Abstract
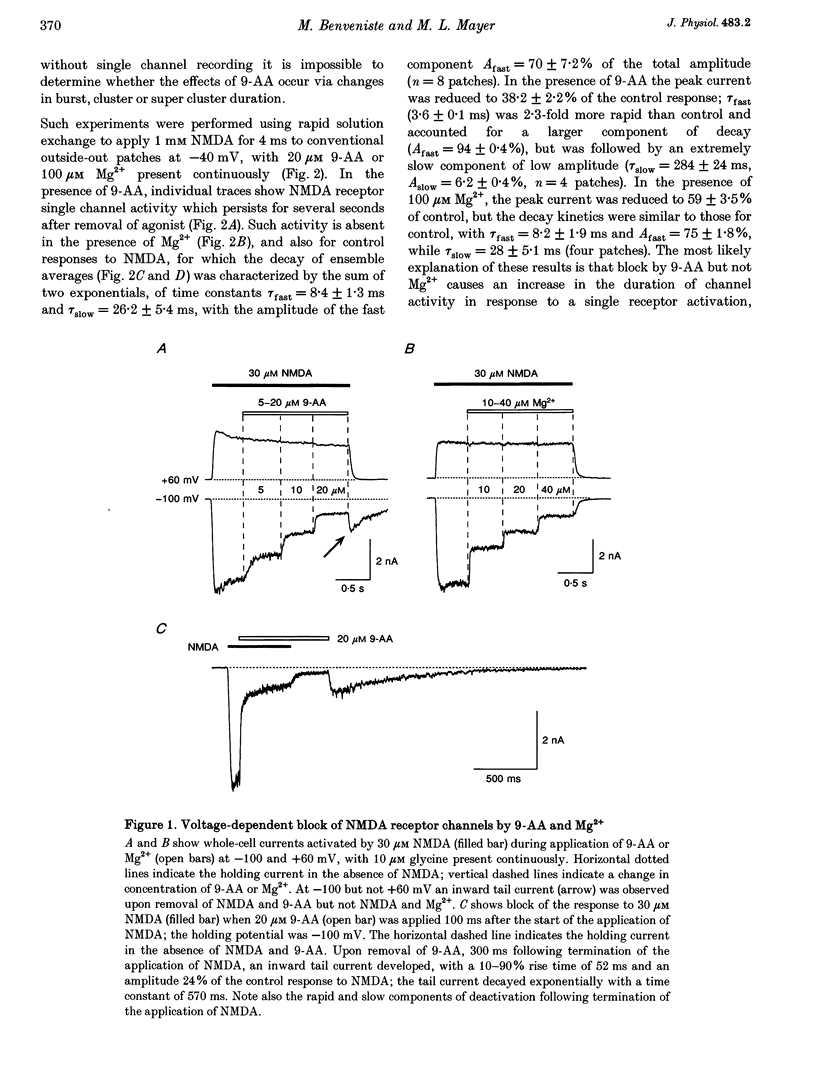
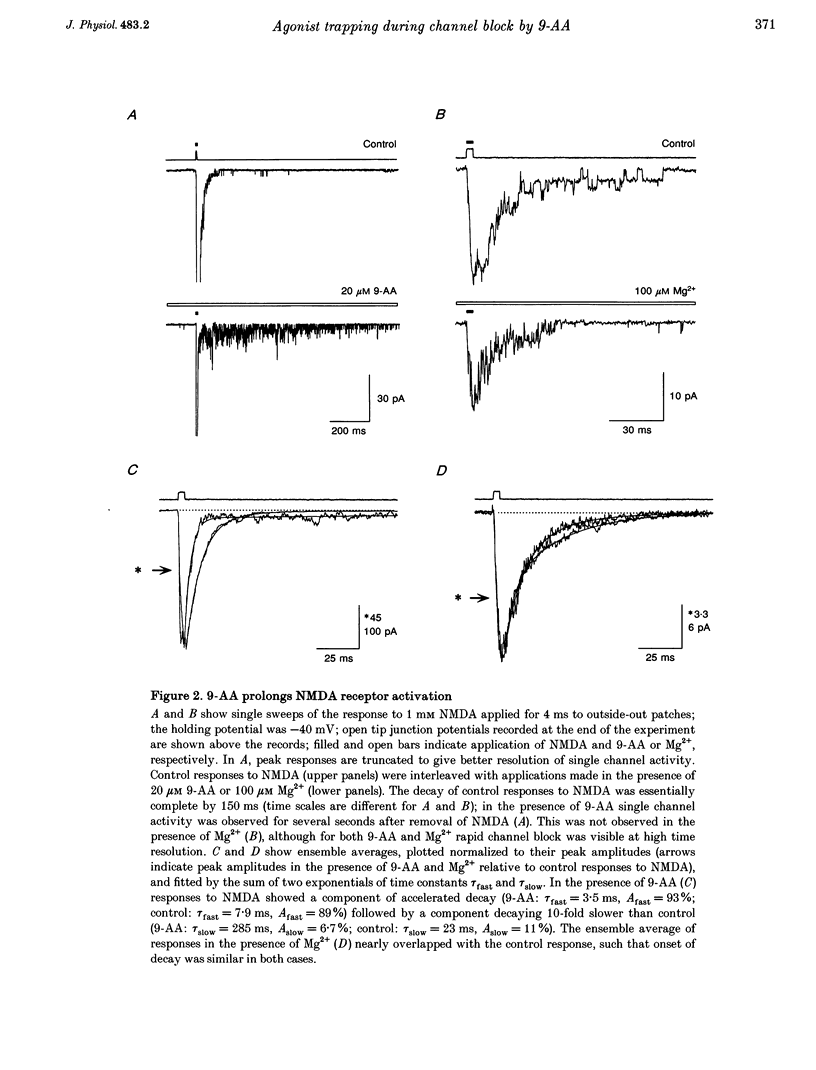
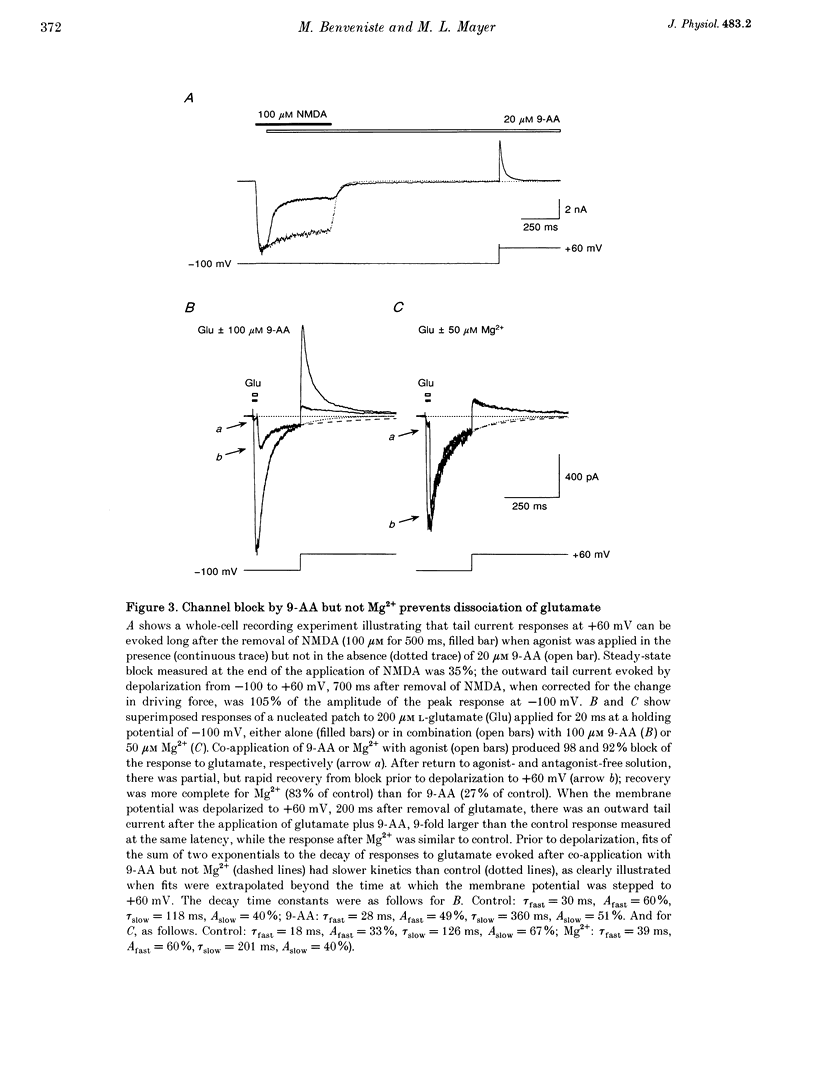
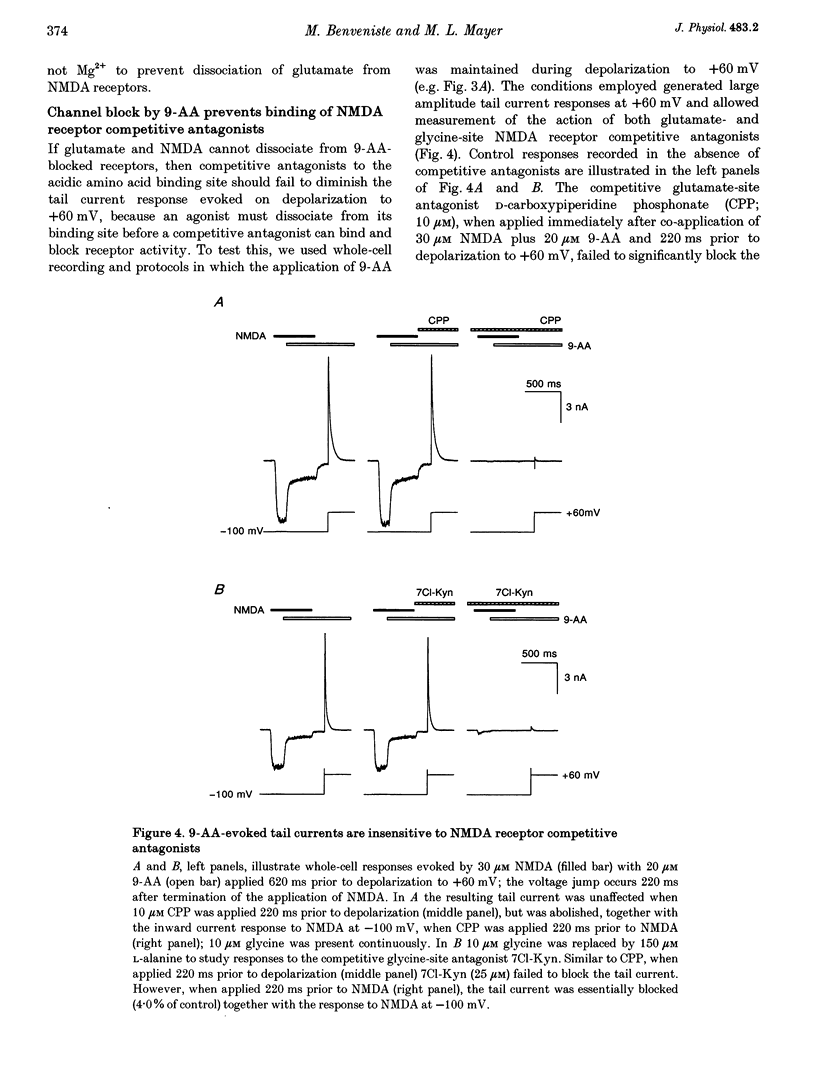
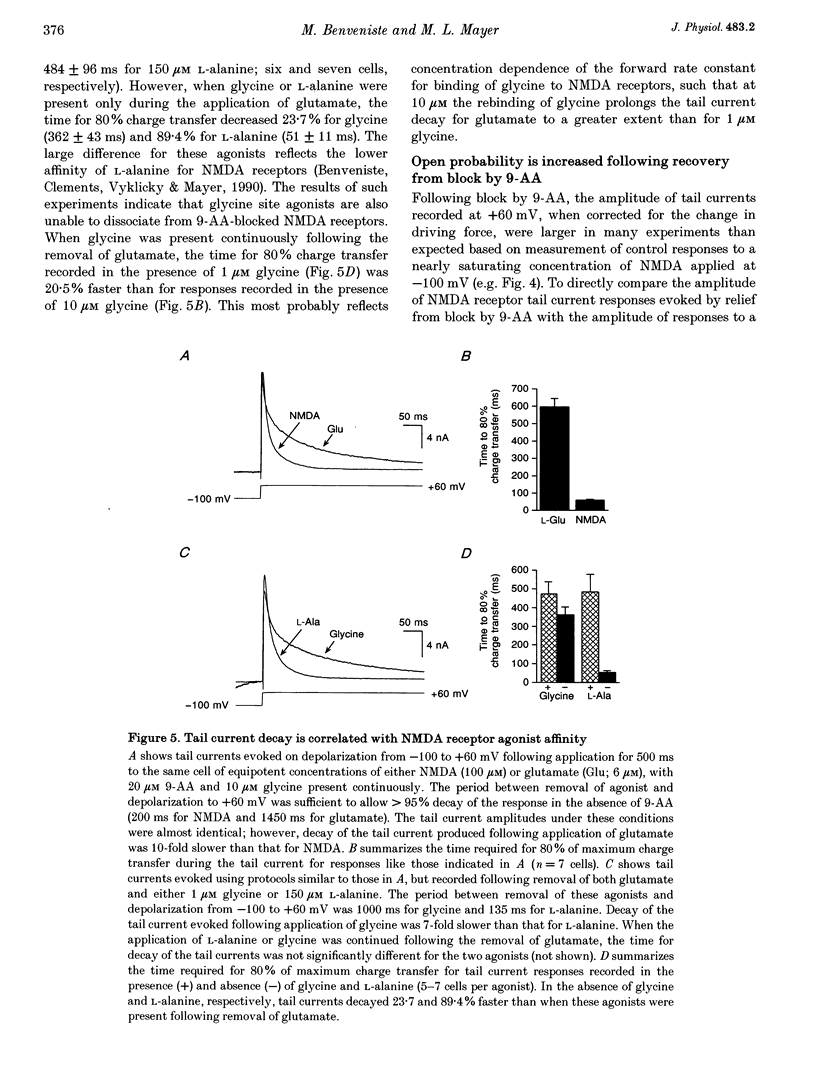
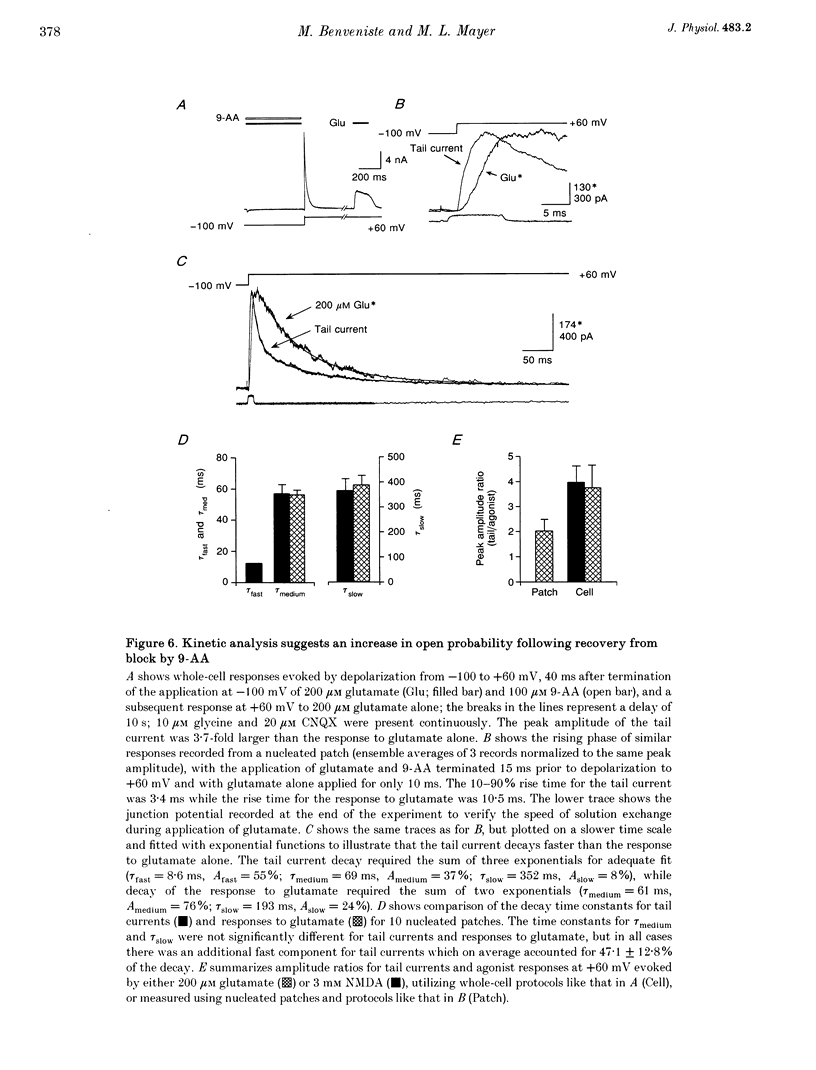
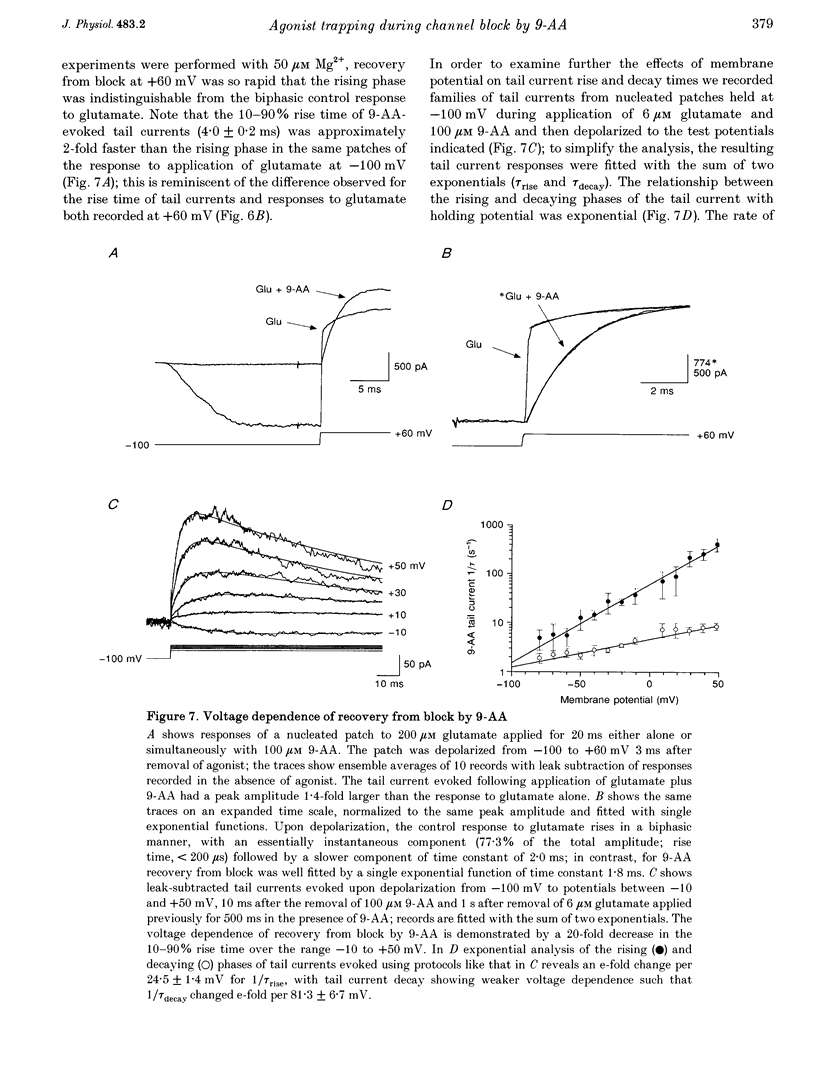
1. N-methyl-D-aspartate (NMDA) receptor responses were recorded from rat hippocampal neurons grown in dissociated culture, using whole-cell, outside-out and nucleated patch recording techniques. Rapid perfusion was used to study voltage-dependent block of NMDA receptors by 9-aminoacridine (9-AA) and by Mg2+. 2. Large amplitude tail currents were evoked on depolarization to +60 mV after application at -100 mV of NMDA and 9-AA but not NMDA and Mg2+. These tail currents were resistant to block by competitive antagonists to the glutamate and glycine binding sites on NMDA receptors and were not evoked when either NMDA or 9-AA were applied alone. 3. The decay kinetics of the tail current were dependent on agonist affinity; the time required for 80% charge transfer was 10-fold briefer for NMDA than for glutamate and 7-fold briefer for L-alanine than for glycine. These results are in accord with a sequential model for block of NMDA receptors by 9-AA, in which neither glutamate nor glycine can dissociate from the open-blocked state of the receptor. 4. Tail current responses had amplitudes 2- to 4-fold larger than responses to maximally effective concentrations of glutamate and glycine, indicating that NMDA receptor channels accumulate in the open-blocked state during co-application of agonist and 9-AA. The rise time and decay kinetics of tail current responses were faster than the response to brief applications of a maximally effective concentration of glutamate. Together, these results suggest that at +60 mV recovery from block by 9-AA occurs faster than the rate of opening of NMDA receptors in response to glutamate. 5. Our experiments suggest that open channel block of NMDA receptors can provide a novel approach for measurement of both open probability and the first latency distribution for ion channel opening in response to the binding of agonists, and provide additional evidence suggesting that the delayed opening of NMDA receptor channels underlies slow activation and deactivation of responses to glutamate.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. A statistical analysis of acetylcholine receptor activation in Xenopus myocytes: stepwise versus concerted models of gating. J Physiol. 1993 Feb;461:339–378. doi: 10.1113/jphysiol.1993.sp019517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Clements J., Vyklický L., Jr, Mayer M. L. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Mienville J. M., Sernagor E., Mayer M. L. Concentration-jump experiments with NMDA antagonists in mouse cultured hippocampal neurons. J Neurophysiol. 1990 Jun;63(6):1373–1384. doi: 10.1152/jn.1990.63.6.1373. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Westbrook G. L. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991 Oct;7(4):605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A. C., Albuquerque E. X. Dynamics of the actions of tetrahydro-9-aminoacridine and 9-aminoacridine on glutamatergic currents: concentration-jump studies in cultured rat hippocampal neurons. J Pharmacol Exp Ther. 1994 Jan;268(1):503–514. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Edmonds B., Colquhoun D. Rapid decay of averaged single-channel NMDA receptor activations recorded at low agonist concentration. Proc Biol Sci. 1992 Dec 22;250(1329):279–286. doi: 10.1098/rspb.1992.0160. [DOI] [PubMed] [Google Scholar]
- Gibb A. J., Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992 Oct;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler N. A., Shirke A. M., Malinow R. The probability of transmitter release at a mammalian central synapse. Nature. 1993 Dec 9;366(6455):569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992 Jun 25;357(6380):686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Bean B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr C. E. High probability opening of NMDA receptor channels by L-glutamate. Science. 1992 Jan 24;255(5043):470–472. doi: 10.1126/science.1346477. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Stevens C. F. A quantitative description of NMDA receptor-channel kinetic behavior. J Neurosci. 1990 Jun;10(6):1830–1837. doi: 10.1523/JNEUROSCI.10-06-01830.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B. U., Konnerth A., Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol. 1991 Apr;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kuryatov A., Laube B., Betz H., Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron. 1994 Jun;12(6):1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Lerma J., Zukin R. S., Bennett M. V. Interaction of Mg2+ and phencyclidine in use-dependent block of NMDA channels. Neurosci Lett. 1991 Feb 25;123(2):187–191. doi: 10.1016/0304-3940(91)90927-l. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Clements J. D., Westbrook G. L., Jahr C. E. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990 Aug 9;346(6284):565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Lester R. A., Jahr C. E. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992 Feb;12(2):635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Tong G., Jahr C. E. Interactions between the glycine and glutamate binding sites of the NMDA receptor. J Neurosci. 1993 Mar;13(3):1088–1096. doi: 10.1523/JNEUROSCI.13-03-01088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D. N., Mody I. Regulation of NMDA channel function by endogenous Ca(2+)-dependent phosphatase. Nature. 1994 May 19;369(6477):235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- Lin F., Stevens C. F. Both open and closed NMDA receptor channels desensitize. J Neurosci. 1994 Apr;14(4):2153–2160. doi: 10.1523/JNEUROSCI.14-04-02153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Miljkovic Z., Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987 Aug;58(2):251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Milburn M. V., Privé G. G., Milligan D. L., Scott W. G., Yeh J., Jancarik J., Koshland D. E., Jr, Kim S. H. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991 Nov 29;254(5036):1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- Neher E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J Physiol. 1983 Jun;339:663–678. doi: 10.1113/jphysiol.1983.sp014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L. M., Wright J. M. Slow voltage-dependent changes in channel open-state probability underlie hysteresis of NMDA responses in Mg(2+)-free solutions. Neuron. 1992 Jan;8(1):181–187. doi: 10.1016/0896-6273(92)90119-x. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Oh B. H., Pandit J., Kang C. H., Nikaido K., Gokcen S., Ames G. F., Kim S. H. Three-dimensional structures of the periplasmic lysine/arginine/ornithine-binding protein with and without a ligand. J Biol Chem. 1993 May 25;268(15):11348–11355. [PubMed] [Google Scholar]
- Sather W., Dieudonné S., MacDonald J. F., Ascher P. Activation and desensitization of N-methyl-D-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol. 1992 May;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Gnatt A., Loewenstein Y., Neville L. F. Excavations into the active-site gorge of cholinesterases. Trends Biochem Sci. 1992 Sep;17(9):353–358. doi: 10.1016/0968-0004(92)90314-y. [DOI] [PubMed] [Google Scholar]
- Tong G., Jahr C. E. Regulation of glycine-insensitive desensitization of the NMDA receptor in outside-out patches. J Neurophysiol. 1994 Aug;72(2):754–761. doi: 10.1152/jn.1994.72.2.754. [DOI] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Vorobjev V. S., Sharonova I. N. Tetrahydroaminoacridine blocks and prolongs NMDA receptor-mediated responses in a voltage-dependent manner. Eur J Pharmacol. 1994 Feb 21;253(1-2):1–8. doi: 10.1016/0014-2999(94)90750-1. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]


