ABSTRACT
Background
Anemia management in chronic kidney disease (CKD) is a significant challenge for healthcare professionals worldwide. The extensive management of CKD and its complications is directly linked with a substantial treatment burden, which impacts quality of life (QoL). This study aimed to assess the prevalence and management of anemia and to evaluate the treatment burden and its impact on the QoL of CKD and dialysis patients in Pakistan.
Methodology
A multicenter prospective observational study was conducted in three hospitals. A total of 170 patients were enrolled, with 156 available for follow-up after six months. Their prior consent was obtained. Each participant was interviewed in person and received a data collection form.
Results
At baseline, the prevalence of anemia among CKD (stage 3–5) and dialysis patients was 78.7% and 94.7%, respectively. Patients on dialysis used more erythropoietin stimulating agents (ESAs), with 38.6% at baseline and 40.8% by month six, compared to non-dialysis CKD patients. Oral iron was used by 6.2% of stage 3, 25% of stage 4, 20% of stage 5 patients, and 6.6% of dialysis patients at baseline. At the six-month follow-up, 42.8% of CKD and 33.8% of dialysis patients achieved the target hemoglobin level. Dialysis patients had a higher treatment burden compared to CKD at baseline (77.4±10.6 vs 59.3±13.3) and at six-month visit (79.3±11.1 vs 59.1±14.5). The multiple regression analysis showed that treatment burden had a significant association with age, disease duration, and comorbidity at baseline. There was a significant negative correlation between overall treatment burden and QoL, indicating that QoL decreases as treatment burden increases.
Conclusion
Anemia was prevalent, and its management was suboptimal in this study. The overall treatment burden score was high in dialysis patients, negatively affecting the QoL.
KEYWORDS: Anemia, chronic kidney disease, dialysis, health-related quality of life, Pakistan
Background
Chronic kidney disease (CKD) is one of the major non-communicable diseases that is linked to high economic and health burdens globally. The global prevalence of CKD is 13.4%, with approximately 4.9–7.1 million patients with end-stage renal disease (ESRD) requiring renal replacement therapy (Odeyemi et al., 2023). CKD is becoming a global health issue, as the occurrence and spread of ESRD have increased continuously during the past three decades (Shaqib, 2022).
Anemia is an inevitable and common complication of CKD (Khalifa et al., 2020). The World Health Organisation (WHO) defines anemia as ‘hemoglobin concentration <13 g/dl in men and <12 g/dl in women of childbearing age’. CKD patients are twice as likely to experience anemia compared to those without renal disease, and the rate of anemia tends to increase as the estimated glomerular filtration rate (eGFR) decreases (Lankhorst & Wish, 2010). There is an exponential relationship between reduced glomerular filtration and anemia. Anemia typically develops when the GFR is reduced to 0.5 mL/s or 0.75 mL/s in patients with diabetic nephropathy (Fathi et al., 2024). It was reported that 80.5% of patients with CKD are affected by anemia (Lankhorst & Wish, 2010). A study from South Ethiopia has shown high anemia incidence, and the intensity of the disease has increased with advanced stages of CKD and in dialysis patients (Adera et al., 2019).
The mechanisms of anemia associated with CKD are multifactorial. Gradual decline in endogenous erythropoietin (EPO) level plays a substantial role in anemia development (Portolés et al., 2021). The other factors are an absolute iron deficiency results from blood loss or decreased iron absorption, an underutilisation of iron stores caused by high hepcidin levels, a low bone marrow response to EPO, a shortened RBC life span, systemic inflammation caused by CKD and related comorbid conditions and vitamin B12 or folic acid shortage (Babitt & Lin, 2012). The early detection and appropriate treatment of anemia can reduce cardiovascular co-morbidities, improve patients’ overall QoL and reduce the mortality rate (Stauffer & Fan, 2014).
Treatment burden is an emerging concept in chronic disease management. Treatment burden is ‘the workload imposed by healthcare on patients and the effect this has on quality of life (QoL)’ (Tran et al., 2015). The burden of treatment is focused on unpleasant feelings associated with receiving treatment. The burden of care may vary based on the kind of treatment a person gets (Liyanage et al., 2015). Many studies have shown that burden is linked to poor adherence to treatment, adverse clinical outcomes, poor satisfaction of patient with care, reduced QoL and a high rate of hospitalisation and fatality (Al-Mansouri et al., 2021). CKD is associated with multiple comorbidities and complications that often lead to polypharmacy and require complex regimens of self-care, other healthcare interventions and actions that pose significant treatment burdens (Kamath & Iyengar, 2017).
Previous studies assessed treatment burden in specific chronic ailments, including diabetes, hypertension and malignancy. Limited data on anemia management, treatment burden, and its effects on health-related quality of life in CKD and dialysis patients is available, especially in resource-constrained regions like Pakistan. Therefore, we designed a multicenter study to assess the management of anemia, the treatment burden, and its outcomes on health-related QoL of CKD and dialysis patients in Pakistan.
Methodology
Study design and setting
It was a multicenter prospective observational study. Data collection was conducted during two visits: one at baseline and the other at the six-month follow-up. The study was conducted in two cities in Pakistan: Sialkot and Islamabad. A total of 170 patients were recruited based on inclusion and exclusion criteria at baseline; however, after losses during follow-up, 156 patients were left for the six-month data collection. All CKD and dialysis patients were recruited using a non-probability convenience sampling technique. The study duration was from December 10, 2022, to May 31, 2023.
Eligibility criteria
Eligibility criteria for CKD non-dialysis and dialysis patients enrolled in the study are as follows: (1) Patients with age ≥18 years, (2) CKD stage 3–5 (non-dialysis) and dialysis patients, (3) Clinically stable patients. Patients who had undergone renal transplantation or with severe digestive tract diseases were excluded.
Study tools
A treatment burden questionnaire (TBQ) was used to assess the treatment-related burden in study participants. This questionnaire was obtained from Mapi Research Trust. It comprised of 15 items that evaluated the following areas: challenges related to taking medication, self-monitoring, adjusting to a certain lifestyle, maintaining lab test schedules, doctor's visits, social life, organisation and administrative load. The five TBQ domains were a social burden, financial burden, administrative burden, medicine burden, and hardship associated with changing one's lifestyle. A Likert-type scale with a range of 0 (not a problem) to 10 (a big problem) was used to score the instrument responses (Duncan et al., 2020; Tran et al., 2014).
Health-related quality of life was assessed through a validated KDQOL-36TM questionnaire, including 12 generic and 24 disease-specific questions covering five dimensions: burden of kidney disease (BKD), physical component summary (PCS), Mental component summary (MCS), symptoms/problems of kidney disease and effects of kidney disease. Patients were asked to indicate their health condition by marking a box opposite the statement for every dimension. All numeric elements were transferred to a value ranging from 0 to 100 points, with a maximum obtainable score of 3600 (Hussien et al., 2021).
Data collection procedure
Patients’ socio-demographics, clinical, laboratory data, and medication history were collected on a standardised data collection form at the baseline visit. Patients were considered anemic if they had hemoglobin concentration <13 g/dl for males and <12 g/dl for females. Questionnaires were distributed among the participants at the study centres to measure treatment burden and HRQOL. The response was obtained in the form of a digit number. TBQ and KDQOL-36TM scores were then calculated using the scoring manual. At month six, data was collected from 156 patients who visited study centres, and their response was recorded. Patients were administered an English version of the questionnaire in a quiet room through face-to-face interviews. The data collection lasted for 15–20 min. This study followed the procedures of KDIGO clinical practice guidelines for anemia.
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 25 was used for data analysis. Continuous variables were calculated as mean and standard deviation, whereas categorical variables were calculated as numbers and percentages. Normality tests were performed to check the distribution of data. Shapiro Wilk test and Q-Q plots indicated that continuous variables were normally distributed. Independent sample t-test or one-way ANOVA was performed to compare continuous and categorical variables. The chi-square test was applied to observe the significant difference between categorical data, and these variables were presented as frequencies and percentages. Multiple regression analysis measured the association between independent variables and treatment burden. Unstandardised co-efficient B was obtained. A p-value of <0.05 was considered statistically significant. Spearman's rank order test was applied to determine the correlation between treatment burden and QoL.
Results
Demographic and clinical features of study participants
Demographic and clinical features of the study population included gender, age, education, marital status, employment, monthly income, BMI, smoking status, disease duration and co-morbidity type. All these are categorical variables, so summary statistics are presented as percentages and frequencies (Table 1).
Table 1.
Demographics and clinical features of study participants (n = 170).
| Variables | Categories | CKD (n=89) N (%) | Dialysis (n=81) N (%) |
|---|---|---|---|
| Age (years) | 18–30 | 16 (18.0) | 16 (19.8) |
| 31–45 | 25 (28.1) | 19 (23.5) | |
| 45–65 | 28 (31.5) | 25 (30.9) | |
| >65 | 20 (22.5) | 21 (25.9) | |
| Gender | Male | 47 (52.8) | 45 (55.6) |
| Female | 42 (47.2) | 36 (44.4) | |
| Marital status | Single | 11 (12.4) | 14 (17.3) |
| Married | 68 (76.4) | 50 (61.7) | |
| Divorced | 3 (3.4) | 5 (6.2) | |
| Widow | 7 (7.9) | 12 (14.8) | |
| Education | No education | 23 (25.8) | 33 (40.7) |
| Primary | 26 (29.2) | 17 (21.0) | |
| Secondary | 29 (32.6) | 17 (21.0) | |
| College/university | 11 (12.4) | 14 (17.3) | |
| BMI | <18.5 | 25 (28.1) | 20 (24.7) |
| 18.5-25 | 42 (47.2) | 36 (44.4) | |
| >25 | 22 (24.7) | 25 (30.9) | |
| Employment status | Unemployed | 50 (56.2) | 57 (70.4) |
| Employed | 39 (43.8) | 24 (29.6) | |
| Income per month | <25000 | 25 (28.1) | 39 (48.1) |
| 25000–50000 | 50 (56.2) | 33 (40.7) | |
| >50000 | 14 (15.7) | 9 (11.1) | |
| Smoking status | Ex-smoker | 18 (20.2) | 15 (18.5) |
| Non-smoker | 63 (70.8) | 58 (71.6) | |
| Current smoker | 8 (9.0) | 8 (9.9) | |
| Duration of disease | <1 year | 51(57.3) | 25 (30.9) |
| ≥ 2 years | 38 (42.7) | 56 (69.1) | |
| Co-morbidity | Yes | 49 (55.1) | 63 (77.8) |
| No | 40 (44.9) | 18 (22.2) | |
| Type of co-morbidity | Hypertension | 53 (59.6) | 61 (75.3) |
| Diabetes mellitus | 51 (57.3) | 54 (66.7) | |
| Eye disease | 6 (6.7) | 12 (14.8) | |
| CVD | 31 (34.8) | 36 (44.4) | |
| Liver disease | 6 (6.7) | 11 (13.6) | |
| Hyperlipidemia | 7 (7.9) | 13 (16) | |
| Others | 6 (6.7) | 10 (12.3) |
Prevalence of anemia
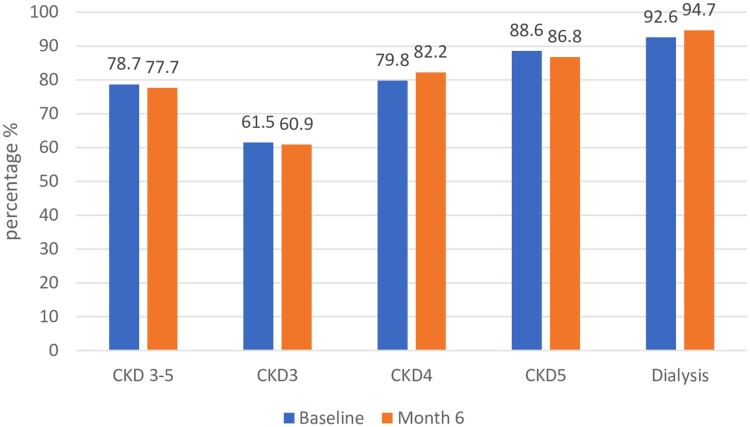
Figure 1 shows anemia prevalence in different stages of CKD and dialysis at baseline and month 6. The prevalence of anemia in patients with CKD stage 3–5 was 78.7% at baseline and 77.7% at month 6. In CKD stage 3, anemia prevalence was 61.5% at baseline and 60.9% at month 6. Anemia prevalence in patients with CKD stage 4 was 79.8% at baseline and 82.2% at month 6. A higher prevalence rate was reported in CKD stage 5 patients (88.6% at baseline; 86.8% at month 6). It was observed that anemia prevalence increased with declining renal function. The prevalence of anemia in dialysis patients was 92.6% at baseline and 94.7% at month 6. No significant change was observed in anemia prevalence between the two visits.
Figure 1.
Prevalence of anemia at baseline and at month 6.
Laboratory parameters of anemic patients
Table 2 shows the laboratory parameters of CKD and dialysis patients. There was a significant difference between CKD and dialysis patients in terms of hemoglobin level (11.1±1 vs. 10.5±1.10), hematocrit (35.5±6.2 vs. 33.0±6), RBC count (4.1±0.5 vs. 3.9±0.6), MCV (75.8±9.0 vs71.9±11.1), MCHC (31.7±2.1 vs. 31.3±3.3) and ferritin level (137±74.9 vs133.1±71.9) as p-value <0.05.
Table 2.
Laboratory parameters of study participants (n = 170).
| Variables | CKD (n=89) | Dialysis (n=81) | P-value |
|---|---|---|---|
| Hemoglobin (g/dl, mean ±SD) | 11.1±1 | 10.5±1.1 | 0.001 |
| Hematocrit (%), (mean± SD) | 35.5±6.2 | 33.0±6.0 | 0.017 |
| RBC (million cells/l, mean ±SD) | 4.1± 0.5 | 3.9± 0.6 | 0.002 |
| MCV (FL/red cell, mean ±SD) | 75.8±9.0 | 71.9±11.1 | 0.024 |
| MCH (pg./cell, mean± SD) | 24.9±4.8 | 23.0±4.1 | 0.012 |
| MCHC (g/dl, mean ±SD) | 31.7±2.1 | 31.35±3.3 | 0.020 |
| TSAT% | 24.3±9.5 | 23.8±10.3 | 0.356 |
| Ferritin(ng/dl) | 137.0±74.9 | 133.1±71.9 | 0.034 |
MCV; mean corpuscular volume, MCH; mean corpuscular hemoglobin, MCHC; mean corpuscular hemoglobin concentration, TSAT; transferrin saturation
Anemia treatment
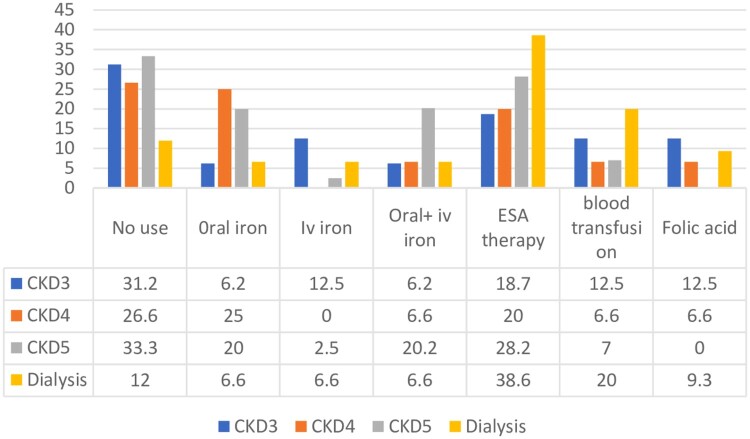
The commonly prescribed medications were oral iron, intravenous iron, intravenous plus oral iron, ESA therapy, blood transfusion, and folic acid. At baseline, no medication was prescribed to 31.2% of stage 3, 26.6% of stage 4, 33.3% of CKD stage 5, and 12% of dialysis patients. The ESA prescribing trend was increased with declining kidney function as a higher percentage of patients, i.e. 28.2% CKD stage 5 and 38.6% dialysis, were receiving ESA therapy. Oral iron was prescribed to 6.2% of stage 3, 25% of stage 4. 20% of CKD stage 5, and 6.6% of dialysis patients. 12.5% of stage 3, 2.5% of stage 5, and 6.6% of dialysis patients took intravenous iron. None of the patients in stage 4 were taking intravenous iron. A high proportion of patients (20.2%) in CKD stage 5 were on oral plus intravenous iron. 6.2% CKD stage 3, 6.6% stage 4, and 6.6% dialysis patients were on intravenous plus oral iron. The blood transfusion rate was high in dialysis patients (20%) compared to CKD. 12.5% of CKD stage 3, 6.6% of stage 4 and 9.3% of dialysis patients used folic acid (Figure 2).
Figure 2.
Anti-anemic medications prescribed at baseline.
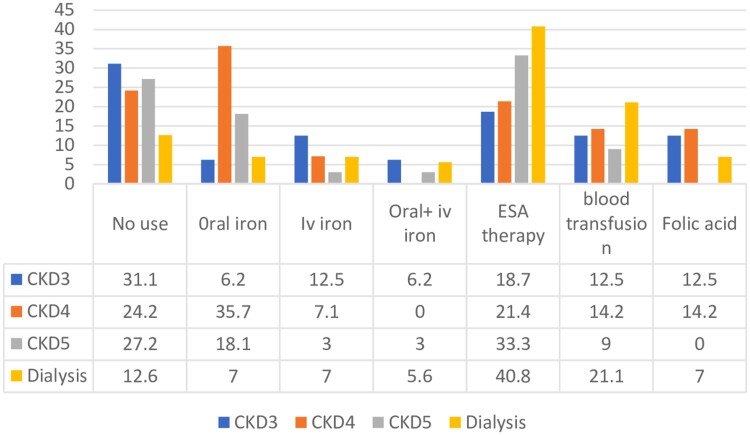
At the month six visit, it was observed that 31.1% stage 3, 24.2% stage 4, 27.2% CKD stage 5 and 12.6% dialysis patients were receiving no therapy for anemia. Oral and intravenous iron use was increased in CKD stage 4 patients at month 6 (35.7% and 7.15%). The utilisation of ESA therapy was also increased in all CKD stage and dialysis patients (Figure 3).
Figure 3.
Anti-anemic medications prescribed at month 6.
Anemia control
Table 3 shows anemia control at baseline and month 6. At baseline, 43.8% of CKD and 55.6% of dialysis patients had a hemoglobin level of ≤ 10.9 g/dl, while 34.9% of CKD and 38.3% of dialysis patients achieved a target hemoglobin level of 11-11.9 g/dl. In CKD, only 15.7% and in dialysis, 6.2% of patients had good anemia control of 12-13.4 g/dl hemoglobin level at baseline. After the month 6 visit, it was observed that 46.9% of CKD and 58.7% of patients had a hemoglobin level of ≤ 10.9 g/dl. Compared to baseline, at the month six visit, the proportion of patients who achieved the target hemoglobin level was high (37% CKD and 36% dialysis). 14.8% CKD and 5.3% dialysis patients achieved 12-13.4 g/dl hemoglobin levels.
Table 3.
Anemia control at baseline and month 6 of study participants.
| Hemoglobin level, g/dl | CKD | Dialysis | ||
|---|---|---|---|---|
| Baseline (89) | Month 6 (81) | Baseline (81) | Month 6 (75) | |
| N (%) | ||||
| ≤10.9 | 39 (43.8) | 38 (46.9) | 45 (55.6) | 44 (58.7) |
| 11-11.9 | 31 (34.9) | 30 (37.0) | 31 (38.3) | 27 (36.0) |
| 12-13.4 | 14 (15.7) | 12 (14.8) | 5 (6.2) | 4 (5.3) |
| ≥13.5 | 5 (5.6) | 1 (1.2) | 0 | 0 |
Comparison of treatment burden in CKD and dialysis patients at baseline
The treatment burden score was summarised as mean and standard deviation. An independent sample or one-way ANOVA test was performed to find the difference in the treatment burden in the study groups. The result shows that dialysis patients had a higher treatment burden at baseline than CKD; the mean TBQ score was 77.4±10.6 vs 59.3±13.3 with p<0. 001. The treatment burden was further categorised into low, moderate, and high burden. Moderate treatment burden was reported by more than 50% of patients (CKD 60.6%; dialysis 75.3%), while 39.3% CKD and 20.9% dialysis reported low burden. A high treatment burden was observed only in 3.7% of dialysis patients (Table 4).
Table 4.
Treatment burden in CKD and dialysis patients at baseline (n = 170).
| Variable | Total (n=170) | CKD (89) | Dialysis (81) | P value |
|---|---|---|---|---|
| Mean ± S. D | ||||
| TBQ global score | 67.9± 15 | 59.3±13.3 | 77.4±10.6 | <0.001 |
| TBQ categories N (%) | ||||
| Low burden | 52 (30.5) | 35 (39.3) | 17 (20.9) | 0.010* |
| Moderate burden | 115 (67.7) | 54 (60.6) | 61 (75.3) | |
| High burden | 3 (1.7) | 0 | 3 (3.7) | |
*One-way ANOVA test
Comparison of treatment burden in CKD and dialysis patients at month 6
The Treatment burden was measured again in the study sample at month six. An independent sample t-test or one-way ANOVA was performed to find the difference between study groups regarding treatment burden. Dialysis patients indicated a higher treatment burden than CKD patients (79.3±11.1 vs 59.1±14.5). A slight difference was observed in low, moderate, and high burden after month 6 (Table 5).
Table 5.
Treatment Burden in CKD and Dialysis at Month 6 (n = 156).
| Variable | Total (156) | CKD (81) | Dialysis (75) | P-value |
|---|---|---|---|---|
| TBQ global score | 68.8±16.4 | 59.1±14.5 | 79.3±11.1 | <0.001 |
| TBQ categories | n (%) | |||
| Low burden | 41 (26.3) | 25 (30) | 16 (21) | 0.030* |
| Moderate burden | 110 (70.5) | 56 (70) | 54 (72) | |
| High burden | 5 (3.2) | 0 | 5 (7) | |
*One-way ANOVA
Association of treatment burden with independent variables at month 6
Multiple regression analysis assessed the relationship between treatment burden and independent variables at the six-month follow-up visit. Only age [p = 0.015; CI(B) 0.647-5.972] and presence of comorbidity [p = 0.046; CI(B) 8.436-7.816] were significantly associated with treatment burden (Table 6).
Table 6.
Multiple regression analysis at month 6.
| Variable | B | Std. Error | P-value | 95% CI range |
|---|---|---|---|---|
| Age in years | 3.310 | 1.347 | 0.015 | 0.647-5.972 |
| Gender | 3.394 | 2.879 | 0.240 | −2.297-9.085 |
| Marital status | −0.396 | 1.847 | 0.830 | −4.049-3.255 |
| Educational level | −1.323 | 1.250 | 0.292 | −3.794-1.148 |
| Employment status | −4.562 | 2.947 | 0.124 | −10.389-1.264 |
| Income | −1.219 | 2.056 | 0.554 | −5.283-2.845 |
| Smoking status | −0.847 | 2.260 | 0.708 | −5.315-3.621 |
| Duration of disease | 3.477 | 2.729 | 0.205 | −1.917-8.871 |
| Comorbidity | −0.310 | 4.110 | 0.046 | 8.436-7.816 |
| Hypertension | 7.104 | 4.120 | 0.087 | −1.041-15.249 |
| Diabetes mellitus | −3.158 | 3.960 | 0.426 | −10.986-4.670 |
| Cardiovascular disease | 0.337 | 3.194 | 0.916 | −5.978-6.652 |
Comparison of HRQOL in CKD and dialysis patients at baseline
An independent sample t-test was performed to observe the difference in study groups regarding quality of life. Table 7 shows that dialysis patients had significantly lower QOL than CKD patients, with a mean score of 2322.8±219.6 vs 2734.2±212.4 (p<0.001). CKD patients had a better quality of life in all five dimensions than dialysis patients. The mean scores of five domains in CKD patients were as follows: PCS,49.8±6.9; MCS, 51.2±8.6; symptoms/problem;85.8±5.7; effects of kidney disease,82.9±9; burden of kidney disease 65±8.6.
Table 7.
Analysis of HRQOL in CKD and Dialysis patients at baseline.
| Variable | Total (170) | CKD (89) | Dialysis (81) | p value |
|---|---|---|---|---|
| (Mean ± S.D) | ||||
| KDQOL-36™ global score | 2447.6±391.3 | 2734.2±212.4 | 2132.9±287 | <0.001 |
| Five domains of KDQOL-36™ instrument score | ||||
| PCS | 55.1±9.1 | 49.8±6.9 | 38.8±9.2 | <0.001 |
| MCS | 52.6±7.8 | 51.2±8.6 | 54.1±6.6 | 0.016 |
| Symptoms/problem | 79.2±9.6 | 85.8±5.7 | 71.1±7.4 | <0.001 |
| Effects of kidney disease | 68.8±18.1 | 82.9±9.0 | 53.3±11.9 | <0.001 |
| Burden of kidney disease | 45.0±24.8 | 65.03±8.6 | 23.1±12.3 | <0.001 |
p<0.05 was considered statistically significant. PCS; physical component summary, MCS; mental component summary.
The mean scores of five domains in dialysis patients were as follows: PCS, 38.8±9.2; MCS,54.1±6.6; symptoms/problem, 71.1±7.4; effects of kidney disease, 53.3±11.9; burden of kidney disease, 23.1±12.3.
Comparison of HRQOL in CKD and dialysis patients at month 6
A little change was reported in mean scores at month 6. CKD patients had better QoL as compared to dialysis patients (p<0.001) (Table 8).
Table 8.
Analysis of HRQOL in CKD and Dialysis patients at Month 6.
| Variable | Total (156) | CKD (81) | Dialysis (75) | P value |
|---|---|---|---|---|
| (Mean ± S.D) | ||||
| KDQOL-36 global score | 2322.9±290.6 | 2548.0±178.0 | 2079±166.4 | <0.001 |
| Five domains of KDQOL-36™ instrument score | ||||
| PCS | 44.5±9.8 | 49.6±6.9 | 38.7±9.2 | .004 |
| MCS | 52.6±8.0 | 51.1±8.8 | 54.3±6.7 | .003 |
| Symptoms/problem | 79.0±9.6 | 85.6±5.9 | 71.9±7.6 | <0.001 |
| Effects of kidney disease | 68.8±17.5 | 82.3±9.1 | 54.2±11.7 | .010 |
| Burden of kidney disease | 45.1±24.5 | 65.0±13.8 | 23.5±12.1 | <0.001 |
P<0.05 was considered statistically significant. PCS; physical component summary, MCS; mental component summary
Correlation between TBQ and KDQOL-36™ in CKD patients at baseline and month six visit
The correlation between treatment burden and QoL was assessed using Spearman’s rank-order test. Table 9 shows a strong negative correlation between the overall TBQ score and KDQOL-36™ score, as the p-value is less than 0.05. Except for MCS, all KDQOL-36™ domains showed a negative correlation with TBQ.
Table 9.
Correlation between TBQ and KDQOL-36™ in CKD patients at baseline and month six visit.
| Variables | Baseline | Month 6 visit | ||
|---|---|---|---|---|
| Correlation coefficient | p-value | Correlation coefficient | p-value | |
| Overall KDQOL score KDQOL-36™ | −.464 | <0.001 | −.488 | <0.001 |
| PCS | −.248 | 0.001 | −.374 | <0.001 |
| MCS | .026 | 0.740 | 0.102 | 0.205 |
| Symptom/problem burden | −.485 | <0.001 | −.552 | <0.001 |
| Effect of kidney disease | −.521 | <0.001 | −.550 | <0.001 |
| Burden of kidney disease | −.509 | <0.001 | −.515 | <0.001 |
p<0.05 was considered statistically significant. PCS; physical component summary, MCS; mental component summary
Discussion
Anemia is the most common and uncontrolled complication in CKD and dialysis patients. Different medications are linked with better control of anemia and patient outcomes among dialysis and CKD patients. In our study, anemia rates were higher in dialysis patients (92.6% at baseline; 94.7% at month 6) compared to CKD patients (78.7% at baseline; 77.7% at month 6) and higher than in a previous Swedish study (60% CKD; 90% dialysis) (Evans et al., 2020). Mean TBQ was also elevated, with 77.4±10.6 in dialysis and 59.3±13.3 in CKD patients.
Demographics showed more male patients (CKD 52.8%; dialysis 55.6%), consistent with studies in Sweden (CKD 63%; dialysis 67%) (Evans et al., 2020), Malaysia (male; 64.1%) (Salman et al., 2016) and Pakistan (male; 62.3%) (Rajput et al., 2020). A strong effect of gender on CKD progression has been noted in males (CKD 63%; dialysis 67%) (Neugarten & Reckelhoff, 2020). Patients aged 45–65 years made up the largest group (CKD 31.5%; dialysis 30.9%), similar to the results of the study conducted elsewhere (Rajput et al., 2020).
Anemia prevalence was comparable between males (baseline 55.2%; month 6, 54.4%) and females (baseline 44.8%; month 6, 44.1%), although other studies reported higher anemia rates in females (Ryu et al., 2017). Another study found similar rates in both genders (Tanaka et al., 2021), with 68.3% of married participants at baseline and 65.4% at month six had anemia. Anemia remained prevalent among participants across both visits, with higher rates in advanced CKD stages: stage 3 (baseline 61.5%; month 6, 66.9%), stage 4 (baseline 79.8%; month 6, 82.2%), and stage 5 (baseline 88.6%; month 6, 86.8%). A similar pattern was observed in a Korean study (stage 3, 46.6%; stage 4, 78.9%; stage 5, 95.6%) (Ryu et al., 2017). The increase in anemia in advanced stages may be due to iron deficiency, reduced erythropoietin production, malnutrition, inflammation, and abnormal red blood cell breakdown (Lankhorst & Wish, 2010).
According to KDIGO guidelines, anemia in CKD is primarily treated with ESA therapy and iron supplementation, while blood transfusions are reserved for emergencies. In our study, ESA therapy use increased slightly over six months: stage 3 (18.7% at both visits), stage 4 (20% at baseline, 21.4% at month 6), and stage 5 (28.2% at baseline, 33.3% at month 6). A similar trend was observed in a Japanese cohort study where 13.7% of patients in stage 3, 15.2% in stage 4, and 21.1% in stage 5 received ESA therapy (Tanaka et al., 2021). Oral iron use was suboptimal in stage 1, while intravenous iron and folic acid were used in a small number of patients. Folic acid is not recommended for CKD-related anemia. Despite KDIGO guidelines, blood transfusions were performed in 12.5% of stage 3, 6.6% of stage 4, and 7% of stage 5 patients have undergone blood transfusion at baseline, with similar rates at month 6, posing risks of immunological reactions and infections. The rate of anemia control in CKD was significantly higher in our study, with 37.1% at baseline and 42.8% at month six, compared to the 8.2% reported in a study conducted in China (Li et al., 2016).
Our results found that 88% of dialysis patients were receiving anemia treatment at baseline, slightly decreasing to 87.4% at six months, higher than a previous report of 77.7% (Borzych-Duzalka et al., 2013). ESA use was low, with 38.6% at baseline and 40.8% at six months, compared to 77% in contrast to another study that found 77% of dialysis patients received ESA therapy (Evans et al., 2020), possibly due to unfamiliarity with guidelines or concerns about side effects. Oral iron was prescribed to 6.6% of dialysis patients, which aligns with another study that reported a 1% iron use in dialysis patients (Evans et al., 2020). Blood transfusion use increased from 20% at baseline to 21.1% at six months, often used when ESA therapy is unsuitable or in emergencies. In the dialysis group, the target hemoglobin level (11–12 g/dl) was achieved by 29.3% of patients at baseline and 33.8% at month 6. Patients with targeted hemoglobin levels increased at the month six visit. The improvement in the rate of anemia control at month six could be due to the increased prescribing rate of ESA.
CKD is a leading cause of morbidity and mortality, with a 36.9% overall mortality rate (Rhee & Kovesdy, 2015). It significantly impacts QoL, which is lower in dialysis patients compared to transplant recipients and non-dialysis CKD patients (Wirkner et al., 2022). Our study found higher QoL scores in CKD patients (2734.2±212) than in dialysis patients (2132.9±287), with QoL declining in both groups over time. Treatment burden, measured by a validated questionnaire, was higher in dialysis patients (mean TBQ score 77.4±10.6) than in CKD patients (59.3±13.3), increasing by the six-month visit. Most CKD (70.5%) and dialysis patients (72%) had a moderate burden, with no CKD patients showing a high burden. A study reported that males experienced more burden than females (Neugarten & Reckelhoff, 2020), but in contrast, our study reported similar percentages of males and females experiencing treatment burden. Less educated and unemployed patients faced higher burdens, likely due to limited disease knowledge and financial strain from complex CKD management. The important consequence of treatment burden is non-adherence. Our study showed that patients with comorbidities experienced a high burden; this finding is in accordance with the previous study results (Sav et al., 2016).
A study conducted in Qatar found that treatment burden was associated with poor HRQOL in non-dialysis CKD patients, indicating that the highest burden was related to medication, lifestyle, and social and economic status (Al-Mansouri et al., 2021). A significant negative correlation was found in our study between treatment burden and QoL (correlation coefficient = −0.464, p < 0.001). Higher treatment burdens were associated with lower QoL across multiple domains, including physical and mental health, symptoms, and the impact of kidney disease. Follow-up data at six months confirmed these trends.
Moreover, variability in anemia treatment practices, such as the low use of ESA or differing adherence to guidelines, may have affected the results, making it difficult to assess the true impact of standard anemia management. Another limitation is not finding the differences in clinical practices between study centres due to time constraints that could have influenced the treatment outcomes and patient experiences.
Conclusion
The current study revealed a high prevalence of anemia, which increased as renal function declined and was more severe in dialysis patients. The commonly prescribed anti-anemic preparations were oral iron, intravenous iron, ESA therapy, blood transfusion and folic acid. Underutilisation of guidelines regarding ESA and iron supplement use was observed. Anemia control was suboptimal in CKD and dialysis patients, with < 50% achieving a target hemoglobin level of 11–12 g/dl. Early identification and proper management can reduce the burden of anemia. A large proportion of patients reported low to moderate treatment burden, and dialysis patients experienced a higher burden than CKD patients. A strong negative correlation was observed between treatment burden and health-related quality of life. It is essential to consider the treatment burden in CKD management strategies. In summary, improving anemia management in CKD patients requires a multi-faceted strategy that prioritises both clinical outcomes and patient experiences. Future studies should aim to bridge the knowledge gap regarding side effects and their influence on QoL, ultimately guiding more effective and patient-centred anemia management practices.
Acknowledgements
Our gratitude goes to colleagues from the Department of Pharmacy, Quaid-i-Azam University, along with doctors and nurses from different study centres, for their kind support throughout our research work.
Authors contribution
Conceptualisation: Amjad Khan, Sadia Ghulam Hussain, Yu Fang
Data curation: Saima Mushtaq, Amjad Khan, Sameen Abbas
Formal analysis: Sadia Ghulam Hussain, Amjad Khan
Investigation: Sadia Ghulam Hussain, Amjad Khan, Saima Mushtaq
Methodology: Sadia Ghulam Hussain, Amjad Khan, Yalin Dong, Weiyi Feng
Project administration: Amjad Khan, Yalin Dong, Weiyi Feng, Yu Fang
Resources: Amjad Khan, Yu Fang
Supervision: Yu Fang
Visualisation: Sameen Abbas
Writing – original draft: Amjad Khan, Sadia Ghulam Hussain
Writing – review & editing: Amjad Khan, Yu Fang
Disclosure statement
No potential conflict of interest was reported by the author(s).
Ethics and consent
The ethical review board of Allama Iqbal Memorial Teaching Hospital, affiliated with KMSMC, has approved this study to be conducted on their premises with Ref No. 3195/KMSMC/2021. Written informed consent was obtained from all individual participants included in the study. All procedures performed in studies involving human participants were by the ethical standards of the institutional and/or national research committee and in line with the Helsinki's declaration.
Data availability statement
All data and supportive materials are available in the manuscript.
References
- Adera, H., Hailu, W., Adane, A., & Tadesse, A. (2019). Prevalence of anemia and its associated factors among chronic kidney disease patients at University of Gondar Hospital, Northwest Ethiopia: A hospital-based cross sectional study. International Journal of Nephrology and Renovascular Disease, 219–228. 10.2147/IJNRD.S216010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mansouri, A., Al-Ali, F. S., Hamad, A. I., Ibrahim, M. I. M., Kheir, N., Ibrahim, R. A., AlBakri, M., & Awaisu, A. (2021). Assessment of treatment burden and its impact on quality of life in dialysis-dependent and pre-dialysis chronic kidney disease patients. Research in Social and Administrative Pharmacy, 17(11), 1937–1944. 10.1016/j.sapharm.2021.02.010 [DOI] [PubMed] [Google Scholar]
- Babitt, J. L., & Lin, H. Y. (2012). Mechanisms of anemia in CKD. Journal of the American Society of Nephrology, 23(10), 1631–1634. 10.1681/ASN.2011111078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzych-Duzalka, D., Bilginer, Y., Ha, I. S., Bak, M., Rees, L., Cano, F., Munarriz, R. L., Chua, A., Pesle, S., & Emre, S. (2013). Management of anemia in children receiving chronic peritoneal dialysis. Journal of the American Society of Nephrology, 24(4), 665–676. 10.1681/ASN.2012050433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, P., Murphy, M., Man, M.-S., Chaplin, K., Gaunt, D., & Salisbury, C. (2020). Development and validation of the multimorbidity treatment burden questionnaire (MTBQ). BMJ Open, 8(4), e019413. 10.1136/bmjopen-2017-019413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, M., Bower, H., Cockburn, E., Jacobson, S. H., Barany, P., & Carrero, J.-J. (2020). Contemporary management of anaemia, erythropoietin resistance and cardiovascular risk in patients with advanced chronic kidney disease: A nationwide analysis. Clinical Kidney Journal, 13(5), 821–827. 10.1093/ckj/sfaa054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathi, A. E., Shahwan, M., Hassan, N., Jairoun, A. A., & Shahwan, M. (2024). Prevalence of anemia in Type 2 diabetic patients and correlation with body mass index and kidney function in Palestine. Diabetes, Metabolic Syndrome and Obesity, 2293–2301. 10.2147/DMSO.S454916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussien, H., Apetrii, M., & Covic, A. (2021). Health-related quality of life in patients with chronic kidney disease. Expert Review of Pharmacoeconomics & Outcomes Research, 21(1), 43–54. 10.1080/14737167.2021.1854091 [DOI] [PubMed] [Google Scholar]
- Kamath, N., & Iyengar, A. A. (2017). Chronic kidney disease (CKD): An observational study of etiology, severity and burden of comorbidities. The Indian Journal of Pediatrics, 84(11), 822–825. 10.1007/s12098-017-2413-2 [DOI] [PubMed] [Google Scholar]
- Khalifa, A. M., Almoallem, R. A., Alruwaili, S. H., Mohammed, A. E., & Alanazi, S. Y. N. R. (2020). Prevalence of anemia: A study on inpatients’ records with chronic kidney disease at Prince Mutaib Hospital, Al-jouf Province, Saudi Arabia. International Journal of Medicine in Developing Countries, 4(1), 206. 10.24911/IJMDC.51-1574930119 [DOI] [Google Scholar]
- Lankhorst, C. E., & Wish, J. B. (2010). Anemia in renal disease: Diagnosis and management. Blood Reviews, 24(1), 39–47. 10.1016/j.blre.2009.09.001 [DOI] [PubMed] [Google Scholar]
- Li, Y., Shi, H., Wang, W.-M., Peng, A., Jiang, G.-R., Zhang, J.-Y., Ni, Z.-H., He, L.-Q., Niu, J.-Y., & Wang, N.-S. (2016). Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease. Medicine, 95(24), e3872. 10.1097/MD.0000000000003872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage, T., Ninomiya, T., Jha, V., Neal, B., Patrice, H. M., Okpechi, I., Zhao, M., Lv, J., Garg, A. X., & Knight, J. (2015). Worldwide access to treatment for end-stage kidney disease: A systematic review. The Lancet, 385(9981), 1975–1982. 10.1016/S0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- Neugarten, J., & Reckelhoff, J. F. (2020). Chapter 7 – Gender issues in chronic kidney disease. In Kimmel P. L. & Rosenberg M. E. (Eds.), Chronic renal disease (2nd ed., pp. 91–109). Academic Press. 10.1016/B978-0-12-815876-0.00007-3 [DOI] [Google Scholar]
- Odeyemi, A., Oladimeji, O. M., Ajibare, A. O., Iyayi, A. A., Oladimeji, A. B., Ojo, O. T., Adebola, A. P., Awobusuyi, J. O., & Adekoya, A. O. (2023). Impact of anemia on The quality of life of chronic kidney disease patients: A single institution experience. West African Journal of Medicine, 40(11), 1253–1261. [PubMed] [Google Scholar]
- Portolés, J., Martín, L., Broseta, J. J., & Cases, A. (2021). Anemia in chronic kidney disease: From pathophysiology and current treatments, to future agents. Frontiers in Medicine, 8, 642296. 10.3389/fmed.2021.642296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput, R., Ahmad, N., Khan, A., Wahid, A., & Atif, M. (2020). Prevalence, risk factors and management of anaemia in non-dialysis chronic kidney disease patients: Findings from a single centre study in Pakistan. Specialty Journal of Medical Research and Health Science, 5(1–2020), 8–15. [Google Scholar]
- Rhee, C. M., & Kovesdy, C. P. (2015). Spotlight on CKD deaths – Increasing mortality worldwide. Nature Reviews Nephrology, 11(4), 199–200. 10.1038/nrneph.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu, S.-R., Park, S. K., Jung, J. Y., Kim, Y. H., Oh, Y. K., Yoo, T. H., & Sung, S. (2017). The prevalence and management of anemia in chronic kidney disease patients: Result from the Korean cohort study for outcomes in patients with chronic kidney disease (KNOW-CKD). Journal of Korean Medical Science, 32(2), 249–256. 10.3346/jkms.2017.32.2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman, M., Khan, A. H., Adnan, A. S., Sulaiman, S. A. S., Hussain, K., Shehzadi, N., Islam, M., & Jummaat, F. (2016). Prevalence and management of anemia in pre-dialysis Malaysian patients: A hospital-based study. Revista da Associação Médica Brasileira, 62(8), 742–747. 10.1590/1806-9282.62.08.742 [DOI] [PubMed] [Google Scholar]
- Sav, A., Whitty, J. A., McMillan, S. S., Kendall, E., Kelly, F., King, M. A., & Wheeler, A. J. (2016). Treatment burden and chronic illness: Who is at most risk? The Patient – Patient-Centered Outcomes Research, 9(6), 559–569. 10.1007/s40271-016-0175-y [DOI] [PubMed] [Google Scholar]
- Shaqib, M. O. (2022). Association between kidney function tests and different GRADES of anemia in diabetic patients.
- Stauffer, M. E., & Fan, T. (2014). Prevalence of anemia in chronic kidney disease in the United States. PLoS One, 9(1), e84943. 10.1371/journal.pone.0084943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, K., Saito, H., Iwasaki, T., Oda, A., Watanabe, S., Kanno, M., Kimura, H., Shimabukuro, M., Asahi, K., & Watanabe, T. (2021). Status of anemia according to underlying renal disease in chronic kidney disease: The Fukushima CKD cohort. Annals of Clinical Epidemiology, 3(1), 27–35. 10.37737/ace.3.1_27 [DOI] [Google Scholar]
- Tran, V.-T., Barnes, C., Montori, V. M., Falissard, B., & Ravaud, P. (2015). Taxonomy of the burden of treatment: A multi-country web-based qualitative study of patients with chronic conditions. BMC Medicine, 13, 1–15. 10.1186/s12916-014-0241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, V.-T., Harrington, M., Montori, V. M., Barnes, C., Wicks, P., & Ravaud, P. (2014). Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Medicine, 12, 1–9. 10.1186/1741-7015-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner, J., Scheuch, M., Dabers, T., Freiin von Rheinbaben, S., Fiene, B., Aymanns, S., Endlich, K., Endlich, N., Lendeckel, U., & Rettig, R. (2022). Comorbid depression and diabetes are associated with impaired health-related quality of life in chronic kidney disease patients. Journal of Clinical Medicine, 11(16), 4671. 10.3390/jcm11164671 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and supportive materials are available in the manuscript.