Abstract
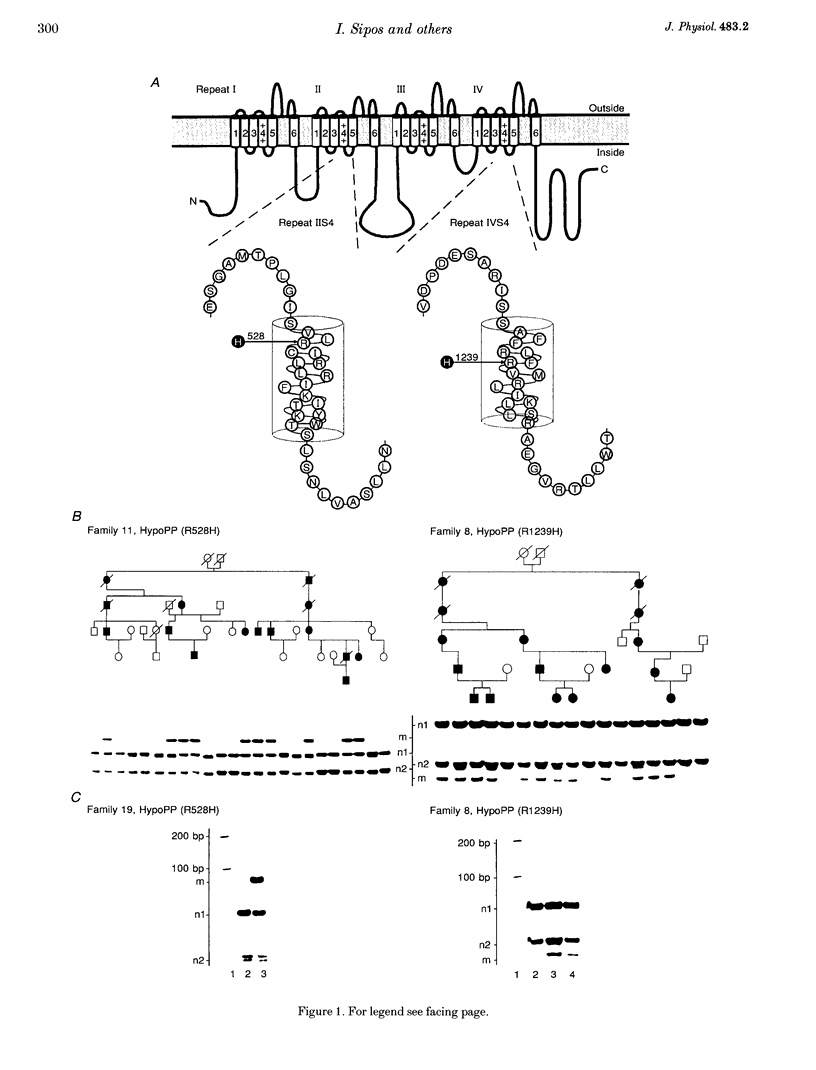
1. Mutations in the gene encoding the alpha 1-subunit of the skeletal muscle dihydropyridine (DHP) receptor are responsible for familial hypokalaemic periodic paralysis (HypoPP), an autosomal dominant muscle disease. We investigated myotubes cultured from muscle of patients with arginine-to-histidine substitutions in putative voltage sensors, IIS4 (R528H) and IVS4 (R1239H), of the DHP receptor alpha 1-subunit. 2. Analysis of the messenger ribonucleic acid (mRNA) in the myotubes from such patients indicated transcription from both the normal and mutant genes. 3. In control myotubes, the existence of the slow L-type current and of two rapidly activating and inactivating calcium current components (T-type with a maximum at about -20 mV and 'third type' with a maximum at +10 to +20 mV) was confirmed. In the myotubes from patients with either mutation, the third-type current component was seen more frequently and, on average, with larger amplitude. 4. In myotubes with the IVS4 mutation (R1239H) the maximum L-type current density was smaller than control (-0.53 +/- 0.31 vs. -1.41 +/- 0.71 pA pF-1). The voltage dependence of activation was normal, and hyperpolarizing prepulses to -120 mV for 20 s did not increase the reduced current amplitude during test pulses. 5. In myotubes with the IIS4 mutation (R528H) the L-type current-voltage relation, determined at a holding potential of -90 mV, was normal. However, the voltage dependence of inactivation was shifted by about 40 mV to more negative potentials (voltage at half-maximum inactivation, V1/2 = -41.5 +/- 8.2 vs. -4.9 +/- 4.3 mV in normal controls).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


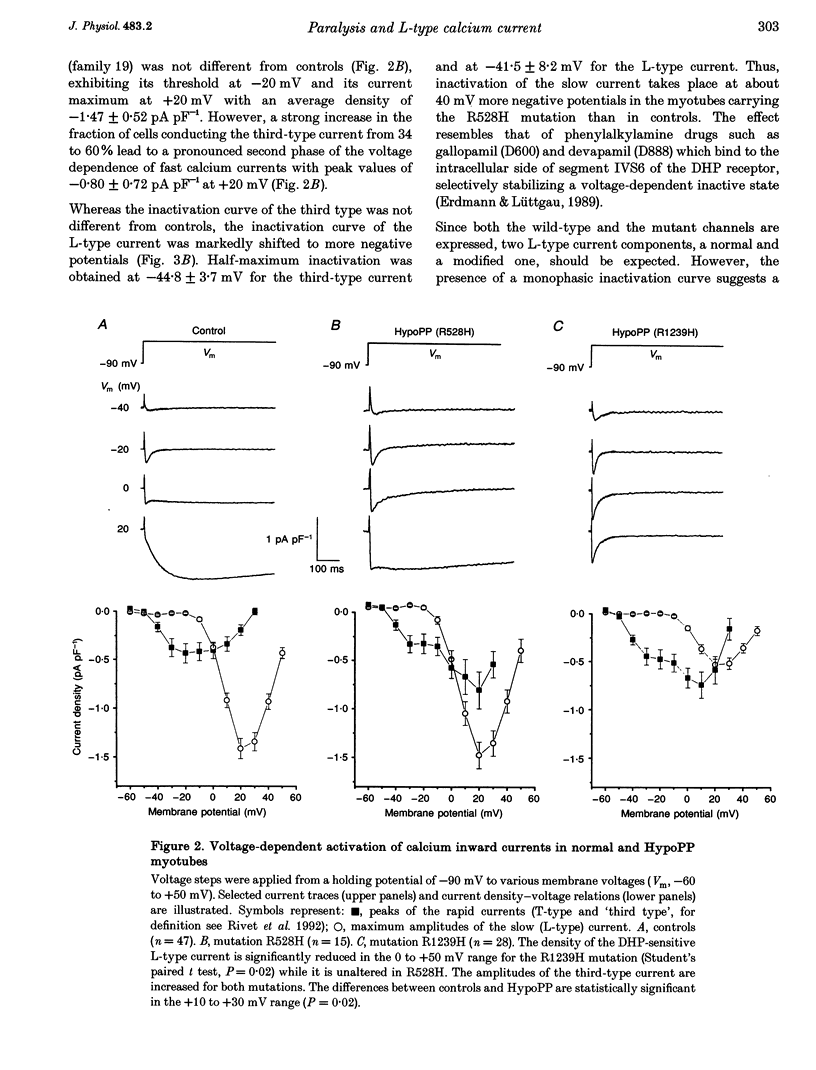
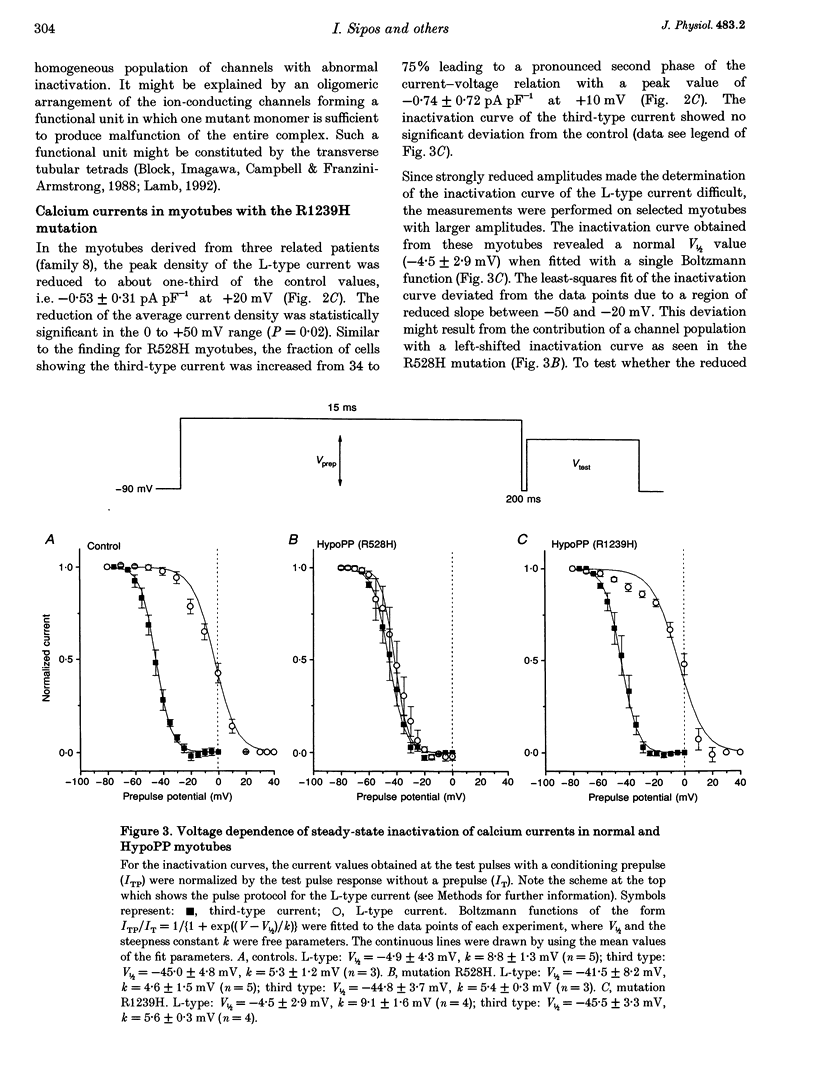


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmeier H., Mutz J. V., Seewald M. J., Melzner I., Rüdel R. Specific modifications of the membrane fatty acid composition of human myotubes and their effects on the muscular sodium channels. Biochim Biophys Acta. 1993 Jan 18;1145(1):8–14. doi: 10.1016/0005-2736(93)90375-a. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Striessnig J. Receptor sites for Ca2+ channel antagonists. Trends Pharmacol Sci. 1992 Jun;13(6):256–262. doi: 10.1016/0165-6147(92)90079-l. [DOI] [PubMed] [Google Scholar]
- Chahine M., George A. L., Jr, Zhou M., Ji S., Sun W., Barchi R. L., Horn R. Sodium channel mutations in paramyotonia congenita uncouple inactivation from activation. Neuron. 1994 Feb;12(2):281–294. doi: 10.1016/0896-6273(94)90271-2. [DOI] [PubMed] [Google Scholar]
- Erdmann R., Lüttgau H. C. The effect of the phenylalkylamine D888 (devapamil) on force and Ca2+ current in isolated frog skeletal muscle fibres. J Physiol. 1989 Jun;413:521–541. doi: 10.1113/jphysiol.1989.sp017667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D., Melzer W., Pohl B., Zöllner P. Modulation of calcium current gating in frog skeletal muscle by conditioning depolarization. J Physiol. 1992 Nov;457:639–653. doi: 10.1113/jphysiol.1992.sp019399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Vale-Santos J., Jurkat-Rott K., Reboul J., Plassart E., Rime C. S., Elbaz A., Heine R., Guimarães J., Weissenbach J. Mapping of the hypokalaemic periodic paralysis (HypoPP) locus to chromosome 1q31-32 in three European families. Nat Genet. 1994 Mar;6(3):267–272. doi: 10.1038/ng0394-267. [DOI] [PubMed] [Google Scholar]
- Heine R., Pika U., Lehmann-Horn F. A novel SCN4A mutation causing myotonia aggravated by cold and potassium. Hum Mol Genet. 1993 Sep;2(9):1349–1353. doi: 10.1093/hmg/2.9.1349. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Biel M., Flockerzi V. Molecular basis for Ca2+ channel diversity. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K., Lehmann-Horn F., Elbaz A., Heine R., Gregg R. G., Hogan K., Powers P. A., Lapie P., Vale-Santos J. E., Weissenbach J. A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet. 1994 Aug;3(8):1415–1419. doi: 10.1093/hmg/3.8.1415. [DOI] [PubMed] [Google Scholar]
- Lamb G. D. DHP receptors and excitation-contraction coupling. J Muscle Res Cell Motil. 1992 Aug;13(4):394–405. doi: 10.1007/BF01738035. [DOI] [PubMed] [Google Scholar]
- Ptácek L. J., Tawil R., Griggs R. C., Engel A. G., Layzer R. B., Kwieciński H., McManis P. G., Santiago L., Moore M., Fouad G. Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell. 1994 Jun 17;77(6):863–868. doi: 10.1016/0092-8674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Rivet M., Cognard C., Imbert N., Rideau Y., Duport G., Raymond G. A third type of calcium current in cultured human skeletal muscle cells. Neurosci Lett. 1992 Apr 13;138(1):97–102. doi: 10.1016/0304-3940(92)90481-l. [DOI] [PubMed] [Google Scholar]
- Ríos E., Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiol Rev. 1991 Jul;71(3):849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Lehmann-Horn F., Ricker K., Küther G. Hypokalemic periodic paralysis: in vitro investigation of muscle fiber membrane parameters. Muscle Nerve. 1984 Feb;7(2):110–120. doi: 10.1002/mus.880070205. [DOI] [PubMed] [Google Scholar]
- Schnier A., Lüttgau H. C., Melzer W. Role of extracellular metal cations in the potential dependence of force inactivation in skeletal muscle fibres. J Muscle Res Cell Motil. 1993 Dec;14(6):565–572. doi: 10.1007/BF00141553. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Mikami A., Niidome T., Numa S., Adams B. A., Beam K. G. Structure and function of voltage-dependent calcium channels from muscle. Ann N Y Acad Sci. 1993 Dec 20;707:81–86. doi: 10.1111/j.1749-6632.1993.tb38044.x. [DOI] [PubMed] [Google Scholar]