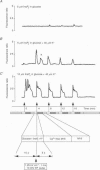
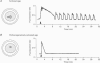
Abstract
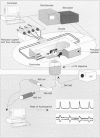
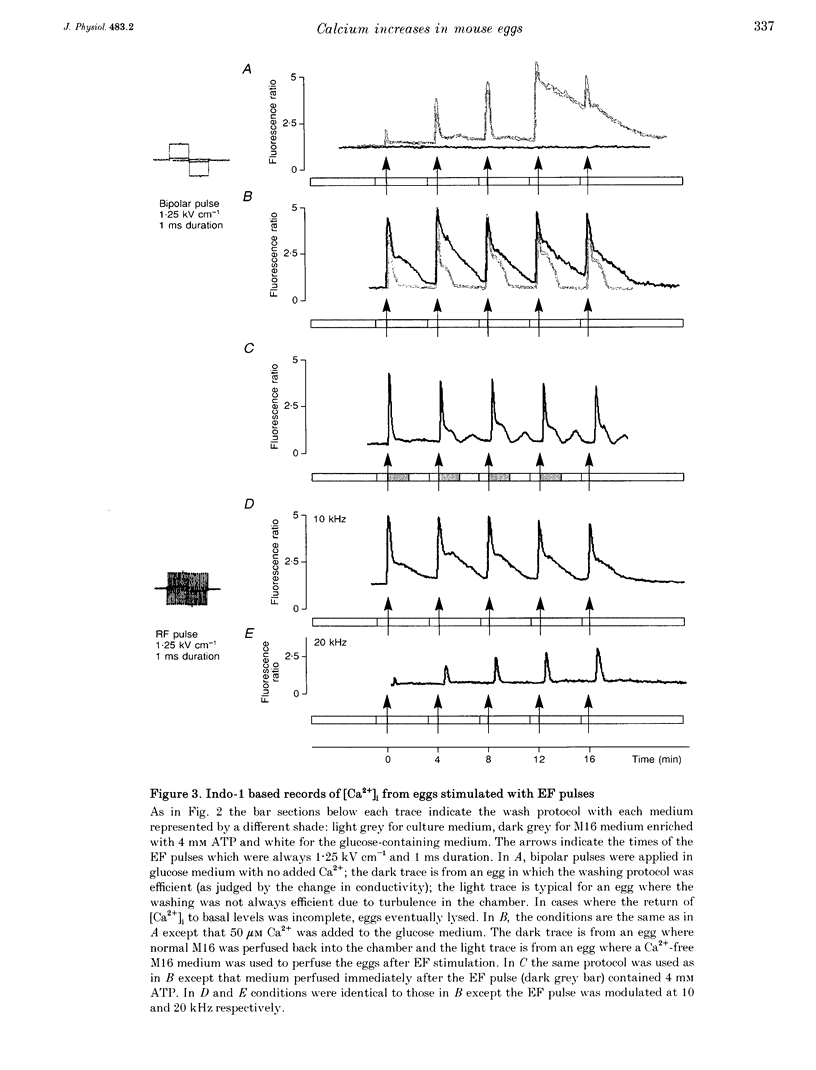
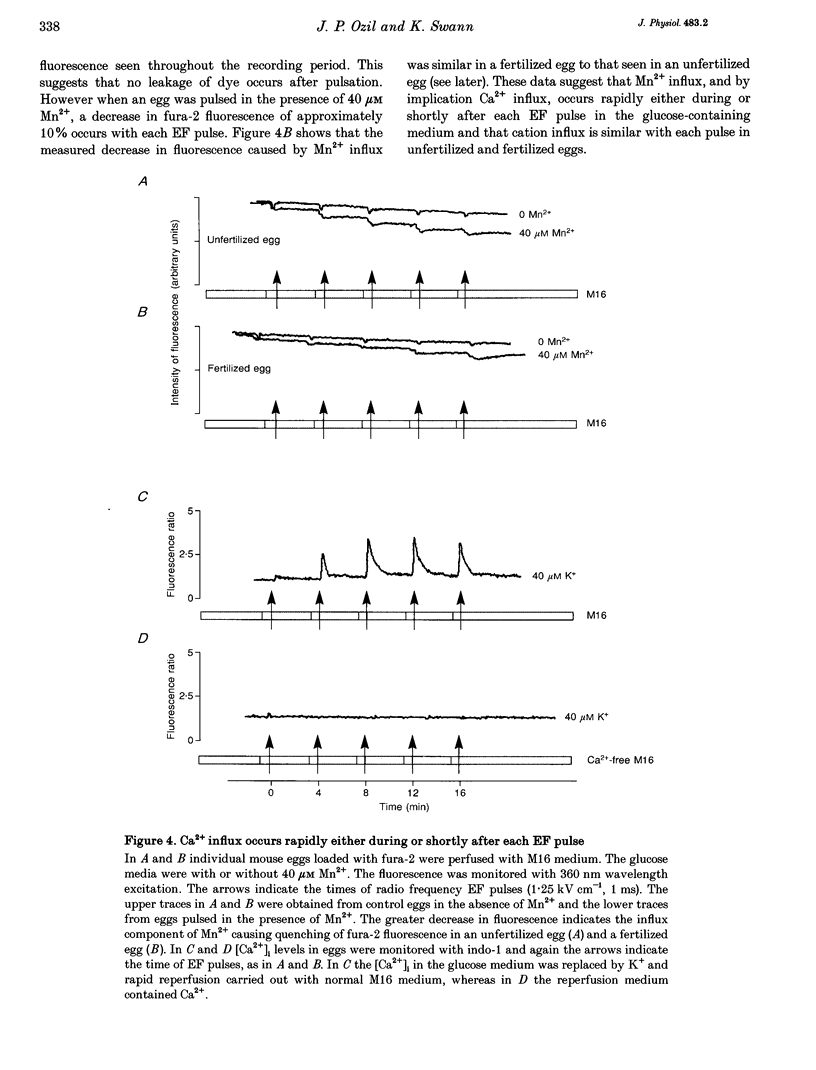
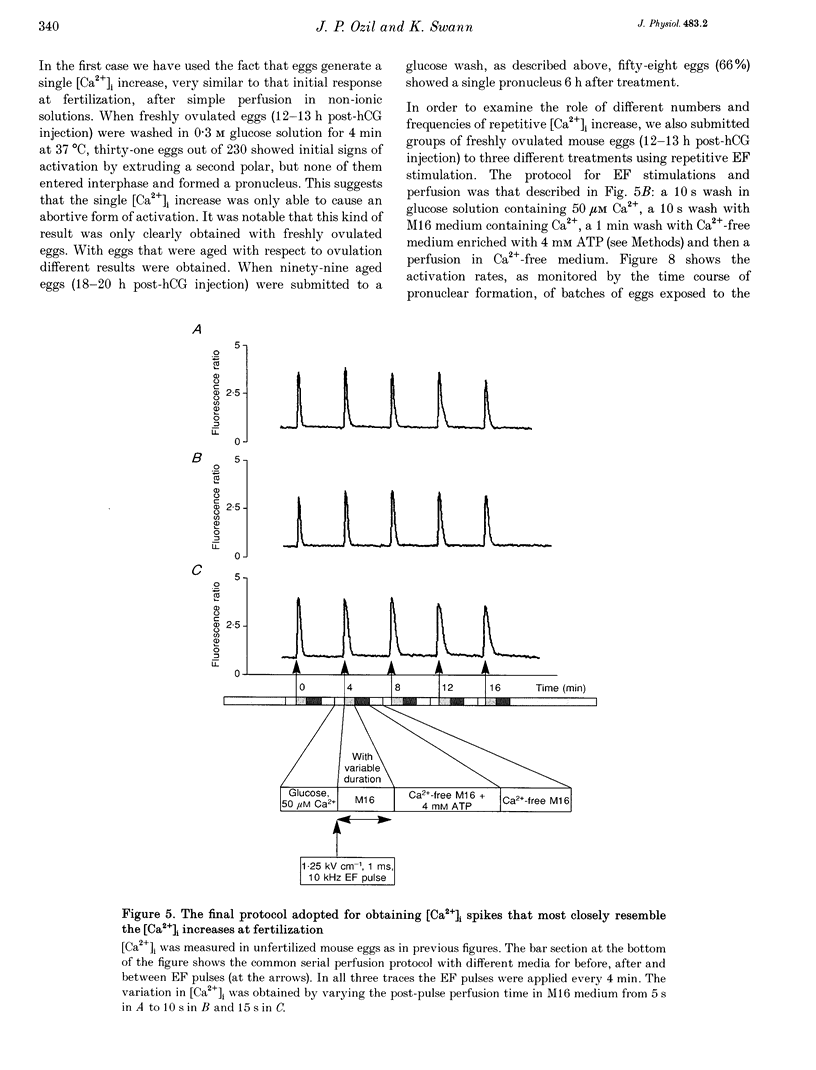
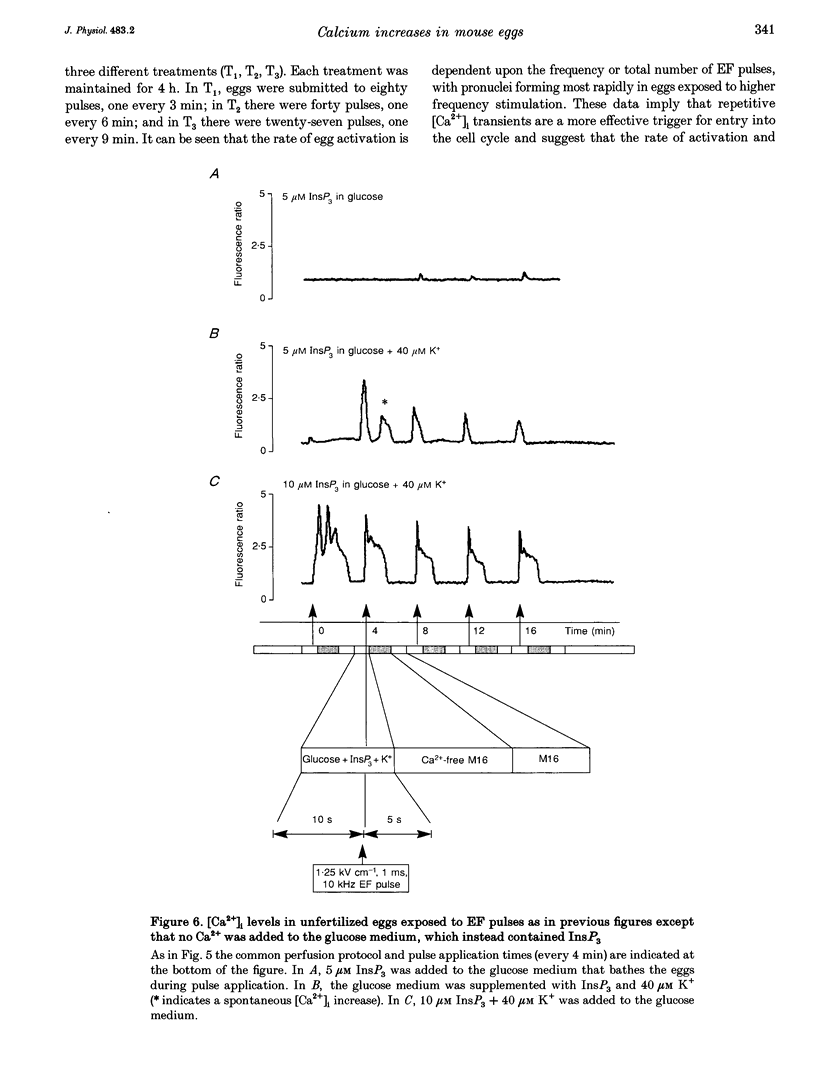
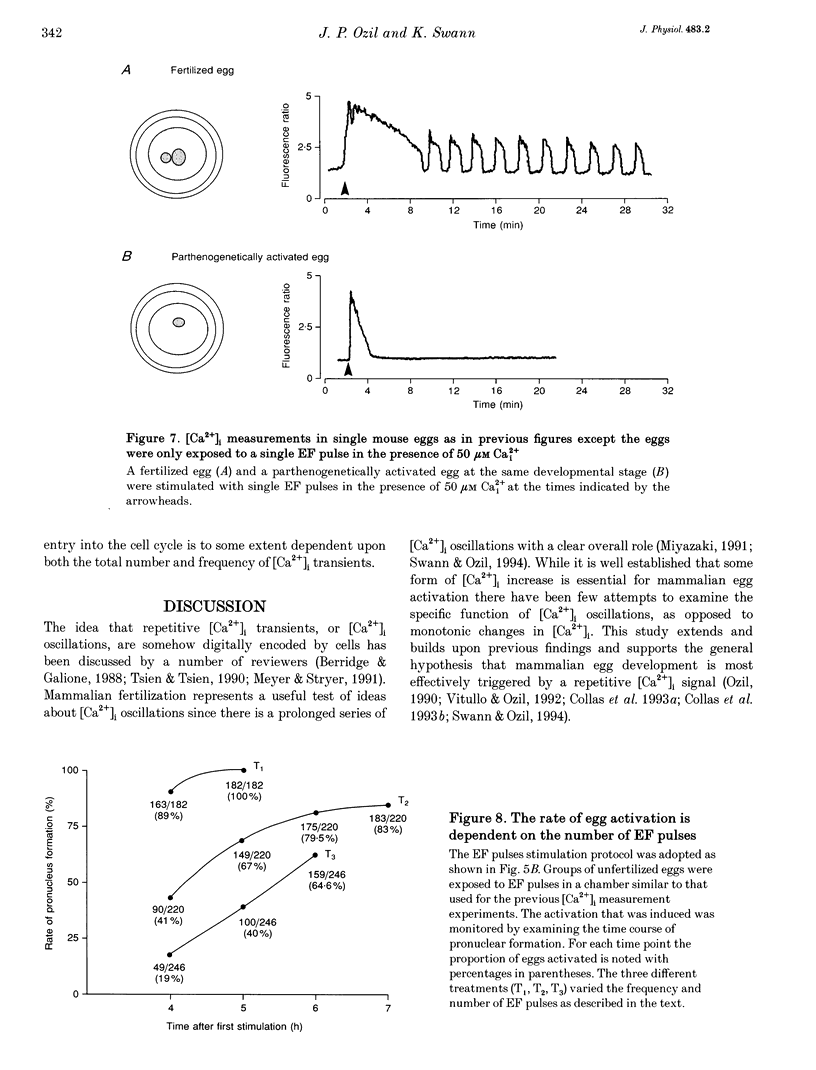
1. We have combined cell membrane electroporation by electrical field (EF) stimulation with a rapid perfusion system in order to stimulate repetitive increases in cytoplasmic free [Ca2+] ([Ca2+]i) in mouse eggs. [Ca2+]i was monitored by ratio fluorescent measurements of intracellular indo-1 on individual eggs. The conditions required to cause different types of [Ca2+]i increases were established and the effects of these [Ca2+]i changes upon egg activation examined. 2. The rapid perfusion of non-ionic medium caused a single [Ca2+]i increase. However, to generate repetitive [Ca2+]i increases, eggs were exposed to EF pulses in the presence of Ca2+ and then washed rapidly with culture medium. Sequential EF pulse application led to prolonged elevation of [Ca2+]i levels and eventual cell lysis unless rapid reperfusion with culture medium was achieved. Transient increases in [Ca2+]i in eggs could also be generated by EF pulses in the presence of inositol 1,4,5-trisphosphate (InsP3). 3. In response to EF stimulation fertilized eggs showed [Ca2+]i increases that were enhanced relative to unfertilized eggs. The responses in these fertilized eggs were often followed by repetitive [Ca2+]i oscillations, despite the fact that the [Ca2+]i oscillations associated with sperm penetration had ceased by this stage. 4. In unfertilized mouse eggs the [Ca2+]i increases appeared to be due to direct cation influx since repeated EF pulses caused repeated influx of Mn2+ as monitored by quenching of fluorescence of fura-2 loaded eggs. 5. Under conditions that stimulated reproducible patterns of [Ca2+]i transients we found that a single large [Ca2+]i transient did not cause significant egg activation, but that inducing repetitive [Ca2+]i transients was effective in activating eggs. The speed of activation as judged by the rate of pronuclear formation was also dependent upon the frequency of pulse application. 6. These data show that combining EF pulses with a rapid and precise sequential perfusion system can be used to manipulate [Ca2+]i levels in mammalian eggs. This provides a means of artificial mimicry of the [Ca2+]i transients seen after fertilization. It appears that Ca2+ influx during EF pulses does not cause significant Ca2+ release from internal stores in unfertilized eggs, but after fertilization Ca2+ influx does induce Ca2+ release. It is also apparent that mouse eggs are more successfully activated by repetitive [Ca2+]i increases than by single large [Ca2+]i rises. We suggest that our data provide direct evidence for the hypothesis that a cellular response to oscillations of intracellular [Ca2+]i can be distinct from that to monotonic rises in [Ca2+]i.
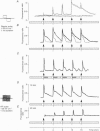
Full text
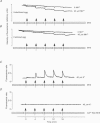
PDF


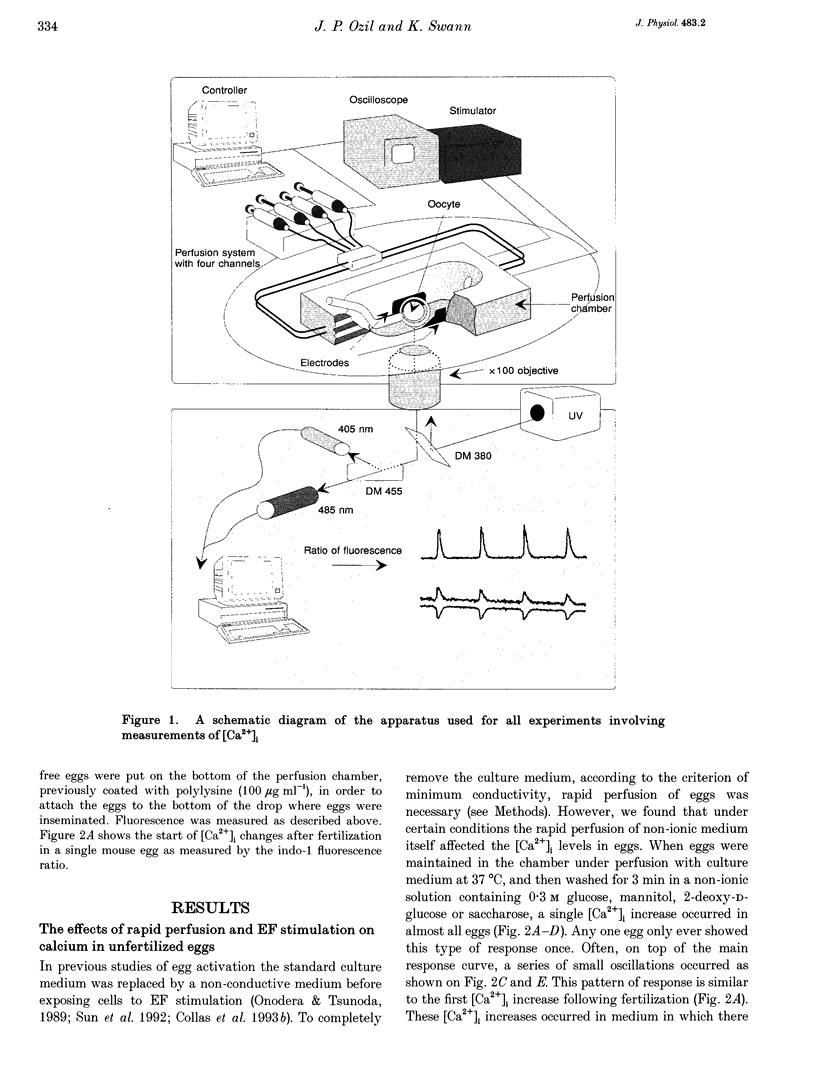

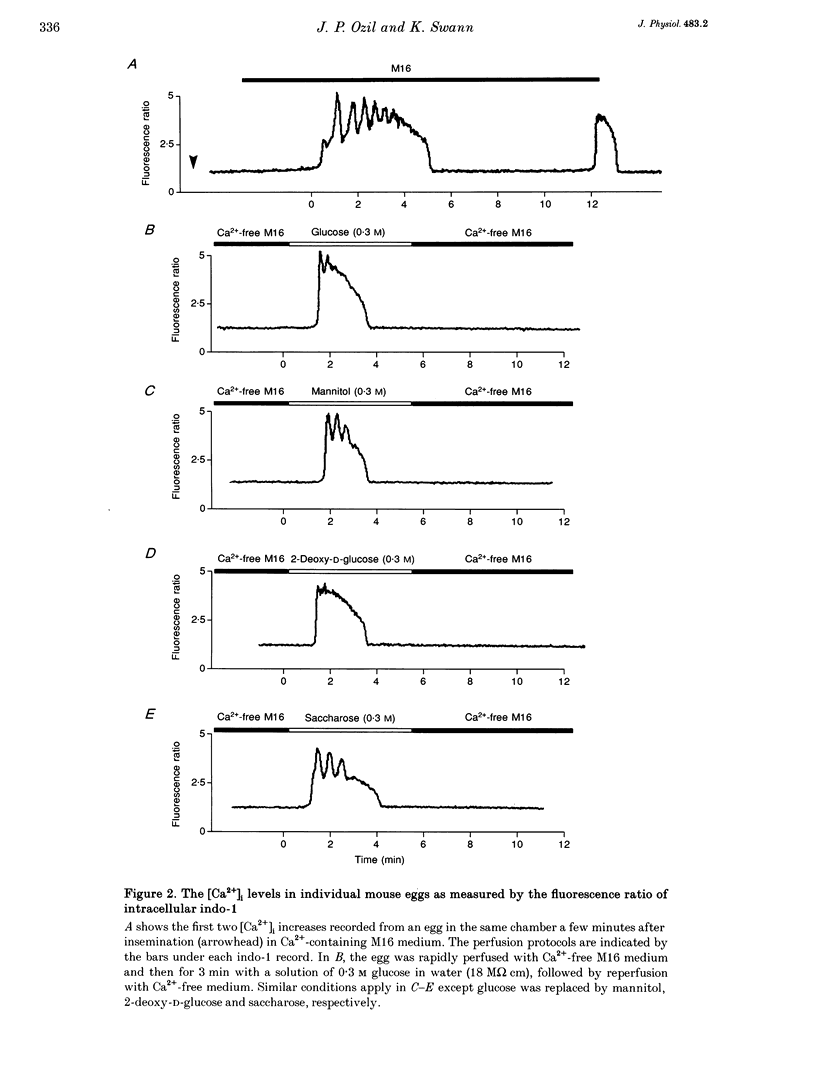





Images in this article
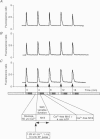
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Galione A. Cytosolic calcium oscillators. FASEB J. 1988 Dec;2(15):3074–3082. doi: 10.1096/fasebj.2.15.2847949. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Rapp P. E. A comparative survey of the function, mechanism and control of cellular oscillators. J Exp Biol. 1979 Aug;81:217–279. doi: 10.1242/jeb.81.1.217. [DOI] [PubMed] [Google Scholar]
- Chang D. C. Cell poration and cell fusion using an oscillating electric field. Biophys J. 1989 Oct;56(4):641–652. doi: 10.1016/S0006-3495(89)82711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek T. R., McGuinness O. M., Vincent C., Moreton R. B., Berridge M. J., Johnson M. H. Fertilisation and thimerosal stimulate similar calcium spiking patterns in mouse oocytes but by separate mechanisms. Development. 1993 Sep;119(1):179–189. doi: 10.1242/dev.119.1.179. [DOI] [PubMed] [Google Scholar]
- Collas P., Fissore R., Robl J. M., Sullivan E. J., Barnes F. L. Electrically induced calcium elevation, activation, and parthenogenetic development of bovine oocytes. Mol Reprod Dev. 1993 Feb;34(2):212–223. doi: 10.1002/mrd.1080340214. [DOI] [PubMed] [Google Scholar]
- Collas P., Sullivan E. J., Barnes F. L. Histone H1 kinase activity in bovine oocytes following calcium stimulation. Mol Reprod Dev. 1993 Feb;34(2):224–231. doi: 10.1002/mrd.1080340215. [DOI] [PubMed] [Google Scholar]
- Cuthbertson K. S., Cobbold P. H. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature. 1985 Aug 8;316(6028):541–542. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- Fulton B. P., Whittingham D. G. Activation of mammalian oocytes by intracellular injection of calcium. Nature. 1978 May 11;273(5658):149–151. doi: 10.1038/273149a0. [DOI] [PubMed] [Google Scholar]
- Galione A., Swann K., Georgiou P., Whitaker M. Regenerative and non-regenerative calcium transients in hamster eggs triggered by inositol 1,4,5-trisphosphate. J Physiol. 1994 Nov 1;480(Pt 3):465–474. doi: 10.1113/jphysiol.1994.sp020375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Igusa Y., Miyazaki S. Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol. 1983 Jul;340:611–632. doi: 10.1113/jphysiol.1983.sp014783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja R., Ito E., Koide S. S. Effect of serotonin and tricyclic antidepressants on intracellular calcium concentrations in Spisula oocytes. Cell Calcium. 1994 Jan;15(1):1–6. doi: 10.1016/0143-4160(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Kline D., Kline J. T. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev Biol. 1992 Jan;149(1):80–89. doi: 10.1016/0012-1606(92)90265-i. [DOI] [PubMed] [Google Scholar]
- Miyazaki S. Repetitive calcium transients in hamster oocytes. Cell Calcium. 1991 Feb-Mar;12(2-3):205–216. doi: 10.1016/0143-4160(91)90021-6. [DOI] [PubMed] [Google Scholar]
- Onodera M., Tsunoda Y. Parthenogenetic activation of mouse and rabbit eggs by electric stimulation in vitro. Gamete Res. 1989 Mar;22(3):277–283. doi: 10.1002/mrd.1120220305. [DOI] [PubMed] [Google Scholar]
- Owen C. S., Shuler R. L. Spectral evidence for non-calcium interactions of intracellular Indo-1. Biochem Biophys Res Commun. 1989 Aug 30;163(1):328–333. doi: 10.1016/0006-291x(89)92139-6. [DOI] [PubMed] [Google Scholar]
- Ozil J. P. The parthenogenetic development of rabbit oocytes after repetitive pulsatile electrical stimulation. Development. 1990 May;109(1):117–127. doi: 10.1242/dev.109.1.117. [DOI] [PubMed] [Google Scholar]
- Quinn P., Barros C., Whittingham D. G. Preservation of hamster oocytes to assay the fertilizing capacity of human spermatozoa. J Reprod Fertil. 1982 Sep;66(1):161–168. doi: 10.1530/jrf.0.0660161. [DOI] [PubMed] [Google Scholar]
- Rickords L. F., White K. L. Electrofusion-induced intracellular Ca2+ flux and its effect on murine oocyte activation. Mol Reprod Dev. 1992 Feb;31(2):152–159. doi: 10.1002/mrd.1080310210. [DOI] [PubMed] [Google Scholar]
- Rickords L. F., White K. L. Electroporation of inositol 1,4,5-triphosphate induces repetitive calcium oscillations in murine oocytes. J Exp Zool. 1993 Feb 1;265(2):178–184. doi: 10.1002/jez.1402650209. [DOI] [PubMed] [Google Scholar]
- Rossignol D. P., Decker G. L., Lennarz W. J., Tsong T. Y., Teissie J. Induction of calcium-dependent, localized cortical granule breakdown in sea-urchin eggs by voltage pulsation. Biochim Biophys Acta. 1983 Dec 19;763(4):346–355. doi: 10.1016/0167-4889(83)90096-4. [DOI] [PubMed] [Google Scholar]
- Shiina Y., Kaneda M., Matsuyama K., Tanaka K., Hiroi M., Doi K. Role of the extracellular Ca2+ on the intracellular Ca2+ changes in fertilized and activated mouse oocytes. J Reprod Fertil. 1993 Jan;97(1):143–150. doi: 10.1530/jrf.0.0970143. [DOI] [PubMed] [Google Scholar]
- Sun F. Z., Hoyland J., Huang X., Mason W., Moor R. M. A comparison of intracellular changes in porcine eggs after fertilization and electroactivation. Development. 1992 Aug;115(4):947–956. doi: 10.1242/dev.115.4.947. [DOI] [PubMed] [Google Scholar]
- Swann K. A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development. 1990 Dec;110(4):1295–1302. doi: 10.1242/dev.110.4.1295. [DOI] [PubMed] [Google Scholar]
- Swann K., Igusa Y., Miyazaki S. Evidence for an inhibitory effect of protein kinase C on G-protein-mediated repetitive calcium transients in hamster eggs. EMBO J. 1989 Dec 1;8(12):3711–3718. doi: 10.1002/j.1460-2075.1989.tb08546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K., Ozil J. P. Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol. 1994;152:183–222. doi: 10.1016/s0074-7696(08)62557-7. [DOI] [PubMed] [Google Scholar]
- Swezey R. R., Epel D. Stable, resealable pores formed in sea urchin eggs by electric discharge (electroporation) permit substrate loading for assay of enzymes in vivo. Cell Regul. 1989 Nov;1(1):65–74. doi: 10.1091/mbc.1.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Electroporation of cell membranes. Biophys J. 1991 Aug;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitullo A. D., Ozil J. P. Repetitive calcium stimuli drive meiotic resumption and pronuclear development during mouse oocyte activation. Dev Biol. 1992 May;151(1):128–136. doi: 10.1016/0012-1606(92)90220-b. [DOI] [PubMed] [Google Scholar]