Abstract
The study of the microbiota and the microbiome, and specifically the intestinal one, has determined great interest due to the possible association of their alterations with numerous diseases. These include entities as diverse as Crohn’s disease, autism, diabetes, cancer or situations as prevalent today as obesity. In view of this situation, different recommendations have been performed regarding the use of probiotics, prebiotics, and postbiotics as modulators of the microbiota and the microbiome, seeking both preventive and therapeutic effects, and faecal material transfer (FMT) is proposed as an alternative. The latter has emerged as the only proven beneficial intervention on the intestinal microbiome, specifically in the treatment of recurrent colitis associated with Clostridioides difficile (R-CDI). In the rest of the entities, the lowering of laboratory costs has favored the study of the microbiome, which is resolved by delivering reports with catalogs of microorganisms, metabolites or supposed biomarkers without consensus on their composition associated with healthy or diseased microbiota and the disease. There is still insufficient evidence in any disease for interventions on the microbiome beyond FMT and R-CDI. Multi- and multi-disciplinary work with extensive research and the application of artificial intelligence in this field may shed light on the questions raised currently. Ethical issues must also be resolved in light of possible interventions within the umbrella of personalized medicine.
Keywords: microbiota, microbiome, Clostridioides difficile, faecal transfer, postbiotics, probiotics, prebiotics
Abstract
El estudio de la microbiota y el microbioma, y en concreto el intestinal, ha despertado gran interés ante la posible asociación de sus alteraciones con numerosas enfermedades. Estas abarcan entidades tan diversas como la enfermedad de Crohn, el autismo, la diabetes, el cáncer o situaciones tan prevalentes en la actualidad como la obesidad. Ante ello, han surgido diferentes recomendaciones en el uso de probióticos, prebióticos y postbióticos como moduladores de la microbiota y el micro-bioma, buscando tanto efectos preventivos como terapéuticos y se propone la trasferencia de materia fecal (TMF) como alternativa. Esta última, se ha erigido como intervención con beneficio demostrado en el tratamiento de la colitis recurrente asociada a Clostridioides difficile (R-CDI). En el resto de las entidades, el abaratamiento de los costes de laboratorio ha favorecido el estudio del microbioma que se resuelve con la emisión de informes con catálogos de microorganismos, metabolitos o supuestos biomarcadores sin que existan consensos sobre su composición que se asocien a microbiotas “sanas” o “patológicas” con enfermedad humana. No existen aún evidencias suficientes en ninguna enfermedad para la intervención sobre el microbioma más allá del TMF y la R-CDI. El trabajo multi y pluridisciplinar con investigaciones amplias y la aplicación de la inteligencia artificial en este terreno pueden arrojar luz a los interrogantes hoy planteados. También deben resolverse cues-tiones éticas a la luz de las posibles intervenciones dentro del paraguas de la medicina personalizada.
Keywords: microbiota, microbioma, Clostridioides difficile, trasferencia fecal, postbióticos, probióticos, prebióticos
INTRODUCTION
The relationship between our health and the set of micro-organisms that reside in different territories of our body (microbiota or colloquially “flora”) has been the subject of study for years. The concept of microbiota has been expanded into that of microbiome, which includes the ecological niche in which the microbiota is found and its interaction with the host [1]. There are currently numerous research lines with essentially three main objectives: 1) to determine whether alterations in the microbiota cause disease; 2) to discern whether their analysis has diagnostic utility in these diseases; and 3) to evaluate the possibility of modifying an altered microbiota as a form of treatment. The latter includes the possibility of administering substances that modify the microbiota (prebiotics), elements of the microbiota (probiotics) or potentially beneficial substances synthesized by the microbiota (postbiotics).
The interest the study of this field is enormous, but so is the confusion in it, a fact that motivates this review promoted by the COVID-19 and Emerging Pathogens Committee of the Illustrious Official College of Physicians of Madrid (ICOMEM). We understand that this confusion has three main causes. The first is due to the extensiveness of the fields of study, ranging from allergies to autism or cancer. The second is due to the economic interests underlying the offer of non-standardized diagnostic techniques with as yet unproven usefulness (studies of the microbiota), and of products aimed at improving its composition (pre, pro and postbiotics). They are often developed and marketing with lack of strict regulation, which facilitates their entry into the market without evidence of clear benefit, with a multitude of products and diverse posology. Finally, the multidisciplinary approach, in the great majority with disconnection between professionals, and the absence of criteria and consensus in the relevance of the studies and analysis of the results.
From the literature reviewed, this Committee only appreciates sufficient evidence on the therapeutic use of the intestinal microbiota for the treatment of recurrent colitis due to Clostridioides difficile (R-CDI) and believes it is necessary to consider the need to regulate not only the research of products intended to modify the microbiota, but also their advertising. The following lines summarize the outcome of the deliberations of the Committee of Experts convened by ICOMEM in response to a series of questions posed by the Committee.
WHAT IS THE MICROBIOME? HOW DOES IT DIFFER FROM THE MICROBIOTA?
The term microbiota is defined as the set of microorganisms (bacteria, fungi, archaea, viruses, and parasites) that reside in our body in different mucous membranes and other territories. Colloquially it is also known as flora. Microbiome refers to a broader concept as it also includes the habitat or ecological niche and the interaction with the host. It refers to the microbial communities found in an ecological niche, their genes and metabolites, as well as the environmental conditions surrounding them. It was first used by Whipps et al. in 1988 [2], referring to the set of microbial communities, the characteristics of their interrelationship, and the properties of the habitat where they are found.
The microbiota is therefore part of the microbiome in defined ecological niches or locations. Among all these locations, the intestinal microbiome stands out for its complexity and diversity, which is the most studied so far. The genitourinary, the oral cavity, the nasopharynx, the respiratory tract, and the skin microbiomes, among others, also stand out. Each of them has its own particular niche and fingerprint in its composition. The microbial communities that make up the microbiome have a symbiotic and mutualistic behavior with human cells and maintain an important dialogue with the immune system [1]. It is therefore considered as an “organ” essential for life and with a clear influence on health and disease. The micro-biome has its own particularities and characteristics inherent in each individual, which may vary depending on the genetic substrate, diet, early exposure, geography, interaction with the environment, or simply the age of the individual.
The vast majority of the microorganisms that make up the microbiome are not able to be cultured in the traditional culture media and conditions in which they are grown. The introduction of massive sequencing techniques and bioinformatics tools for massive data analysis (metaomic techniques) and, more recently, artificial intelligence (AI), have brought about a revolution and a great advance in the knowledge of the microbiota and in the catalogs of its composition [3]. However, it is more difficult to characterize the conditions of the microbiome and the effects derived from its composition. Recent studies suggest that, more than microbial composition, the importance of the microbiome lies in its functionality, since different microbial species can perform equivalent metabolic functions and the same species can perform different functions. Figure 1 shows the main groups of microorganisms found in the intestinal microbiota and their taxonomic update.
Figure 1.
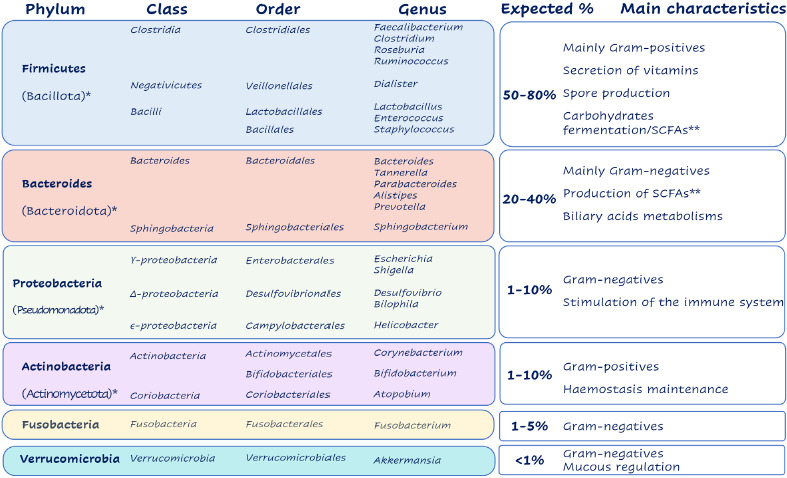
Main groups of microorganisms found in the intestinal microbiota and their taxonomic update
*updated taxonomic designation; ** Short-chain fatty acids
WHAT IS THE FUNCTION OF THE GUT MICROBIOME?
The intestinal microbiome represents a complex ecosystem that, in a symbiotic manner, contributes to numerous functions of the host physiology. To date, its participation in the absorption and regulation of nutrient metabolism, the synthesis of essential vitamins, the elimination of toxic compounds, the strengthening of the intestinal barrier and protection against pathogenic microorganisms and the development of the immune system is well known. However, the mechanisms of this contribution are far from being sufficiently elucidated [4]. The participation of the intestinal microbiota in these functions is synthesized below.
Nutrition and metabolism. The intestinal microbiota provides energy and nutrients to the organism, intervening in the absorption and metabolism mainly of carbohydrates, but also of lipids and proteins, besides being able to interfere in the metabolism of some drugs. It has been demonstrated that commensal species of Bifidobacterium can synthesize vitamin K and water-soluble B vitamins. Other groups such as Bacteroides can generate short-chain fatty acids (SCFA) by fermentation of non-digestible carbohydrates, also collaborating with Bifidobacterium species in the fermentation of oligosaccha-rides. The fatty acids generated, in the form of acetate, propionate and butyrate, constitute a primary energy source for the colonic epithelium and can also reach systemic availability. In acetate form they become substrate for lipogenesis and gluconeogenesis [5,6]. In butyrate form they can prevent lactic acid accumulation. Some microorganisms such as those of the genus Oxalobacter and certain Bifidobacterium prevent the formation of oxalates. In relation to lipid metabolism, they enhance lipid hydrolysis by regulation of the colipase required by pancreatic lipases for lipid digestion. Finally, it affects protein metabolism through proteinases and peptidases that potentiate those of the host [7].
Immunomodulation. The intestinal microbiota also works in conjunction with the innate and adaptive immune system of the host. It contributes to the maintenance of intestinal lymphoid tissue (GALT, gut-associated lymphoid tissue), modulates the expression of T cells, mainly T-helper (Th) and T-regulatory (Treg) cells, and influences innate lymphoid cells (ILC). It also interacts with IL10-producing macrophages [6].
Protection against infection. This is a multifactorial action in which the direct action of the microbiota and the interaction with the host’s defense mechanisms must be considered. In the former, the intestinal microbiota enters into competition for nutrients against possible pathogenic micro-organisms. In addition, some microorganisms have the function of synthesizing proteins with antimicrobial activity. However, interaction with host defense mechanisms is essential. The microbiota induces the production of IgA, improving the barrier function exerted by the intestinal mucous layer. Also the production of lactic acid during metabolism, enhances the activation of host lysozyme which acts as an antimicrobial agent.
However, it should not be forgotten that the microbiome constitutes a dynamic ecosystem, rapidly changing according to changes in diet or exposure to chemical agents, which can cause not only positive but also negative actions [8].
WHAT EXTRINSIC AND INTRINSIC FACTORS OF THE INDIVIDUAL INFLUENCE THE GUT MICROBIOME?
Several factors have been identified that influence the composition of the intestinal microbiota. It is not well understood, however, which are the ultimate determinants affecting its stability and the fluctuations that occur throughout life. The factors most frequently implicated include the following:
Diet. The basic composition of the human microbiota is affected by diet over the long term, especially diets with a high proportion of fat and protein versus a high-fibre diet. Marked differences in gut microbiota have been found to be associated with these dietary patterns [9].
Host genetics. In particular, the genetic determinants of the immune system, which plays a key role in selecting the bacteria that reside in the body [10].
Use of antimicrobials. They significantly alter the intestinal microbiota, affecting its diversity and composition. This effect appears both in the short and long term [11].
Lifestyle and environment. Factors related to lifestyle, diet and medications in general have a profound impact on the gut microbiota [11].
This is another crucial factor affecting the diversity of the microbiome. Its composition has been observed to vary with age, possibly reflecting changes in the immune system, diet and general health [12].
Stress. Both chronic and acute, can influence the gut micro-biota. Although the exact mechanism is not fully elucidated, stress is thought to affect the intestinal barrier and the interactions of the microbiota with the nervous system [13].
Geographic factors. Geographic location and local dietary habits account for a significant part of the variation in gut microbiota [14].
Sex. Sex differences exist in the composition of the gut microbiota, although studies show mixed results and the underlying mechanism is still under investigation [15].
CAN THE HEALTH OF THE INTESTINAL MICROBIOME BE IMPROVED? HOW?
Based on the factors that modify the microbiota and the microbiome, some interventions have been postulated to improve their “health”. However, their effect on overall health is still far from being well defined. Some of these interventions are summarized in Table 1. Although these strategies are promising, further research is essential to fully understand their impact on global health and disease prevention. Individualization of these interventions, taking into account the uniqueness of each person’s microbiota, could be key in the quest for optimal health and lasting well-being [16-21].
Table 1.
Interventions on the microbiome to improve your health
| Intervention | Comments | Ref. |
|---|---|---|
| Prebiotics | Non-digestible compounds, present in the diet, that stimulate the growth or activity of microorganisms of the microbiota, resulting in a possible health benefit. Generally, plant fibres that we do not digest and that are food for bacteria. Examples: inulin (fructooligosaccharide) and oligosaccharides from breast milk. | 16 |
| Probiotics | A live microorganism that, when administered in adequate amounts, is intended to improve health problems such as obesity. Examples: There are many types of probiotics, generally they are of the species Lactobacillus or Bifidobacterium or related such as Lactecaseibacillus rhamnosus GG. The Escherichiacoli Nissle 1917 strain is also used. | 18 |
| Postbiotics | Inactivated microorganisms and their metabolites that confer a potential beneficial effect on intestinal health by providing possible anti-inflammatory, immunomodulatory and intestinal barrier protective effects. Examples: Current focus is on gut-derived bacteria such as Akkermansiamuniciphila, a strict anaerobic bacterium that releases metabolites with potentially health-promoting activity, including short-chain fatty acids | 21 |
| Dietary fibre | Its consumption through fibre-rich foods may improve glucose metabolism. It is associated with an increase in bacteria to which beneficial effects are attributed. | 17 |
| Dietary components and dietary habits | Healthy dietary habits, such as a diet rich in plant foods, can promote a diverse and health-promoting microbial ecosystem. Vegetarian and vegan diets are associated with an increase in certain potentially beneficial bacteria (such as Bacteroidetes) and may promote the production of short-chain fatty acids, which are considered beneficial. | 19, 20 |
IS THERE A LINK BETWEEN THE GUT MICROBIOME AND DISEASE? WHAT IS THE EVIDENCE BASE?
The involvement of the microbiota in health or disease is postulated on three basic pillars: the influence on the construction, maintenance and modulation of immunity. Its action is positive in the digestive process and in the generation of energetic and vitamin resources essential for life or, on the contrary, negative in the production of potentially toxic substances that can alter its normal functionalism. The biological aggressiveness of many microorganisms is manifested when they colonize or when they undergo translocation and colonize extraintestinal tissues. When studying the relationship between the microbiota and health or disease, methodological problems arise in the study of a microbiome of a changing nature and its possible influence on future disease. Its follow-up over time would show greater certainty, and always including large numbers of study subjects and their controls to assert the relationship.
The ultimate example of a pathogenic dysbiosis and its eradication by manipulation of the microbiota is R-CDI. From this immediate evidence, we can jump to the already tested scenario of stool transplantation (or transfer) for the improvement of autism [22]. Between these two extremes, the relationship between the microbiota and almost every type of disease is raised.
The lack of antigenic stimuli due to decreased diversity and the alteration of what would be a “healthy” microbiome in the early stages of life, including gestation, have been linked to reduced immune efficiency and increased risk of disease or earlier onset of disease. These include atopic eczema [23,24], food allergies [25], asthma [26-28] and metabolic diseases as important as obesity [29,30] and type-1 diabetes [31-33], in the genesis of which, in addition to genetic susceptibility, there is a background of inflammation [34,35] or autoimmune aggression [36]. These “dysbioses” seem to be strongly influenced by the type of delivery (natural or cesarea) [37,38], the lack of maternal exposure during gestation to domestic animals, receiving antibiotics during this period or in the first months of life [39,40] and, of course, an artificial neonatal diet. What we would understand as a more “healthy” state would favor the development of “allergies” [28,41]. On the other hand, some of the relationships that have been established between infant dysbiosis and diseases have not been confirmed in observational studies in real life, as is the case of the use of antibiotics and obesity [42].
There are direct pathogenic aspects of the microbiota in the newborn and especially in the premature infant that pre-dispose to necrotizing enterocolitis, characterized by a pathological predominance of Enterobacterales and Staphylococcus with respect to Bifidobacterium and Bacteroidetes [43-45], contrary to what occurs in healthy children. Immune dysfunction against this dysbiosis is suspected as the pathogenesis [46]. Late-onset sepsis, which sometimes occurs, would be an example of bacterial translocation. The same bacteria isolated during sepsis have been found in the pre-sepsis intestine of these children [47,48].
Inflammatory bowel disease (Crohn’s disease and ulcerative colitis) shares the same suspected pathogenesis with the interaction of microbiota, genetic susceptibility and inadequate immune-inflammatory response [49-51]. Dysbiosis due to an increase of proteobacterias and decrease of Bacteroidetes and Firmicutes [52] is pointed to in its genesis, as well as the interaction between diet and microbiota and its consequences on intestinal permeability [50]. Irritable bowel syndrome, with such a high prevalence, is a permanent focus of studies looking for a direct relationship with the microbiota, without evidence that allows definitive treatment actions [53].
Obesity deserves a separate mention. At the moment, it is not clear what the profile of the microbiota attributed to obesity is, although in animal models it seems to be related to a higher proportion of Firmicutes, to the detriment of Bacteroidetes. On the other hand, bariatric/metabolic surgery changes the ratio between these two groups of bacteria, with predominance of Bacteroidetes [54-56]. These changes may be long-lasting and determinant in weight loss and metabolic comorbidity control. However, there is little evidence to support a causal or associative relationship between the microbiome and obesity. Although there is evidence that the gut micro-biome may affect the risk of adiposity-related comorbidities, such as type-2 diabetes and inflammation, there is no evidence that manipulation of the gut microbiome is an effective treatment for obesity [57-58].
Another field that opens a huge range of studies is the influence of the microbiota with neurogenesis, behavior and diseases of the nervous system through the so-called “gut-brain microbiome axis” and again the invitation to modify this “environmental” part of the individual to control the alterations of its genetic disposition [59]. Depression, autism, attention deficit disorder, schizophrenia, Parkinson’s disease and neuro-degenerative disorders, including Alzheimer’s disease, do not escape this relationship.
Evidence has shown bidirectional communication between the gut microbiome and the central nervous system, making it a target for ameliorating the development and progression of neurodegenerative diseases [60,61]. This communication would be mediated by the immune system, vagus nerve, enteric nervous system, neuroendocrine and circulatory systems [60]. In mouse models it has been observed that bacterial metabolites generate responses in neurotransmitters (serotonin, dopamine, noradrenaline, etc.) and in the immune system, regulating neurophysiological function and cognition [61]. Likewise, alterations in the gut microbiota have been associated with autism spectrum disorders, anxiety, depressive behaviors, impaired physical performance and motivation, as well as neurodegenerative diseases such as Alzheimer’s disease and patients with Parkinson’s disease) [62]. Aging, a key player in neurodegenerative diseases, shows that the gut microbiome of centenarians has a great similarity with those of young individuals.
On the other hand, the relationship of microorganisms and cancer has a long history focused on treatment. Today, the role that microbiota can play in direct or inducing and especially modulating causation through the immune system and the production of functional metabolites is recognized [63,64]. The concept of the immune-oncology-microbiota axis is created. Much of this relationship is still maintained with the intestinal microbiota (the colon harbors 97% of the bacterial microbiota and is the organ with the highest production of biologically active substances with immunomodulatory activity). The rest corresponds to bacterial microbiota of other organs.
Once again, a strictly intratumoral microbiome appears, found in more than thirty types of tumors [65,66] whose pathogenic role remains to be defined both in oncogenesis and in the interference in the response to chemotherapy or immuno-therapy, and therefore of a possible therapeutic interventionism. The specificity of the intratumoral microbiome is postulated as a localization or body mapping tool when investigating this microbiota in blood.
There are very few microorganisms recognized as directly oncogenic, also called oncomicrobes: Epstein Barr virus; HBV; HCV; Kaposi’s herpesvirus; HIV-1; human papillomavirus; HTL-1 virus; Opistorchis viverrini and Clonorchis sinensis, Schistosoma haematobium and Helicobacter pylori [67], but there are many others that could act as facilitators through their active metabolites or by immunomodulation [68-71]. In any case, we would be at a point that opens the way for the future, but still without clinically validated tools for therapeutic interventionism [65,66,72-77].
CAN THE MICROBIOME HELP DIAGNOSE DISEASE OR BE USEFUL IN DISEASE MONITORING? WHAT ARE THE INDICATIONS FOR MICROBIOME STUDIES IN THE CLINIC?
Microbiome analysis has the potential to revolutionize many aspects of healthcare, from diagnosis and treatment to prevention. However, it is still at a very early stage and further clinical research and technological developments are needed for full implementation. Therefore, at present, microbiome analysis should not be recommended in clinical practice as we lack the necessary knowledge to translate the results of its analysis into clinical decisions. Some relevant findings of recent research are schematically presented below:
Inflammatory Bowel Disease (IBD). The microbiome plays a role in the pathogenesis of IBD. Microbial biomarkers in blood and other body fluids are emerging as promising tools for the diagnosis and prognosis of IBD, although their clinical application is still under development [78].
Diagnosis of precancerous lesions. This is a hot area. There are numerous groups interested in developing microbiota-based diagnostic tools for colon cancer. For example, it has been observed that the abundance of Fusobacterium nucleatum is higher in patients with colorectal cancer than in controls [79]. Similarly, it has been observed that the determination of metabolites produced by anal bacteria can help identify subjects with precancerous lesions [80].
Predictor of response to immunotherapy in cancer. The role of the microbiome in response to immunotherapy is an area of growing interest in oncology. Studies in preclinical, observational models, and pilot clinical trials suggest that it is possible to predict the response to immunotherapy based on the composition of the microbiome, and to modify this response by microbiota transplantation. Further studies are needed to introduce this tool into the clinic [81,82].
Personalized Medicine. Host gene-microbiome interactions appear to influence the development of chronic multifactorial diseases such as type-1 diabetes, ulcerative colitis, and Crohn’s disease. Analysis of the microbiome could help identify subjects at risk for developing these diseases and become a tool for diagnosis and personalized medicine [83].
HOW IS THE INTESTINAL MICROBIOME STUDIED? WHAT LABORATORY REPORTS ARE ISSUED AND HOW SHOULD THEY BE INTERPRETED?
The study of the microbiome can be approached with different techniques. Typically, mass sequencing is performed after amplification of the gene encoding the 16S ribosomal subunit (16S rDNA for bacteria), or of the ITS1-2 intergenic space (for fungi) [84]. First, the total DNA of microorganisms from a clinical sample is obtained, then the chosen target is amplified by PCR, and finally mass sequencing is performed. After passing the quality filters of the process and discarding incomplete or chimeric sequences, each amplicon (DNA fragment amplified by PCR, approximately 300 base pairs – bp –) is assigned to a specific microorganism after matching it to databases with bioinformatics tools. The final step consists of assigning values of alpha diversity (total number of different taxa and balance in their abundance) and beta diversity (statistical differences in groups of subjects). This is the most common technique because of its low cost (approx. 50 €/sample in reagents) and relative simplicity, but in most cases it cannot discern the complete taxonomy, and generally only reaches genus without descending to species. The new massive sequencing platforms, such as PacBio®, perform sequences up to 1,500 bp, allowing a more accurate taxonomic assignment as they can read the complete 16S rDNA gene and reach the species level with high confidence.
There is a more complex approach called “shotgun” in which all the DNA is sequenced without a previous amplification step, but again with short sequences of 300 bp [84]. With this strategy we can identify all microorganisms, including viruses, and metabolism, virulence, or antibiotic resistance genes. However, it has as a disadvantage the difficulty in the bioinformatic reconstruction of the individual genomes of each microorganism.
Both with the amplification of 16RNA sequences and with a “shotgun” approach we can know the composition and abundance of each taxon in a sample in which we want to characterize the microbiome, with the limitation that the process has not been standardized.
Other study techniques collect the expression of genes (transcriptomics), the proteins synthesized (proteomics), the metabolites of the entire ecosystem (metabolome) and finally the connections between them (interactome). Current interest in the study of the gut microbiome is focused on metabolites of exclusive microbial production that also have an impact on health and disease such as SCFAs and trimethylamine N-oxide (TMAO)
It is important to emphasize that, despite technical advances, there is no consensus on the normality of the microbiota in its composition, and this prevents the preparation of a report to grant the degree of “normality”. There are diagnostic laboratories that make reports on the composition of the microbiota based on internal criteria of normality, but in no case do they have, for the moment, any validity for therapeutic decisions. These reports are only based on the abundance of each bacterial taxon, attributing to them a functionality that has not been proven, for example, they refer to the abundance of proteolytic bacteria only with the abundance of certain taxa. The problem with these reports is that they often have therapeutic implications, in many cases with antimicrobials, without prior consensus or evidence of their usefulness.
CAN THE MODULATON OF INTESTINAL MICROBIOME BE USED TO TREAT DISEASES? WHAT IS A FECAL TRANSPLANT/TRANSFER AND WHAT ARE ITS INDICATIONS?
Modulation of the intestinal microbiome has become a potential therapeutic target to treat putative diseases associated with its dysbiosis. Among the therapeutic interventions targeting the gut microbiome, fecal microbiota transplantation (FMT) has attracted the interest of the scientific and clinical communities. Its use has been documented in at least 85 diseases, with heterogeneous results [85].
FMT can be defined as the transfer of fecal matter from a healthy donor to a sick person, with the aim of restoring or modifying his or her microbiota. The procedure is performed by introducing the processed product from the feces of the healthy donor into the gastrointestinal tract of the recipient, either through colonoscopy [86], enema [87], nasoduodenal tube [88] or more recently, through orally administered capsules [89-91].
FMT is believed to have originated in 4th century China, where fecal material was administered orally to treat patients with diarrhea [92]. In the current medical literature, FMT was first used to treat pseudomembranous colitis in 1958 in Denver, USA [93]. Most of the clinical experience of FMT has originated in the treatment of R-CDI, where it has demonstrated high success rates (around 90%) in both randomized clinical trials and long case series [94,95]. The European Society for Clinical Microbiology and Infectious Diseases (ECCMID) treatment guidelines recommend fecal transplantation for R-CDI [96,97]. The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) guidelines [98] also include a strong recommendation for its use in second and subsequent episodes of R-CDI. R-CDI nonresponsive to standard therapies is the only indication approved by the US Food and Drug Administration (FDA) for FMT since 2013 [99].
Despite its high efficacy, the exact mechanisms by which FMT acts on R-CDI are not well understood. Some of the proposed mechanisms include direct competition of C. difficile with the administered microbiota, reconstitution of alpha diversity that helps prevent C. difficile colonization, changes in bile acid metabolism, and repair of the intestinal barrier by stimulating the mucosal immune system [100-102]. FMT in R-CDI has been limited by practical barriers such as donor selection, product preparation, storage, delivery, and in some cases, patient reluctance [88,103,104]. Stool donors should undergo periodic thorough controls in which the presence of pathogenic microorganisms is ruled out both in the stool and by serology.
Due to increasing clinical demand, the FMT has gradually evolved. The use of frozen material versus fresh stool was the first step in its modernization, allowing the creation of stool banks [94]. There are several ways of processing stool depending on the route of administration. For administration by colonoscopy, the stool is mixed with a solvent (water or saline), whereas nowadays it is most commonly administered in capsules. For this purpose, the stool is dissolved with a cryoprotectant, then freeze-dried and finally encapsulated with the resulting powder. The stool or its derivatives have a viability of 6 months but there are currently protocols that will enlarge this period.
The development of FMT in oral capsules has represented a great advance in the treatment and prevention of R-CDI, overcoming the main disadvantages of other routes of administration. Several studies have shown that encapsulated TFM has similar efficacy (around 90%) and produces fewer adverse events [89,91,95,105-108].
Current data suggest that FMT is a safe therapeutic method with few adverse effects, although its long-term outcomes have not been fully elucidated [109]. In a systematic review collecting FMT-related adverse effects reported over 20 years (2000-2020) and including 5,688 procedures [110], 19% of adverse events were found, of which the most frequent were diarrhea (10%), abdominal discomfort, pain, and cramping (7%). Serious adverse events related to FMT were reported in 1.4% of patients undergoing FMT. The mortality rate was low (0.13%), mostly as a result of aspiration pneumonia related to the route of FMT administration. This suggests that most FMT-related deaths could be avoided by paying attention to risks related to the route of administration. Of note, serious adverse effects related to FMT occurred in patients with mucosal barrier injury. Another recent review and meta-analysis with 5,099 patients showed that serious adverse events developed in less than 1% of patients [111].
In conclusion, FMT is proposed as a therapeutic action with great potential in the prevention or treatment of many diseases, although the only current clinical indication is the treatment of R-CDI. However, much effort is still needed to unravel the mechanisms of action and to further update the guidelines for FMT on an international scale. It is hoped that with advances in technology and knowledge, FMT will become a standardized treatment in which the current regimen will be replaced by well-defined microbial consortia, metabolites or laboratory-synthesized compounds that can be easily administered.
WHAT ARE PROBIOTICS, PREBIOTICS AND POSTBIOTICS AND WHAT ARE THEIR CLINICAL INDICATIONS?
Prebiotics are non-digestible dietary ingredients that facilitate intestinal health by stimulating the growth of beneficial bacteria such as bifidobacteria and lactobacilli. An example of these would be human milk oligosaccharides, unique for their high concentration in the human species, synthesized in the mammary gland, and the first prebiotics ingested by the newborn and infant. Like probiotics, their real benefit is controversial, although by favoring intestinal colonization by bifidobacteria, they could improve infant colic, prevent early constipation and intestinal infections. For this reason, the companies that manufacture them are incorporating them into milk formulas and as food supplements. Prebiotics are also present in foods rich in fibre.
Probiotics are live microorganisms that, when administered in adequate amounts, may offer significant health benefits to the host. They are found in fermented foods and can be purchased as dietary supplements. Although the actual benefits are controversial, they would contribute to the stability of the microbiota, strengthen the integrity of the intestinal barrier and enhance immune function. Lactecaseibacillus rhamnosus GG is the strain with the most evidence of therapeutic and/or preventive benefit for different types of diarrhea (community, traveler’s and post-antibiotic), functional digestive disorders, and immune boosting (against allergies and respiratory infections).
Postbiotics are metabolic products or cellular components of inactive microorganisms of the intestinal microbiota. An example is SCFA, metabolites formed in the colon after enzymatic degradation by the microbiota (Table 1). Their beneficial effects would be observed both at the intestinal level (inhibition of pathogens, barrier effect, increased intestinal transit) and as anti-inflammatory, immunomodulators and protectors of the intestinal barrier. Their use in the management of gastrointestinal disorders and their potential in improving general health are being increasingly investigated [21].
Research in this area is difficult due to the absence of strict regulation of their use. Facilitating their entry into the market without clear evidence of benefit is the multitude of different products and posologies. They have been studied in multiple indications, such as prevention of recurrences of IBD [112], recurrences of bacterial vaginosis [113], prevention of C. difficile diarrhea [114] or prevention of recurrent urinary tract infection [115]. There is great heterogeneity in the results and products investigated, resulting in a low quality of evidence, although the most recent investigations show promising data on the use of probiotics for the prevention of bacterial vaginosis [113] and recurrent urinary tract infection [115].
There is no firm evidence to recommend the use of prebiotics, probiotics or postbiotics in clinical practice. Even so, the prescription of probiotics has increased a lot and it is important to know their safety. Although adverse reactions have rarely been demonstrated, there are doubts as to whether their administration is safe. In theory, due to their composition, probiotics could be implicated in five types of adverse effects: 1) infectivity and/or pathogenicity as they are live products; 2) production of undesirable metabolites; 3) possibility of transmission of genes that confer resistance to antibiotics; 4) excessive immunostimulation or immunosuppression in sensitized individuals; and 5) adverse reaction associated with their excipients. In case of doubt, it should be taken into account which are the potential subjects at risk of suffering complications (Table 2).
Table 2.
Patients potentially at risk of complications derived from the use of probiotics [116].
| Immunocompromised (including severely malnourished and oncology patients) |
|---|
| Premature infants (*) |
| Neonates with severe pathology |
| Cardiopathies (valvular alterations and their replacement, history of endocarditis) |
| Pregnant women (*) |
| Patients in ICU (severe pathologies and central catheter carriers) |
| Patients undergoing surgery (*) |
| Severe risk of intestinal translocation (acute abdomen, intestinal fistula, neutropenia or severe risk of doing so due to chemotherapy or radiotherapy) |
| Administration of probiotics through jejunostomy |
| Concomitant administration of broad-spectrum antibiotics to which they are resistant (*) (Lactobacilli often have natural resistance to vancomycin) |
| Probiotics with high intestinal mucosal binding capacity or known pathogenicity |
(*) Relative risk. In general, their use is considered safe in the following groups.
WHAT IS THE INTEREST OF THE MICROBIOTA AND THE USE OF PROBIOTICS AND PREBIOTICS IN PEDIATRICS?
Microbial colonization of the digestive tract during infancy is an essential process for our life. It is very important that, in the immediate neonatal period, a healthy and healthy microbiota is established, which will maintain its beneficial effect in the child’s life, protecting him/her from intestinal infections and favoring his/her immune development [116]. The intestinal microbiome would have an important role in the maintenance of a healthy gut-brain axis.
The intestinal microbiota is fundamentally established in the perinatal period (between 22 weeks of gestation and 8 days of life), when the most important intestinal colonization of the individual occurs, especially during delivery. Although for years it seemed that the fetus developed in a sterile environment, DNA from different bacterial species has recently been detected in amniotic fluid, placenta, umbilical cord blood and meconium. This suggests that the microbiota may be initiated in gestation. The neonatal microbiome is very dynamic and changes from the initial intrauterine colonization until 3 years of life, when it becomes similar to that of the adult.
Several protective factors favor the establishment of a healthy microbiome. Thus, while vaginal delivery, maternal contact, and breastfeeding favor this physiological process, birth by cesarean section, artificial breastfeeding, and early exposure to antibiotics hinder it [117]. The following are some proven findings:
In vaginal delivery, the newborn is colonized by microorganisms from the maternal vaginal, fecal and cutaneous microbiota. It incorporates Escherichia coli, Staphylococcus, Streptococcus, and anaerobic bacteria of the Bacteroides and Bifidobacterium spp. type.
The continuous contact of the newborn with its mother and breastfeeding expose it to a large number of microorganisms that are also incorporated into its initial microbiota: Staphylococcus, Streptococcus, Serratia, Pseudomonas, Corynebacterium, and Propionobacterium.
Breast milk contains prebiotics that promote the growth of Bifidobacterium in the infant’s intestine.
In children born by cesarean section, intestinal colonization by the bacteria that appear to be most beneficial (Lactobacillus, Bifidobacterium, and Bacteroides) is delayed.
Artificially breastfed infants, compared to breastfed infants, have less Bifidobacterium which facilitates intestinal colonization by enterobacteria and the acquisition of antibiotic resistance genes.
Early exposure of the newborn to antibiotics alters the intestinal microbiota and can have permanent effects on the microbiome, especially on Bifidobacterium which sometimes persists for the first 12 months of life.
There is increasing interest in the use of probiotics and prebiotics in pediatrics, including in neonates and premature infants [118,119]. Many studies have shown some benefit in the prevention and/or treatment of digestive and nutritional diseases in children [120]. Recently, the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) [121,122] has published Clinical Practice Guidelines on the use of probiotics to unify criteria for the appropriate management of acute diarrhea, antibiotic-associated diarrhea, prevention and treatment of necrotizing enterocolitis of prematurity, H. pylori infection, and functional digestive disorders such as infant colic, functional abdominal pain, and functional constipation. Although there is a high level of agreement among the clinical practice recommendations of the Scientific Societies, there are also differences. Comparison of the studies is difficult due to their heterogeneity in terms of indications, doses, duration, and composition of the probiotics used, which makes it difficult to recommend them routinely.
In older children and adolescents, the use of probiotics is being evaluated, as in adults, for many other pathologies: allergic (allergic rhinitis), cutaneous (acne), nephrotic syndrome, endocrinological (obesity and diabetes), neurological diseases (TDH, autism), etc. In some cases, the results are encouraging, but for the moment there is insufficient information for its recommendation and routine use.
It is important to insist that the use of probiotics as treatment and/or prevention of specific diseases should be done in a personalized way, knowing well the product, its doses, benefits and risks, and the maximum time of administration. This is the only way to better understand the expected benefit of these products and others that will probably be available in the future.
WHAT ARE LIVE BIOTHERAPEUTIC AGENTS OR SYNTHETIC MICROBIOTAS?
Synthetic microbiota (SynCom) is a laboratory-created, structurally defined and/or controlled microbial community consisting of relatively few microorganisms that have been cultured and that acts as a substitution for the original functions and structure of the microbiome. These communities when created for therapeutic purposes are also known as “bio-therapeutic agents”.
The SynCom approach has the great advantage that we can manipulate this community by simply adding, deleting or substituting one or a few strains to achieve the desired functions. Furthermore, these manipulations can affect the genomes of the strains; for example, certain functions can be eliminated or enhanced by gene silencing or expression enhancement, respectively. Because SynCom member microorganisms can be cultured, the strains that comprise SynCom are ideal for dissecting the structural complexity and associated functions of the microbiota using reductionist approaches. Although many strains exist, the most commonly used synthetic microbiotas are of murine (ASF, OMM, GM15) or human (SIHUMI, SIM, MET-1, or B4PC2) origin [123, 124]. All of them need first to grow the microorganisms, stabilize them, and co-culture them so that all of them are represented and in previously established proportions.
The FDA authorized in April 2023 a product (VOWST®) containing encapsulated Firmicutes spores (between 1x106 and 3x107 CFU/ml) to prevent R-CDI in individuals 18 years of age and older after failure of antibacterial treatment [125]. Ingestion of the capsules (4 per day for 3 consecutive days) should be done 2-4 days after completing antimicrobial treatment for R-CDI. Their efficacy in clinical trials was demonstrated by lower R-CDI [12,45] compared to placebo (39.8%) (https://www.fda.gov/vaccines-blood-biologics/vowst). Earlier (November 2022) the FDA cleared a stool suspension (Rebyota®) from qualified donors to prevent R-CDI in individuals 18 years and older [124]. It is prepared for rectal administration, 150 ml containing between 1x108 and 5x1010 UCF/ml of fecal microorganisms including more than 1x105 CFU/mL of Bacteroides. It should be administered 24 to 72 hours after the last dose of antibiotic for R-CDI. Its efficacy in clinical trials was 70.6% versus 57.5% in the placebo group (https://www.fda.gov/vaccines-blood-biologics/vaccines/rebyota). The estimated price in the United States for one dose of VOWST® is $17,000 and that of Rebyota® is $9,000 [126].
WHAT ROLE DOES THE GUT MICROBIOME PLAY IN DRUG EFFICACY?
The microbiome can condition the pharmacokinetics and metabolism of drugs, especially those with an enterohepatic route of elimination, affecting their effectiveness, but also causing unexpected side effects [127]. The gut hosts the most abundant microbiota in our body, however, effects may also be at the local level following interaction with vaginal, skin, or respiratory tract microbiota. Altered pharmacokinetics of tacrolimus and other immunosuppressants have been reported [128], highlighting that these products are also of microbial origin. Although microorganisms can metabolize drugs, the main mechanism by which their action is affected is sequestration inside the microbial cell.
Another aspect to consider is the opposite, the impact of drugs on the microbiota. Many compounds, beyond antimicrobials, may have inhibitory activity by causing death or exerting a decelerating effect on metabolism, while others may be metabolic accelerators. In recent years, the study of omeprazole or metformin has focused on the intestinal microbial ecosystem [128,129]. Finally, the major focus of current research is how the microbiota conditions the response to oncological treatment, particularly immunosuppressive treatment. Thus, there are proposals for fecal transfer from immunomodulator responders to non-responders. In some cases, adequate responses have been achieved with this strategy.
WHAT IS THE FUTURE OF THE APPLICATION OF ARTIFICIAL INTELLIGENCE IN MICROBIOME RESEARCH AND ITS POTENTIAL ROLE IN THE CLINIC? ETHICAL CONSIDERATIONS
Research in relation to the microbiota, beyond FMT, is in full swing. Metabolites associated with the intestinal microbiota, such as TMAO or SCFAs, may have a potential therapeutic impact on the future of some diseases. Other potential strategies would come from the use of micro RNA (miRNA) that would allow the manipulation of the intestinal microbiome, hyaluronan, nanomedicine or extracellular vesicles [130]. Much of the knowledge about microbiota-host interactions comes from animal models. There are methodological complexities and manifest limitations inherent in translating reductionist animal models to complex human disease. Although it is a gateway to research and future treatments, at present there are no robust conclusions in either the diagnosis or treatment of diseases that have been linked to alterations in the microbiota. Therefore, great expectation has been created in the possible use of AI to facilitate the knowledge of the microbiome and its relationship with health and disease.
AI and in particular machine learning (machine-learning) and deep learning (deep-learning), is opening new frontiers in microbiota research. The use of these tools can improve the understanding, diagnosis and treatment of diseases related to the human microbiome [131]. AI allows the analysis of high volume and complex datasets by identifying patterns and associations limited to other traditional statistical methods, as well as assisting in the sequencing of metagenomic data by allowing the identification and classification of microbial species. These tools can be used to design predictive models and improve the diagnostic accuracy of some microbiota-related diseases. In addition, they can contribute to personalized treatments by improving knowledge of individual microbiome profiles. There are international experiences and networks to promote research and work on the identification of predictive and discriminatory “omics” features, the improvement of repeatability and comparability, the development of automation procedures, and the definition of priority areas for the development of novel machine learning methods targeting the microbiome [132].
Despite the potential of AI application in microbiota research, there are important challenges related to data quality and standardization, improving algorithms into applicable models that better handle the variability and complexity of microbiome data, combining them with other data such as genomic, proteomic and metabolomic data, and ensuring their ethical use in research. It would be important to start identifying potential ethical issues early on rather than waiting for problems to arise. In this sense, this new technology leads to try to answer the following points that can be generalized to the study of the microbiome [133,134]:
Personal identity: The microbiome of each individual is unique. If this microbiome, together with genes, conditions our susceptibility to disease and response to treatment, it can be considered part of our identity.
Privacy: The guarantee of confidentiality is fundamental to the practice of medicine and research. Therefore, in both clinical and research settings, everything related to confidentiality is fully applicable to the microbiome. Patients reasonably expect that their information will not be disclosed.
Ownership: If someone’s microbiome is unique and valuable, to whom does it belong? Do we have ownership rights over our microbiome because our body is its host? When knowledge from a microbiome study has commercial value, who should get the benefits?
Research with human subjects, creation of biobanks: Having treatments to prevent diseases requires advances in basic science and translational research including biobanks, sample banks and research with human subjects. In this respect, autonomy gives primacy to informed consent and privacy in the current ethics of all research, including therefore all microbiome research. But aside from the above basic consideration, questions arise about the rules and governance of biobanks and sample banks. Who should be given access to biological samples, what information should be shared, what projects should the samples support, and who should make these decisions?
The biomedical research community will need to answer these questions as microbiome research progresses, avoiding both exaggeration of the risks and ignorance of the real problems.
CONCLUSIONS
Although there is evidence of the possible association between the composition of the gut microbiota and numerous diseases, beyond those affecting the digestive tract, and the possible benefit on its modulation, it is still too early to establish general recommendations for action. Only FMT in the treatment of R-CDI has demonstrated unequivocal benefits, and in the rest of the diseases it is necessary to carry out extensive studies with ambitious designs that allow conclusions to be drawn. Part of the difficulty lies in the definition of what is a healthy microbiota (or state of eubiosis) or a diseased microbiota (state of dysbiosis), which is covered by the standardized application of laboratory techniques for its study and interpretation. Around this, decision making has arisen in the face of reports with catalogs of microorganisms that are part of the microbiota that in many cases favor the use of probiotics, prebiotics and postbiotics as a medicine of complacency with the patient. Efforts should be made by scientific societies and regulatory agencies in the analysis of the evidence and the promotion of research by funding agencies. The application of AI, the reduction in the cost of laboratory techniques, and work in multi- and multidisciplinary teams can facilitate this work. Also, and given that action on the microbiome can be considered part of personalized medicine, it is necessary to clarify numerous questions that arise from the ethical point of view. On this depends a better knowledge of the microbiome and the role of prevention and treatment of possible diseases associated with the composition of the microbiota, without falling into a pseudo-medicine that does not add value to scientific progress.
FUNDING
Nothing to declare
CONFLICTS OF INTEREST
The authors declare the absence of conflicts of interest.
References
- 1.Graham DB, Xavier RJ. Conditioning of the immune system by the microbiome. Trends Immunol. 2023. Jul;44(7):499-511. doi: 10.1016/j.it.2023.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Whipps JM, Lewis K, Cooke RC. Mycoparasitism and plant disease control 161–187. In: Burge, NM (editor), Fungi in Biological Control Systems. Manchester University Press; 1988. P. 176. [Google Scholar]
- 3.Malakar S, Sutaoney P, Madhyastha H, Shah K, Chauhan NS, Banerjee P. Understanding gut microbiome-based machine learning platforms: A review on therapeutic approaches using deep learning. Chem Biol Drug Des. 2024. Mar;103(3):e14505. doi: 10.1111/cbdd.14505. [DOI] [PubMed] [Google Scholar]
- 4.Heintz-Buschart A, Wilmes P. Human gut microbiome: function matters. Trends in microbiology 2018; 26:563-574. 10.1016/j.tim.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1-10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 6.Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical frontier. Gut 2016; 65:330-339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol 2015; 21(29): 8787-8803 doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh A, Bäckhed F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Mol cell 2020; 78:584-596. doi: 10.1016/j.molcel.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011. Oct 7;334(6052):105-8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurilshikov A, Wijmenga C, Fu J, Zhernakova A. Host Genetics and Gut Microbiome: Challenges and Perspectives. Trends Immunol. 2017;38:633-647. doi: 10.1016/j.it.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients.2019; 11:1613. doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-30. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-70. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tandon D, Haque MM, Shaikh S, Dubey AK, Mande SS. A snapshot of gut microbiota of an adult urban population from Western region of India. PLoS One. 2018;13:e0195643. doi: 10.1371/journal.pone.0195643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YS, Unno T, Kim BY, Park MS. Sex Differences in Gut Microbiota. World J Mens Health. 2020;38:48-60. doi: 10.5534/wjmh.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401-12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 17.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015; 22:971-82. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Kong C, Gao R, Yan X, Huang L, Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2019;60:175-184. doi: 10.1016/j.nut.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, et al. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients. 2019. Oct 7;11(10):2393. doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, et al. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front Nutr. 2019;6:47. doi: 10.3389/fnut.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunningham M, Azcarate-Peril MA, Barnard A, Benoit V, Grimaldi R, Guyonnet D, et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021. Aug;29(8):667-685. doi: 10.1016/j.tim.2021.01.003. Epub 2021 Feb 4. PMid: . [DOI] [PubMed] [Google Scholar]
- 22.Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017. Jan 23;5(1):10. doi: 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Karlsson C, Olsson C, Adlerberth I, Wold AE, Strachan DP, et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J Allergy Clin Immunol. 2008. Jan;121(1):129-34. doi: 10.1016/j.jaci.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Forno E, Onderdonk AB, McCracken J, Litonjua AA, Laskey D, Delaney ML, et al. Diversity of the gut microbiota and eczema in early life. Clin Mol Allergy. 2008. Sep 22;6:11. doi: 10.1186/1476-7961-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua X, Goedert JJ, Pu A, Yu G, Shi J. Allergy associations with the adult fecal microbiota: Analysis of the American Gut Project. EBioMedicine. 2015. Nov 27;3:172-179. doi: 10.1016/j.ebiom.2015.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016. Oct;22(10):1187-1191. doi: 10.1038/nm.4176. Epub 2016 Sep 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015. Jun 23;6:7320. doi: 10.1038/ncomms8320. [DOI] [PubMed] [Google Scholar]
- 28.Kemter AM, Nagler CR. Influences on allergic mechanisms through gut, lung, and skin microbiome exposures. J Clin Invest. 2019. Feb 25;129(4):1483-1492. doi: 10.1172/JCI124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008. Mar;87(3):534-8. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 30.Scheepers LE, Penders J, Mbakwa CA, Thijs C, Mommers M, Arts IC. The intestinal microbiota composition and weight development in children: the KOALA Birth Cohort Study. Int J Obes (Lond). 2015. Jan;39(1):16-25. doi: 10.1038/ijo.2014.178. [DOI] [PubMed] [Google Scholar]
- 31.Brown CT, Davis-Richardson AG, Giongo A, Gano KA, Crabb DB, Mukherjee N, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One. 2011;6(10):e25792. doi: 10.1371/journal.pone.0025792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011. Jan;5(1):82-91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunne JL, Triplett EW, Gevers D, Xavier R, Insel R, Danska J, et al. The intestinal microbiome in type 1 diabetes. Clin Exp Immunol. 2014. Jul;177(1):30-7. doi: 10.1111/cei.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agustí A, García-Pardo MP, López-Almela I, Campillo I, Maes M, Romaní-Pérez M, et al. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front Neurosci. 2018. Mar 16;12:155. doi: 10.3389/fnins.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knip M, Honkanen J. Modulation of Type 1 Diabetes Risk by the Intestinal Microbiome. Curr Diab Rep. 2017. Sep 23;17(11):105. doi: 10.1007/s11892-017-0933-9. [DOI] [PubMed] [Google Scholar]
- 37.Khashan AS, Kenny LC, Lundholm C, Kearney PM, Gong T, Almqvist C. Mode of obstetrical delivery and type 1 diabetes: a sibling design study. Pediatrics. 2014. Sep;134(3):e806-13. doi: 10.1542/peds.2014-0819. [DOI] [PubMed] [Google Scholar]
- 38.Pallasmaa Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011. Jun 15;474(7351):307-17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livanos AE, Greiner TU, Vangay P, Pathmasiri W, Stewart D, McRitchie S, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol. 2016. Aug 22;1(11):16140. doi: 10.1038/nmicrobiol.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raymond SL, Rincon JC, Wynn JL, Moldawer LL, Larson SD. Impact of Early-Life Exposures to Infections, Antibiotics, and Vaccines on Perinatal and Long-term Health and Disease. Front Immunol. 2017. Jun 23;8:729. doi: 10.3389/fimmu.2017.00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez FD, Vercelli D. Asthma. Lancet. 2013. Oct 19;382(9901):1360-72. doi: 10.1016/S0140-6736(13)61536-6. Epub 2013 Sep 13. [DOI] [PubMed] [Google Scholar]
- 42.Gerber JS, Bryan M, Ross RK, Daymont C, Parks EP, Localio AR, et al. Antibiotic Exposure During the First 6 Months of Life and Weight Gain During Childhood. JAMA. 2016. Mar 22-29;315(12):1258-65. doi: 10.1001/jama.2016.2395. [DOI] [PubMed] [Google Scholar]
- 43.Madan JC, Salari RC, Saxena D, Davidson L, O’Toole GA, Moore JH, et al. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012. Nov;97(6):F456-62. doi: 10.1136/fetalneonatal-2011-301373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mai V, Torrazza RM, Ukhanova M, Wang X, Sun Y, Li N, et al. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS One. 2013;8(1):e52876. doi: 10.1371/journal.pone.0052876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cernada M, Bäuerl C, Serna E, Collado MC, Martínez GP, Vento M. et al. Sepsis in preterm infants causes alterations in mucosal gene expression and microbiota profiles compared to non-septic twins. Sci Rep. 2016. May 16;6:25497. doi: 10.1038/srep25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collado MC, Cernada M, Neu J, Collado MC, Cernada M, Neu J, Pérez-Martínez G. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res. 2015. Jun;77(6):726-31. doi: 10.1038/pr.2015.54. [DOI] [PubMed] [Google Scholar]
- 47.Carl MA, Ndao IM, Springman AC, et al. Sepsis from the gut: the enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin Infect Dis. 2014. May;58(9):1211-8. doi: 10.1093/cid/ciu084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart CJ, Embleton ND, Marrs ECL, mith DP, Fofanova T, Nelson A, et al. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome. 2017. Jul 12;5(1):75. doi: 10.1186/s40168-017-0295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002. Aug 8;347(6):417-29. doi: 10.1056/NEJMra020831 [DOI] [PubMed] [Google Scholar]
- 50.Llewellyn SR, Britton GJ, Contijoch EJ, Vennaro OH, Mortha A, Colombel JF, et al. Interactions Between Diet and the Intestinal Microbiota Alter Intestinal Permeability and Colitis Severity in Mice. Gastroenterology. 2018. Mar;154(4):1037-1046.e2. doi: 10.1053/j.gastro.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011. Jun 15;474(7351):307-17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity. 2014. Jun 19;40(6):843-54. doi: 10.1016/j.immuni.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev. 2017. Nov 8;81(4):e00036-17. doi: 10.1128/MMBR.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hjorth MF, Blædel T, Bendtsen LQ, Lorenzen JK, Holm JB, Kiilerich P, et al. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int J Obes (Lond). 2019. Jan;43(1):149-157. doi: 10.1038/s41366-018-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy R, Tsai P, Jüllig M, Liu A, Plank L, Booth M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes Surg. 2017. Apr;27(4):917-925. doi: 10.1007/s11695-016-2399-2. [DOI] [PubMed] [Google Scholar]
- 56.Wang FG, Bai RX, Yan WM, Yan M, Dong LY, Song MM. Differential composition of gut microbiota among healthy volunteers, morbidly obese patients and post-bariatric surgery patients. Exp Ther Med. 2019. Mar;17(3):2268-2278. doi: 10.3892/etm.2019.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathur R, Barlow GM. Obesity and the microbiome. Expert Rev Gastroenterol Hepatol. 2015;9(8):1087-99. doi: 10.1586/17474124.2015.1051029. [DOI] [PubMed] [Google Scholar]
- 58.Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab. 2015. Sep;26(9):493-501. doi: 10.1016/j.tem.2015.07.002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cenit MC, Sanz Y, Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. 2017. Aug 14;23(30):5486-5498. doi: 10.3748/wjg.v23.i30.5486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loh JS, Mak WQ, Tan LKS, Ng CX, Chan HH, Yeow SH, et al. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. 2024. Feb 16;9(1):37. doi: 10.1038/s41392-024-01743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sittipo P, Choi J, Lee S, Lee YK. The function of gut microbiota in immune-related neurological disorders: a review. J Neuroinflammation. 2022. Jun 15;19(1):154. doi: 10.1186/s12974-022-02510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Socała K, Doboszewska U, Szopa A, Serefko A, Włodarczyk M, Zielińska A, et al. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res. 2021. Oct;172:105840. doi: 10.1016/j.phrs.2021.105840. [DOI] [PubMed] [Google Scholar]
- 63.Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021. Mar 26;371(6536):eabc4552. doi: 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garrett WS. Cancer and the microbiota. Science. 2015. Apr 3;348(6230):80-6. doi: 10.1126/science.aaa4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020. May 29;368(6494):973-980. doi: 10.1126/science.aay9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020. Mar;579(7800):567-574. doi: 10.1038/s41586-020-2095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt B):1-441. PMid: . [PMC free article] [PubMed] [Google Scholar]
- 68.Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J, et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature. 2020. Apr 580(7802):269-273. doi: 10.1038/s41586-020-2080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science. 2019. Feb 15;363(6428):eaar7785. doi: 10.1126/science.aar7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barrett M, Hand CK, Shanahan F, Murphy T, O’Toole PW. Mutagenesis by Microbe: the Role of the Microbiota in Shaping the Cancer Genome. Trends Cancer. 2020. Apr;6(4):277-287. doi: 10.1016/j.trecan.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 71.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000. Jan 7;100(1):57-70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 72.Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell. 2019. Feb 21;176(5):998-1013.e16. doi: 10.1016/j.cell.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017. Sep 15;357(6356):1156-1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017. Dec 15;358(6369):1443-1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, Kim JI, et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019. Oct;574(7777):264-267. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018. Apr;8(4):403-416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parhi L, Alon-Maimon T, Sol A, Nejman D, Shhadeh A, Fainsod-Levi T, et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 2020. Jun 26;11(1):3259. doi: 10.1038/s41467-020-16967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dubinsky M, Braun J. Diagnostic and Prognostic Microbial Bio-markers in Inflammatory Bowel Diseases. Gastroenterology. 2015. Oct;149(5):1265-1274.e3. doi: 10.1053/j.gastro.2015.08.006. Epub 2015 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao L, Chen Z, et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018. Apr 11;6(1):70. doi: 10.1186/s40168-018-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serrano-Villar S, Tincati C, Raju SC, Sáenz JS, Moreno E, Bargiela R, et al. Microbiome-derived cobalamin and succinyl-CoA as biomarkers for improved screening of anal cancer. Nat Med. 2023. Jul;29(7):1738-1749. doi: 10.1038/s41591-023-02407-3. Epub 2023 Jul 18. Erratum In: Nat Med. 2024 Jan;30(1):303. PMid: . [DOI] [PubMed] [Google Scholar]
- 81.McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019. Feb;20(2):e77-e91. doi: 10.1016/S1470-2045(18)30952-5. [DOI] [PubMed] [Google Scholar]
- 82.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021. Feb 5;371(6529):602-609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 83.Virgin HW, Todd JA. Metagenomics and personalized medicine. Cell. 2011. Sep 30;147(1):44-56. doi: 10.1016/j.cell.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alarcón Cavero T, D’Auria G, Delgado Palacio S, Del Campo Moreno, R, Ferrer Martínez, M. Microbiota. 59. Del Campo Moreno R (coordinadora). Procedimientos en Microbiología Clínica. Cercenado E, Cantón R (editores). Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC). 2016. [Google Scholar]
- 85.Wang Y, Zhang S, Borody TJ, Zhang F. Encyclopedia of fecal micro-biota transplantation: a review of effectiveness in the treatment of 85 diseases. Chin Med J (Engl). 2022;135(16):1927-39. doi: 10.1097/CM9.0000000000002339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of Oral Capsule-vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2017;318(20):1985-93. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients With Recurrent Clostridium difficile Infection: A Randomized Clinical Trial. JAMA. 2016;315(2):142-9. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 88.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013. Jan 31;368(5):407-15. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 89.Reigadas E, Bouza E, Olmedo M, Vazquez-Cuesta S, Villar-Gomara L, Alcala L, et al. Faecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection: Experience with Lyophilized Oral Capsules. J Hosp Infect. 2020. Jun;105(2):319-324. doi: 10.1016/j.jhin.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 90.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772-8. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 91.Staley C, Hamilton MJ, Vaughn BP, Graiziger CT, Newman KM, Kabage AJ, et al. Successful Resolution of Recurrent Clostridium difficile Infection using Freeze-Dried, Encapsulated Fecal Microbiota; Pragmatic Cohort Study. Am J Gastroenterol. 2017;112(6):940-7. doi: 10.1038/ajg.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang F, Luo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107(11):1755. doi: 10.1038/ajg.2012.251 [DOI] [PubMed] [Google Scholar]
- 93.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44(5):854-9. PMid: [PubMed] [Google Scholar]
- 94.Quraishi MN, Widlak M, Bhala N, Moore D, Price M, Sharma N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. 2017;46(5):479-93. doi: 10.1111/apt.14201. [DOI] [PubMed] [Google Scholar]
- 95.Ramai D, Zakhia K, Fields PJ, Ofosu A, Patel G, Shahnazarian V, Lai JK, Dhaliwal A, Reddy M, Chang S. Fecal Microbiota Transplantation (FMT) with Colonoscopy Is Superior to Enema and Nasogastric Tube While Comparable to Capsule for the Treatment of Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2021. Feb;66(2):369-380. doi: 10.1007/s10620-020-06185-7. [DOI] [PubMed] [Google Scholar]
- 96.Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20 Suppl 2:1-26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 97.van Prehn J, Reigadas E, Vogelzang EH, Bouza E, Hristea A, Guery B, et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. 2021;27 Suppl 2:S1-s21. doi: 10.1016/j.cmi.2021.09.038. [DOI] [PubMed] [Google Scholar]
- 98.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-94. doi: 10.1093/cid/ciy149. [DOI] [PubMed] [Google Scholar]
- 99.U.S. Food and Drug Administration . Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Micro-biota for Transplantation to Treat Clostridium difficile Infection. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-regarding-investigational-new-drug-requirements-use-fecal-microbiota.
- 100.Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13(9):508-16. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Seekatz AM, Aas J, Gessert CE, Rubin TA, Saman DM, Bakken JS, et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio. 2014;5(3):e00893-14. doi: 10.1128/mBio.00893-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seekatz AM, Rao K, Santhosh K, Young VB. Dynamics of the fecal microbiome in patients with recurrent and nonrecurrent Clostridium difficile infection. Genome Med. 2016;8(1):47. doi: 10.1186/s13073-016-0298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58(11):1515-22. doi: 10.1093/cid/ciu135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moayyedi P, Yuan Y, Baharith H, Ford AC. Faecal microbiota transplantation for Clostridium difficile-associated diarrhoea: a systematic review of randomised controlled trials. Med J Aust. 2017;207(4):166-72. doi: 10.5694/mja17.00295. [DOI] [PubMed] [Google Scholar]
- 105.Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection. 2012;40(6):643-8. [DOI] [PubMed] [Google Scholar]
- 106.Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92(2):117-27. doi: 10.1007/s15010-012-0307-9. [DOI] [PubMed] [Google Scholar]
- 107.Wang S, Xu M, Wang W, Cao X, Piao M, Khan S, et al. Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PLoS One. 2016;11(8):e0161174. doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iqbal U, Anwar H, Karim MA. Safety and efficacy of encapsulated fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review. Eur J Gastroenterol Hepatol. 2018;30(7):730-4. doi: 10.1097/MEG.0000000000001147. [DOI] [PubMed] [Google Scholar]
- 109.Ooijevaar RE, van Nood E, Goorhuis A, Terveer EM, van Prehn J, Verspaget HW, et al. Ten-Year Follow-Up of Patients Treated with Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection from a Randomized Controlled Trial and Review of the Literature. Microorganisms. 2021;9(3). doi: 10.3390/microorganisms9030548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marcella C, Cui B, Kelly CR, Ianiro G, Cammarota G, Zhang F. Systematic review: the global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol Ther. 2021;53(1):33-42. doi: 10.1111/apt.16148. [DOI] [PubMed] [Google Scholar]
- 111.Rapoport EA, Baig M, Puli SR. Adverse events in fecal microbiota transplantation: a systematic review and meta-analysis. Ann Gastroenterol. 2022;35(2):150-63. doi: 10.1111/apt.16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, Chen N, Niu F, Li Y, Guo K, Shang X, et al. Probiotics therapy for adults with diarrhea-predominant irritable bowel syndrome: a systematic review and meta-analysis of 10 RCTs. Int J Colorectal Dis. 2022. Nov;37(11):2263-2276. doi: 10.1007/s00384-022-04261-0. [DOI] [PubMed] [Google Scholar]
- 113.Chieng WK, Abdul Jalal MI, Bedi JS, Zainuddin AA, Mokhtar MH, Abu MA, et al. Probiotics, a promising therapy to reduce the recurrence of bacterial vaginosis in women? a systematic review and meta-analysis of randomized controlled trials. Front Nutr. 2022. Sep 20;9:938838. doi: 10.3389/fnut.2022.938838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Valdés-Varela L, Gueimonde M, Ruas-Madiedo P. Probiotics for Prevention and Treatment of Clostridium difficile Infection. Adv Exp Med Biol. 2018;1050:161-176. doi: 10.1007/978-3-319-72799-8_10. [DOI] [PubMed] [Google Scholar]
- 115.Gupta V, Mastromarino P, Garg R. Effectiveness of Prophylactic Oral and/or Vaginal Probiotic Supplementation in the Prevention of Recurrent Urinary Tract Infections: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis. 2024. May 15;78(5):1154-1161. doi: 10.1093/cid/ciad766. PMid: . [DOI] [PubMed] [Google Scholar]
- 116.Alvarez Calatayud G, Leis Trabazo R, Diaz Martin JJ. Modulación de la microbiota intestinal. Uso de probióticos y prebióticos en Pediatría. Protocolos. Asociación Española de Pediatria. 2022. Available at: https://www.aeped.es/sites/default/files/documentos/39_microbiota.pdf
- 117.Diez-Delgado J. Probióticos y Prebióticos en el periodo peri-natal. Evidencia científica. An Microbiota Probióticos Prebióticos.2024;5(1):153-157. [Google Scholar]
- 118.De Veaux A, Ryou J, Dantas G, Warner BB, Tarr PI. Microbiome-targeting therapies in the neonatal intensive care unit: safety and efficacy. Gut Microbes.2023;15(1):2221758. doi: 10.1080/19490976.2023.2221758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiruvolu A, Hendrikson H, Hanson R, Reedy A, Reis J, Desai S, et al. Effects of prophylactic probiotics supplementation on infants born very preterm or very low birth weight. J Perinatol.2023;43(5): 635-41.doi: 10.1038/s41372-023-01657-w. [DOI] [PubMed] [Google Scholar]
- 120.Diaz Martin J. Evidencia científica sobre el empleo de probióticos en Gastroenterología Pediátrica. Guias de práctica clínica. An Microbiota Probióticos Prebióticos 2024;5(1):79-82. [Google Scholar]
- 121.Szajewska H, Berni Canani R, Domelloof M, et al. ; ESPGHAN Special Interest Group on Gut Microbiota and Modifications. Probiotics for the management of pediatric gastrointestinal disorders: Position Paper of the ESPGHAN Special Interest Group on Gut Microbiota end Modifications. J Pediatr Gstroenterol Nutr. 2023; 76(2):232-247. doi: 10.1097/MPG.0000000000003633. [DOI] [PubMed] [Google Scholar]
- 122.Directrices mundiales de la organización Mundial de Gastroenterología Probióticos y prebióticos . Avalaible at: https://www.world-gastroenterology.org/guidelines/probiotics-and-prebiotics-prebiotics-and-prebiotics-spanish. Feb 2023.
- 123.Bolsega S, Bleich A, Basic M. Synthetic Microbiomes on the Rise-Application in Deciphering the Role of Microbes in Host Health and Disease. Nutrients. 2021. Nov 21;13(11):4173. doi: 10.3390/nu13114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Torres-Carrillo N, Martínez-López E, Torres-Carrillo NM, López-Quintero A, Moreno-Ortiz JM, González-Mercado A, et al. Pharmacomicrobiomics and Drug-Infection Interactions: The Impact of Commensal, Symbiotic and Pathogenic Microorganisms on a Host Response to Drug Therapy. Int J Mol Sci. 2023. Dec 4;24(23):17100. doi: 10.3390/ijms242317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anonimous . Live fecal microbiota (Rebyota) for prevention of CDI recurrence. Med Lett Drugs Ther. 2023. Mar 6;65(1671):35-36. doi: 10.58347/tml.2023.1671b. [DOI] [PubMed] [Google Scholar]
- 126.Anonimous . Live fecal microbiota oral capsules (Vowst) for prevention of CDI recurrence. Med Lett Drugs Ther. 2023; 65:81-82. doi: 10.58347/tml.2023.1677a. [DOI] [PubMed] [Google Scholar]
- 127.Manes A, Di Renzo T, Dodani L, Reale A, Gautiero C, Di Lauro M, et al. Pharmacomicrobiomics of Classical Immunosuppressant Drugs: A Systematic Review. Biomedicines. 2023. Sep 18;11(9):2562. doi: 10.3390/biomedicines11092562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020. Aug;69(8):1510-1519. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang Y, An Y, Dong Y, Chu Q, Wei J, Wang B, et al. Fecal micro-biota transplantation: no longer cinderella in tumour immuno-therapy. EBioMedicine. 2024. Feb;100:104967. doi: 10.1016/j.ebiom.2024.104967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gebrayel P, Nicco C, Al Khodor S, Bilinski J, Caselli E, Comelli EM, et al. Microbiota medicine: towards clinical revolution. J Transl Med. 2022;20(1):111. doi: 10.1186/s12967-022-03296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hernández Medina R, Kutuzova S, Nielsen KN, Johansen J, Hansen LH, Nielsen M, et al. Machine learning and deep learning applications in microbiome research. ISME Commun. 2022;2(1):98. doi: 10.1038/s43705-022-00182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.D’Elia D, Truu J, Lahti L, Berland M, Papoutsoglou G, Ceci M, et al. Advancing microbiome research with machine learning: key findings from the ML4Microbiome COST action. Front Microbiol. 2023. Sep 25;14:1257002. doi: 10.3389/fmicb.2023.1257002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rhodes R. Ethical issues in microbiome research and medicine. BMC Med. 2016. Oct 12;14(1):156. doi: 10.1186/s12916-016-0702-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ejtahed HS, Parsa M, Larijani B. Ethical challenges in conducting and the clinical application of human microbiome research. J Med Ethics Hist Med. 2023. Jul 7;16:5. doi: 10.18502/jmehm.v16i5.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]


