, statistically significant differences were observed for NLR levels in patients with articular involvement (3.13) and ILD (3.68), compared with patients without these clinical manifestations (1.9 and 2.55, respectively) (Figure 1).
Figure 3.
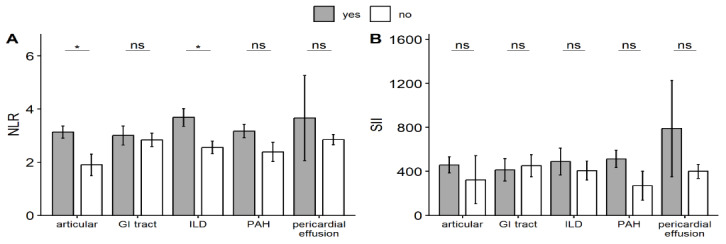
Serum levels of inflammatory indexes in relation to clinical manifestations; NLR-neutrophil-lymphocyte ratio, SII-systemic immune-inflammation index, GI-gastrointestinal, ILD-interstitial lung disease, PAH-pulmonary arterial hypertension
Among all clinical features analyzed, superior levels for both NLR and SII were registered in patients with pulmonary involvement.
The highest value for NLR was observed in association with ILD, while the highest value for SII was observed in patients with PAH.
In addition, both NLR and SII registered higher values in association with the presence of low ejection fraction and pericardial effusion, although without statistically significant differences (Table 3).
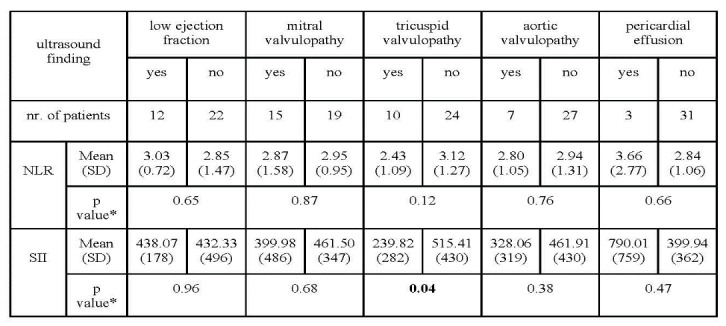
Table 3. Inflammatory indexes values in relation to pathologic heart ultrasound findings
Interestingly, lower values for NLR and SII were obtained among patients with mitral, tricuspid or aortic valvulopathy.
This difference was statistically significant with regard to SII in patients with tricuspid valvulopathy.
Furter analysis compared measurements of heart ultrasound parameters with regard to the presence of cardiac abnormalities or SSc disease manifestations.
Among these comparisons and excluding the self-evident relation of PAH and increased volume of right ventricle, only cases with low ejection fraction recorded statistically significant differences with higher values of the mean estimated pulmonary arterial systolic pressure and interventricular septum compared to patients with normal ejection fraction (Table 4).
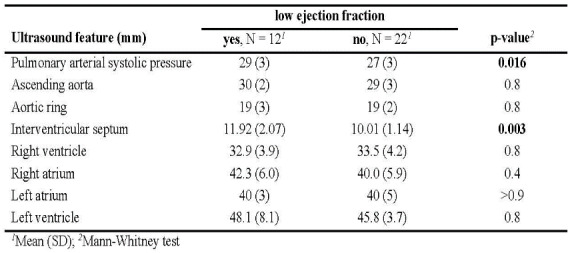
Table 4. Ultrasound measurements with regard to the presence of low ejection fraction
Numerically higher mean values were observed also for measurements of ascending aorta, right atrium and left ventricle in patients with low ejection fraction, while patients with aortic and mitral valve insufficiency registered numerically higher values of estimated pulmonary arterial systolic pressure.
Discussion
Assessment of disease severity in SSc requires a detailed examination of components of both disease activity and damage. In the early phases of the disease, the main pathologic process driving clinical symptoms is the disease activity (i.e., inflammation).
In time, irreversible organ damage develops and constitutes the predominant disease feature associated with morbidity.
It is essential to evaluate the disease severity and identify prognostic factors in the first years of onset since it is known that the majority of SSc organ involvement will occur in the first two to five years [12, 13].
A well-established disease severity scale is the Medsger scale which includes nine organ systems.
Each organ domain has individual parameters that can be assessed and are then scored on a 0-4 scale [14].
It is observed through prospective studies that increased morbidity and mortality are associated with a higher disease severity at baseline.
It is thus essential to identify the patients at risk to develop severe organ manifestations and life-threatening complications which should benefit from close monitoring and more aggressive immunosuppressive therapy.
Prognostic factors associated with a poor prognosis and worse survival are diffuse cutaneous SSc, cardiac and lung involvement [13, 15, 16].
Although SSc survival has improved over time, the main causes of death are still related to heart, lung and kidneys complications [17].
In the study based on the EULAR Scleroderma Trials and Research (EUSTAR) database, which included 5860 patients, 55% of deaths (N=234) were attributed to SSc.
Of these, 35% were related to ILD, 26% to PAH and 26% to heart involvement; the main cardiac causes were heart failure and arrythmia [18].
Serum levels of C reactive protein and erythrocyte sedimentation rate can signify disease activity and are increased especially in the early course of the disease [19, 20].
Apart from these routinely used inflammatory markers, other laboratory test derived from the complete blood count report can be utilized to assess disease activity and predict severity in SSc.
Some of these tests include the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), neutrophil-to-monocyte ratio (NMR) and the systemic immune-inflammation index (SII).
The NLR and SII have been shown to be able to predict outcomes in different clinical settings such as coronary artery disease and cancer.
Also, it has been previously been proven that NLR may reflect disease activity in systemic autoimmune diseases such as RA [8], SLE [10, 11], ankylosing spondylitis [21] and SSc [7].
In a meta-analysis performed by Wang et al., NLR was significantly higher SLE patients with active disease compared to inactive disease and in those with lupus nephritis as opposed to those without lupus nephritis [11].
Studies showed positive correlations of NLR with DAS28 score in RA patients [21] and SSDAI in Sjögren’s syndrome patients [22].
A recent meta-analysis by Mangoni et al. which included 16 studies offers insight into the clinical associations of SII and several autoinflammatory and autoimmune diseases [23].
Neutrophiles participate in both initiation and progression of the inflammatory process through the release of proinflammatory cytokines.
High neutrophile activity coupled with increased lymphocyte apoptosis are signs of uncontrolled systemic inflammation and explains why the NLR reflects a pro-inflammatory status.
In addition to this, it is known that neutrophils induce endothelial cell apoptosis which is considered one of the initial pathogenic events in SSc [24].
Studies on SSc patients focused mainly on NLR, NMR and PLR, which revealed promising results regarding the potential use as activity and prognosis markers [7, 25, 26, 27, 28, 29, 30, 31].
Literature review revealed that currently there is a research gap regarding the use of SII in assessment of SSc patients.
We analyzed both NLR and SII and compared results based on the presence of clinical manifestations.
Our study showed that for both inflammatory indexes, the presence of joint, lung and pericardial involvement was associated with higher values.
Statistical significance was observed only for NLR levels with regard to the presence of articular involvement and ILD.
Studies that include NLR in their analysis have consistently shown that compared to healthy controls, SSc patients have significantly higher NLR values [7, 25, 26, 27, 28, 29, 30, 31].
Regarding the relation with certain clinical manifestations, studies have shown that higher values of NLR are associated with interstitial lung disease [7, 27, 28, 29, 30, 31], cardiac disease [7, 31], digital ulcers [26, 28, 31] and muscle weakness [25].
Moreover, in studies by Jung et al and Atilla et al, cut-off values of NLR have been calculated for the prediction of ILD [29, 30].
Importantly, the level of NLR could be correlated in some studies with established disease activity scores such as Medsger severity score and EUSTAR activity index [7, 26].
This study included patients with a comprehensive cardiovascular and pulmonary assessment.
Inflammatory tests derived from complete blood count included both NLR and SII, the latter being a novel inflammatory marker studied in SSc.
One important aspect of the design of this study is the inclusion of patients at first admission to the rheumatology department, without prior therapies for SSc limiting the influence of specific medications on inflammatory marker levels.
One important limitation of this study consists in the lack of a control group, coupled with a small study population.
Also, due to the retrospective design of the study, data collection was unable to retrieve activity scores or Rodnan skin score for all patients and thus correlations could not be performed between these scores and inflammatory markers.
Future research agenda could include the study of how inflammatory indexes correlate with disease progression in time, ability to prediction of future organ involvement and the sensitivity to therapy response.
Conclusion
Increased levels of NLR and SII are observed in the presence of specific clinical manifestations related to systemic scleroderma.
Multiple cardiopulmonary manifestations are associated with higher levels of inflammatory indexes which in turn reflect an active disease.
The development of novel and accessible prognostic markers for disease activity and severity is a crucial step for improving the management of patients with systemic scleroderma.
Conflict of interests
The authors have no conflict of interest to declare.
References
- 1.Ciechomska M, van Laar, O’Reilly S. Current frontiers in systemic sclerosis pathogenesis. Exp Dermatol. 2015;24(6):401–406. doi: 10.1111/exd.12673. [DOI] [PubMed] [Google Scholar]
- 2.Walker UA, Tyndall A, Czirják L, Denton C, Farge-Bancel D, Kowal-Bielecka O, Müller-Ladner U, Bocelli-Tyndall C, Matucci-Cerinic M. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis. 2007;66(6):754–763. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano Y. The Pathogenesis of Systemic Sclerosis: An Understanding Based on a Common Pathologic Cascade across Multiple Organs and Additional Organ-Specific Pathologies. J Clin Med. 2020;19(9):2687–2687. doi: 10.3390/jcm9092687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobolewski P, Maślińska M, Wieczorek M, Łagun Z, Malewska A, Roszkiewicz M, Nitskovich R, Szymańska E, Walecka I. Systemic sclerosis-multidisciplinary disease: clinical features and treatment. Reumatologia. 2019;57(4):221–233. doi: 10.5114/reum.2019.87619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glynn P, Hale S, Hussain T, Freed BH. Cardiovascular Imaging for Systemic Sclerosis Monitoring and Management. Front Cardiovasc Med. 2022;9:846213–846213. doi: 10.3389/fcvm.2022.846213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai CS, Lee DC, Shah SJ. Systemic sclerosis and the heart: current diagnosis and management. Curr Opin Rheumatol. 2011;23(6):545–554. doi: 10.1097/BOR.0b013e32834b8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nejatifar F, Mirbolouk N, Masooleh IS, Kazemnejad E, Ghavidel-Parsa B, Ghanbari AM, Zayeni H. Association between neutrophil/lymphocyte ratio and disease severity in scleroderma patients. Heliyon. 2023;9(10):e20576–e20576. doi: 10.1016/j.heliyon.2023.e20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Li J, Sun L, Wang T, Liang W. The association between neutrophil-to-lymphocyte ratio and disease activity in rheumatoid arthritis. Inflammopharmacology. 2023;31(5):2237–2244. doi: 10.1007/s10787-023-01273-2. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Wang J, Li YY, Li KP, Zhang Q. The association between systemic immune-inflammation index and rheumatoid arthritis: evidence from NHANES 1999-2018. Arthritis Res Ther. 2023;25(1):34–34. doi: 10.1186/s13075-023-03018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozdemir A, Baran E, Kutu M, Celik S, Yılmaz M. Could systemic immune inflammation index be a new parameter for diagnosis and disease activity assessment in systemic lupus erythematosus? Int Urol Nephrol. 2023;55(1):211–216. doi: 10.1007/s11255-022-03320-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Wang C, Jia X, Yang M, Yu J. Relationship between Neutrophil-to-Lymphocyte Ratio and Systemic Lupus Erythematosus: A Meta-analysis. Clinics (Sao Paulo) 2020;75:e1450–e1450. doi: 10.6061/clinics/2020/e1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roofeh D, Khanna D. Management of Systemic Sclerosis: The First Five Years. Curr Opin Rheumatol. 2020;32(3):228–237. doi: 10.1097/BOR.0000000000000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geirsson AJ, Wollheim FA, Akesson A. Disease severity of 100 patients with systemic sclerosis over a period of 14 years: using a modified Medsger scale. Ann Rheum Dis. 2001;60(12):1117–1122. doi: 10.1136/ard.60.12.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medsger TA, Bombardieri S, Czirjak L, Scorza R, Della Rossa, Bencivelli W. Assessment of disease severity and prognosis. Clin Exp Rheumatol. 2003;21(3 Suppl 29):S42–6. [PubMed] [Google Scholar]
- 15.De Almeida, Porel T, Mounié M, Alric L, Astudillo L, Huart A, Lairez O, Michaud M, Prévot G, Ribes D, Sailler L, Gaches F, Adoue D, Pugnet G. Sine scleroderma, limited cutaneous, and diffused cutaneous systemic sclerosis survival and predictors of mortality. Arthritis Res Ther. 2021;23(1):295–295. doi: 10.1186/s13075-021-02672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Dhaher FF, Pope JE, Ouimet JM. Determinants of morbidity and mortality of systemic sclerosis in Canada. Semin Arthritis Rheum. 2010;39(4):269–277. doi: 10.1016/j.semarthrit.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Rubio-Rivas M, Simeón-Aznar CP, Velasco C, Marí-Alfonso B, Espinosa G, Corbella X, Colunga-Argüelles D, Egurbide-Arberas MV, Ortego-Centeno N, Vargas-Hitos JA, Freire M, Ríos-Blanco JJ, Trapiella-Martínez L, Rodríguez-Carballeira M, Fonollosa-Pla V;, Autoimmune Diseases. Changes in the pattern of death of 987 patients with systemic sclerosis from 1990 to 2009 from the nationwide Spanish Scleroderma Registry (RESCLE) Clin Exp Rheumatol. 2017;106(4):40–47. [PubMed] [Google Scholar]
- 18.Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, Bancel DF, Allanore Y, Müller-Ladner U, Distler O, Iannone F, Pellerito R, Pileckyte M, Miniati I, Ananieva L, Gurman AB, Damjanov N, Mueller A, Valentini G, Riemekasten G, Tikly M, Hummers L, Henriques MJ, Caramaschi P, Scheja A, Rozman B, Ton E, Kumánovics G, Coleiro B, Feierl E, Szucs G, Von Mühlen, Riccieri V, Novak S, Chizzolini C, Kotulska A, Denton C, Coelho PC, Kötter I, Simsek I, de la, Hachulla E, Seibold JR, Rednic S, Stork J, Morovic-Vergles J, Walker UA. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69(10):1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 19.Muangchan C, Harding S, Khimdas S, Bonner A;, Pope J. Association of C-reactive protein with high disease activity in systemic sclerosis: results from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken) 2012;64(9):1405–1414. doi: 10.1002/acr.21716. [DOI] [PubMed] [Google Scholar]
- 20.Ross L, Stevens W, Rabusa C, Wilson M, Ferdowsi N, Walker J, Sahhar J, Ngian GS, Zochling J, Roddy J, Tymms K, Major G, Strickland G, Proudman SM, Nikpour M. The role of inflammatory markers in assessment of disease activity in systemic sclerosis. Clin Exp Rheumatol. 2018;113(4):126–134. [PubMed] [Google Scholar]
- 21.Mercan R, Bitik B, Tufan A, Bozbulut UB, Atas N, Ozturk MA, Haznedaroglu S, Goker B. The Association Between Neutrophil/Lymphocyte Ratio and Disease Activity in Rheumatoid Arthritis and Ankylosing Spondylitis. J Clin Lab Anal. 2016;30(5):597–601. doi: 10.1002/jcla.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu ZD, Sun Y, Guo J, Huang YL, Qin BD, Gao Q, Qin Q, Deng AM, Zhong RQ. Red blood cell distribution width and neutrophil/lymphocyte ratio are positively correlated with disease activity in primary Sjögren's syndrome. Clin Biochem. 2014;47(18):287–290. doi: 10.1016/j.clinbiochem.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Mangoni AA, Zinellu A. The diagnostic role of the systemic inflammation index in patients with immunological diseases: a systematic review and meta-analysis. Clin Exp Med. 2024;24(1):27–27. doi: 10.1007/s10238-024-01294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes TC, Spiller DG, Anderson ME, Edwards SW, Moots RJ. Endothelial activation and apoptosis mediated by neutrophil-dependent interleukin 6 trans-signalling: a novel target for systemic sclerosis. Ann Rheum Dis. 2011;70(2):366–372. doi: 10.1136/ard.2010.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakr BR, Rabea RE, ElHamid SM. Value of hematological parameters as biomarkers of disease manifestations and severity in systemic sclerosis. Egypt Rheumatol. 2021;43(2):159–165. [Google Scholar]
- 26.Yayla ME, İlgen U, Okatan İE, UsluYurteri E, Torgutalp M, Keleşoğlu Dinçer, Aydemir Gülöksüz, Sezer S, Turgay TM, Kınıklı G, Ateş A. Association of simple hematological parameters with disease manifestations, activity, and severity in patients with systemic sclerosis. Clin Rheumatol. 2020;39(1):77–83. doi: 10.1007/s10067-019-04685-0. [DOI] [PubMed] [Google Scholar]
- 27.Tezcan D, Turan Ç, Yılmaz S, Sivrikaya A, Gülcemal S, Limon M, Ecer B. What do simple hematological parameters tell us in patients with systemic sclerosis. Acta Dermatovenerol Alp Pannonica Adriat. 2020;29(3):101–107. [PubMed] [Google Scholar]
- 28.Kim A, Kim Y, Kim GT, Ahn E, So MW, Sohn DH, Lee SG. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio as potential makers for digital ulcers and interstitial lung disease in patients with systemic sclerosis: cross-sectional analysis of data from a prospective cohort study. Rheumatol Int. 2020;40(7):1071–1079. doi: 10.1007/s00296-020-04604-6. [DOI] [PubMed] [Google Scholar]
- 29.Jung JH, Lee YM, Lee EG, Yoo WH, Lee WS. Neutrophil-to-lymphocyte Ratio in Diagnosis of Systemic Sclerosis for Prediction of Interstitial Lung Disease. J Rheum Dis. 2017;24(3):138–142. [Google Scholar]
- 30.Atilla N, Yıldırım Çetin, Balkarlı A. Association of neutrophil/lymphocyte ratio with the degree ofinterstitial lung disease in systemic sclerosis. Turk J Med Sci. 2016;46(6):1871–1874. doi: 10.3906/sag-1601-87. [DOI] [PubMed] [Google Scholar]
- 31.Esheba N, Shahba A. Assessment of Neutrophil Lymphocyte Ratio in Systemic Sclerosis Patients in Tanta University Hospital: A Promising Marker in Predicting Disease Severity. Egypt J Rheumatol Clin Immunol. 2016;4(1):33–41. [Google Scholar]


