Abstract
Joint hypermobility is very common in the general population as is arthralgia. Increased awareness of hypermobility and hypermobile Ehlers Danlos Syndrome (hEDS) among patients and providers has led to a surge in demand for evaluation. Many patients with hypermobility meet clinical criteria for a diagnosis of hypermobile spectrum disorder (HSD) or hEDS, but monogenic connective tissue diseases (CTD) are rare. Genetic testing is not recommended for patients with HSD/hEDS unless another underlying CTD is suspected. Given the high prevalence of HSD/hEDS in the general population, primary care providers should be familiar with HSD/hEDS evaluation, management, and indication for referral to a CTD specialist.
Introduction
Approximately 10–30% of the population has some degree of joint hypermobility (JH), but only 1:500 patients meet diagnostic criteria for hypermobility spectrum disorder (HSD) or hypermobile Ehlers Danlos Syndrome (hEDS).1–3 The cause of HSD and hEDS is unknown.4 There is no genetic test for HSD or hEDS. Joint hypermobility can also be seen in rare disorders such as other forms of Ehlers Danlos Syndrome, Marfan Syndrome, and Loeys-Dietz Syndrome but these are readily clinically recognizable with distinctive features not found in HSD/hEDS. Genetic testing is only recommended when another connective tissue disorder (CTD) is suspected.5 Regardless of the cause, management of JH is symptomatic and follows general good practice for joint health. The goal of this article is to empower general practitioners to evaluate JH and recognize signs and symptoms that suggest an alternative diagnosis that should prompt referral to a specialty center.
Assessing Joint Hypermobility
Joint hypermobility occurs when a joint can be being actively or passively moved “beyond normal limits along physiological axes.”6 It may be caused by an inherent laxity of the connective tissue, as in heritable CTD, but it can also be a manifestation of an acquired condition of the muscles, joints, or bones such as past trauma, age-related joint degeneration, hypotonia, or other neuromuscular conditions.
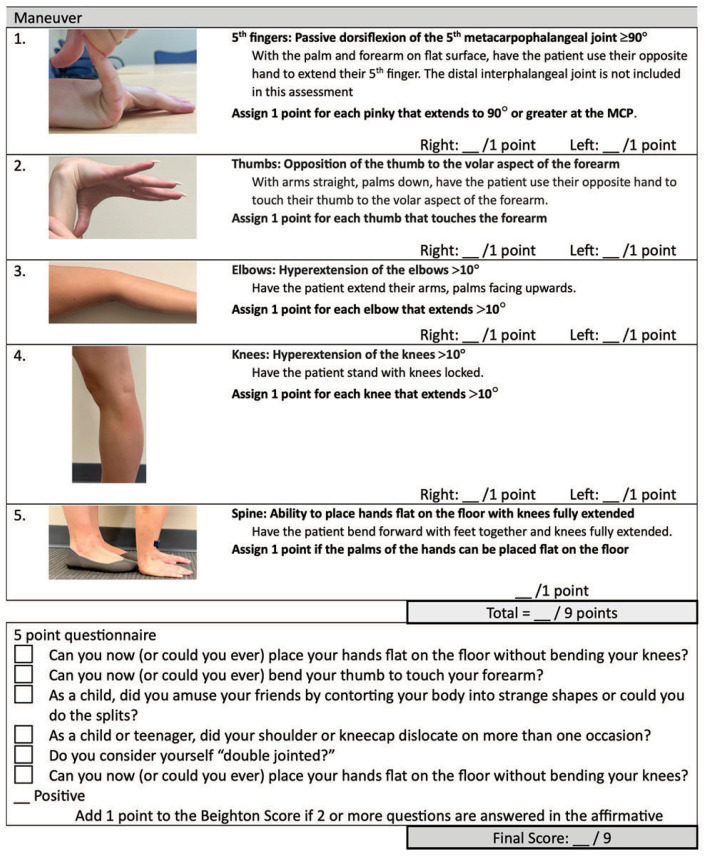
The Beighton score (BS) is used to diagnose HSD, hEDS, and pediatric hypermobility.7–9 There are other tools available for the assessment of JH but these have not been validated for diagnosis of hEDS or pediatric hypermobility.7,9
The Beighton score (BS) is a quick, easy tool to assess JH in adults and children ≥ 5 years.7 It is the recommended tool for diagnosing HSD, hEDS, and pediatric hypermobility.7–9 The BS incorporates of five maneuvers, with a score of 1 given for each positive maneuver, yielding a possible score of 0–9 (Table 1). The BS is not reliable in children under 5.9,10 A score of 6 or more should be used for pediatric patients age 5 to 17 or skeletal maturity. Skeletal maturity is defined as a growth velocity <1cm/yr using two measurements at least three months apart or a mature bone age on X-ray studies. A score of 5 or more is required from age 18 to 50 and 4 or more for patients older than 50. For adults within 1 point of the age-specific cutoff, a 5 point questionnaire can be administered - one additional point can be added to the score if the patient answers “yes” to at least two questions (Table 1).11
Table 1.
Beighton Score
Diagnosis
Adults
For adults and adolescents who have reached skeletal maturity, hEDS should be diagnosed based on the 2017 criteria.7,12
HSD is currently described as any generalized, peripheral, localized, or historic JH in the absence of an underlying etiology.12 While no strict or consistent definition exists in the literature, HSD is generally considered an appropriate diagnosis in patients with a BS that meets age-specific cut-offs but does not meet the systemic criteria required for hEDS. HSD is not an appropriate diagnosis if there is an alternative explanation for JH (past trauma, age-related joint degeneration, hypotonia, and other neuromuscular conditions).
Diagnosis of hEDS requires a positive age adjusted BS, and two of the following: A) chronic musculoskeletal pain or recurrent joint dislocations; B) five systemic symptoms (Table 2); or C) a family history of a first degree relative (parent, child, or sibling) who meets 2017 diagnostic criteria.7 The final diagnostic criteria is exclusion of alternate diagnoses. Most alternate diagnosis can be excluded based on history and exam. Symptoms of an alternate diagnosis that should prompt referral to a CTD specialist are discussed in more detail below.
Table 2.
Systemic Criteria for Hypermobile Ehlers Danlos
| 1. | Unusually soft or velvety skin This should be assessed on the volar aspect of the forearm. Skin softness and texture is subjective but is marked in patients with hEDS. A high threshold for positivity is recommended |
| 2. | Mild skin hyperextensibility Skin extensibility can be affected by location and extrinsic factors such as sun exposure and weight fluctuations. This should be measured in the middle of the volar surface of the non-dominant forearm. Skin should stretch >1.5cm to be considered positive. Stretch >2cm should prompt consideration for alternative diagnosis such as classic EDS or classic-like EDS |
| 3. | Unexplained striae in adolescents, men, or prepubertal women without a history of significant gain or loss of body fat or weight |
| 4. | Bilateral piezogenic papules of the heel |
| 5. | Recurrent or multiple abdominal hernia(s) (e.g., umbilical, inguinal, crural) Congenital umbilical hernias are common particularly in preterm infants and are generally not counted towards this criterion unless they recur |
| 6. | Atrophic scarring involving at least two sites without papyraceous or hemosideric scars Atrophic scars are typically wide and sunken due to impaired wound healing and subsequent dermal atrophy. Linear scars are more reliable than elliptical scars (such as from shave biopsies) which are more difficult to assess without knowing the size of the original wound. Scars that result from multiple incisions, wound infections, or inflammatory conditions should not be considered. |
| 7. | Pelvic floor, rectal, and/or uterine prolapse in children, men or nulliparous women without a history of morbid obesity or other known predisposing medical conditions Pregnancy, morbid obesity, and other conditions such as chronic constipation predispose patients to pelvic organ prolapse. This criterion should be considered positive in the absence of those predisposing conditions. |
| 8. | Dental crowding and high or narrow palate This is a subjective measure but history of extensive orthodontia, tooth extractions, or palate expansion suggest the presence of significant crowding |
| 9. | Arachnodactyly, as defined by a bilateral positive wrist (Steinberg) sign and/or bilateral positive thumb (Walker) sign. Wrist sign is only considered positive if the tip of the thumb overlaps the entire nail of the pinky. Thumb sign is only considered positive if the entire distal phalanx of the adducted thumb extends beyond the ulnar border of the palm. |
| 10. | Arm span-to-height ≥1.05 |
| 11. | Mitral valve prolapse (MVP) mild or greater based on strict echocardiographic criteria |
| 12. | Aortic root dilatation with Z-score > +2 |
The history and exam should also evaluate for acquired connective tissue disorders such as autoimmune disorders, age-related degeneration, instability caused by trauma, hypotonia, and neuromuscular disorders. While patients with autoimmune disorders may have underlying JH, they also typically have joint pain due to their autoimmune disorder, so the criterion regarding pain is not considered in diagnosis of hEDS. Because the pain criterion is not considered in diagnosis, patients with a coexisting acquired connective tissue disorder, such as lupus or rheumatoid arthritis, can only be diagnosed with hEDS if there is a positive family history of hEDS in a first degree assessed by the 2017 criteria. A first degree relative with HSD or a subjective report of hypermobility does not fulfill this criteria. If symptoms of an autoimmune disorder are present (morning stiffness lasting >30 min, pain that improves with activity, joint swelling in the absence of trauma) this should prompt a rheumatologic evaluation. Adult patients should also be evaluated for a history of joint trauma and age related joint degeneration.
A convenient hEDS diagnosis checklist can be found on the Ehlers Danlos Society website at https://www.ehlers-danlos.com/.
Pediatric Patients
JH is naturally prevalent in young children and adolescents and typically declines with age.10,13 Infants and toddlers have insufficient skeletal maturity to meaningfully assess ligamentous laxity, therefore, JH cannot be accurately assessed in children <5 years of age.10 Tone should be assessed in infants and children with apparent hypermobility as hypotonia may give the appearance of an intrinsic connective tissue abnormality.
In 2023, pediatric-specific guidelines were released for patients ages 5–17 years old or until skeletal maturity is established as described above.9 In accordance with the 2017 hEDS diagnostic criteria, the 2023 pediatric guidelines recommend a minimum BS of 6 for diagnosis and classify patients as either pediatric generalized JH (in the absence of musculoskeletal complications) or pediatric hypermobile spectrum disorder (in the presence of musculoskeletal complications). These are further subdivided into patients with skin and soft tissue abnormalities and core comorbidities (Table 3).
Table 3.
Pediatric Diagnosis
| Skin and Soft Tissue Abnormalities | Musculoskeletal Complications | Core Comorbidities | |
|---|---|---|---|
| Asymptomatic | |||
| pGJH | Absent | Absent | Absent |
| pGHJ with skin involvement | Present | Absent | Absent |
| Symptomatic | |||
| pGHJ with core comorbidities | Absent | Absent | Present |
| pGJH with core comorbidities and skin involvement | Present | Absent | Present |
| pHSD, Musculoskeletal subtype | Absent | Present | Absent |
| pHSD Musculoskeletal subtype with skin involvement | Present | Present | Absent |
| pHSD: systemic subtype | Absent | Present | Present |
| pHSD: Systemic subtype with skin invoivement | Present | Present | Present |
pGJH – Pediatric Generalized Joint Hypermobility
pHSD – Pediatric Hypermobility Spectrum Disorder
A convenient Pediatric Hypermobility diagnosis checklist can be found on the Ehlers Danlos Society website at https://www.ehlers-danlos.com.
This diagnostic framework “provides the foundation for appropriate current treatment and support, but not a lifelong diagnosis which may result in over medicalization and potential harms.”9 It also allows for diagnostic flexibility, so patients can be reclassified as their musculoskeletal system matures and symptoms change.
A convenient hEDS diagnosis checklist can be found on the Ehlers Danlos Society website (https://www.ehlers-danlos.com/).
Genetic Testing
Currently, there is no genetic testing for diagnosis HSD/hEDS. Hypermobility and hEDS have a clear autosomal dominant pattern of inheritance but despite years of work, no genetic cause has been identified. The genetic etiology is likely heterogeneous and hypothesized to be due multiple benign rare or common variants within connective tissue genes. Genetic testing is not recommended for patients with HSD or hEDS unless there are signs/symptoms of a distinct connective tissue disorder.5
At the Connective Tissue Disorder Center at Washington University in St. Louis, we evaluate hundreds of patients for concerns for possible connective tissue disorder annually. Genetic testing is only recommended for a small proportion of individuals with HSD/hEDS and atypical symptoms. However, genetic counseling is provided to patients with HSD/hEDS and some elect to undergo genetic evaluation. In review of our testing over the last year, of the 102 tests sent on patients without clinical suspicion of a specific disorder, none were diagnostic: half were negative and the other half had variants of uncertain significance (VUS) that were not felt to suggest an alternate diagnosis. Of those patients, a change in clinical management was only recommended in three patients who had variants of uncertain significance in FBN1 or FBN2 without a clinical diagnosis of Marfan or Congenital Contractural Arachnodactyly. While an adverse vascular event was considered unlikely, those periodic screening with echocardiograms were recommended in those patients.
Genetic counseling should be provided to all patients undergoing genetic testing. While genetic testing is generally thought to be low risk, it can have lasting implications on the individual’s health care, life and long-term disability insurance, military service, and for family members. Importantly, genetic testing can never rule out a genetic condition. All genetic testing has lab and test specific limitations that should be understood by ordering providers in order to assess risk of an underlying genetic disorder not evaluated by any given test. These risks and limitations should be discussed with patients prior to genetic testing to obtain informed consent.
Additionally, identification of variants of uncertain significance can cause high anxiety in any population.14–16 Increased anxiety is seen in patients with HSD/hEDS.17,18 In our experience, many patients with HSD/hEDS experience significant anxiety related to VUS despite extensive pre- and post-test genetic counseling. Identification of a VUS can also lead to medically unnecessary testing due to patients’ and/or providers’ perception of the risk associated with VUS.
Rare Connective Tissue Diseases
Diagnosis of HSD and hEDS requires the reasonable exclusion of other heritable disorders of connective tissue.7 HSD and hEDS are clinically distinct from other rare forms of EDS and other CTD such as Marfan Syndrome and Loeys-Dietz Syndrome. Reasonable exclusion of other heritable CTD can usually be made based on history and exam, with referral to a CTD specialist made if there are any concerning signs or symptoms for an alternate diagnosis.
The most common concern among the patients we see and their referring providers is regarding vascular Ehlers Danlos Syndrome (vEDS). Patients with vEDS rarely have generalized hypermobility or significant arthralgia. Hypermobility in patients with vEDS is typically limited to the distal extremities and is rarely, if ever, the presenting symptom for patients with vEDS. Patients with vEDS also typically have exceptionally thin, translucent skin with pronounced visible vasculature. Visible veins are also common in hEDS particularly in patients with fair skin and the skin is often soft, velvety, and doughy but these characteristics are much more pronounced in patients with vEDS. We recommend evaluation for vEDS in patients with a personal history or first degree relative with an unprovoked, spontaneous arterial rupture at a young age (<40y), carotid-cavernous sinus fistula, hollow organ rupture, or a family history of vEDS.
Symptoms of Classic Ehlers Danlos Syndrome (cEDS) can overlap with hEDS including generalized JH. However, cEDS can easily be distinguished from hEDS by severe skin hyperextensibility and fragility: Skin hyperextensibility >2cm at the volar surface of the non-dominant wrist is suggestive of cEDS. Patients with cEDS also demonstrate extreme skin fragility with tearing of the skin with minor trauma not expected to break the skin. Patients develop papyraceous (tissue paper) or hemosideric scaring, frequently on the shins, forehead, chin, knees, and elbows. Patients with severe skin hyperextensibility, skin tearing, or papyraceous or hemosideric scaring should be referred to a CTD specialist for evaluation.
Marfan syndrome can be evaluated using the 2010 Ghent criteria.19 Key characteristics that differentiate Marfan Syndrome from HSD/hEDS include arachnodactyly, pectus or chest asymmetry, hindfoot deformity, spontaneous pneumothorax, and ectopia lentis. Arachnodactyly can be assessed by the wrist and thumb sign. The thumb sign is considered positive when the entire distal phalanx extends beyond the ulnar border of the palm and the wrist sign is positive when the tip of the thumb covers the entire fingernail of the fifth finger. These should be present bilaterally to count towards the systemic criteria. Hindfoot deformity is distinguished from pes planus with pronation by the presence abduction of the forefoot. A systemic score of 7 or higher is indicative of a diagnosis of Marfan Syndrome and necessitates genetic testing and evaluation of the aortic root and ascending aorta. Patients with aortic root dilation (Z = >2) or ectopia lentis should be referred to a CTD specialist for evaluation.
The presentation of Loeys-Dietz Syndrome (LDS) has more overlap with hEDS but typically includes features of Marfan Syndrome and may include additional symptoms such as craniosynostosis, cleft palate or bifid uvula, and arterial tortuosity. Musculoskeletal and craniofacial manifestations of LDS are variable. Patients with a personal or family history of arterial tortuosity or aneurysms or dissections should be referred for evaluation.
Severe musculoskeletal manifestations other than hypermobility may also prompt referral to a CTD specialist. Tall stature is often familial even in the absence of a CTD but may raise suspicion for Marfan Syndrome or Loeys-Dietz Syndrome as would a marfanoid habitus. Musculoskeletal features that may prompt consideration for an alternate diagnosis include severe or early onset (prior to puberty) scoliosis, pectus carinatum, pectus excavatum, and multiple or recurrent hernias. Flat feet with or without pronation is common in HSD/hEDS and in the absence of other concerning findings is not indicative of an alternate diagnosis. However, patients with a true hindfoot deformity (pes planus with pronation and forefoot abduction) warrant additional evaluation. Ligamentous tears in pediatric patients with hypermobility or multiple ligamentous tears in adults are not uncommon in patients with HSD/hEDS. Adults with ligamentous tears should also have an evaluation for joint pathology as some common ligamentous tears in adults are more strongly associated with age related degeneration. With the exception of vascular manifestations, most signs and symptoms of an alternate diagnosis are readily identifiable on clinical exam and are typically much more pronounced in patients with other CTD. A thorough family history may suggest vascular manifestations of a CTD and a one time screening echocardiogram is recommended in adults with hEDS which would identify aortic pathology. See Table 4 for additional symptoms that may suggest an alternative diagnosis.
Table 4.
Symptoms of other Connective Tissue Disorders
| Symptom | Considerations |
|---|---|
| Skin hyperextensiblity1 | cEDS, Classical-like EDS |
| Skin tearing with minor trauma2 | cEDS |
| Papyraceous or hemosideric scaring | cEDS |
| Skin tears at birth | Dermatosparaxis EDS |
| Uterine or bowel rupture | vEDS |
| Aortic dilation, aneurysm, dissections3 | vEDS, Marfan Syndrome, Loey Dietz |
| Tall Stature, marfanoid habitus | Marfan Syndrome, Loeys-Dietz Syndrome |
| Hindfoot deformity4 | Marfan Syndrome, Loeys-Dietz Syndrome |
| Cleft palate/bifid uvula, craniosynostosis | Loeys-Diet Syndrome |
| Pneumothorax | Marfan Syndrome, Loeys-Diet, Birt-Hogg-Dubé5 |
| Lens dislocation | Marfan Syndrome, Loeys-Dietz Synrome, Homocystinuria, non-syndromic ectopia lentis |
| Corneal rupture | Brittle cornea syndrome |
| Atraumatic retinal detachment <30y | Stickler Syndrome |
| Short stature | Spondylodysplastic EDS, Skeletal Dysplasia |
| Atraumatic fractures or fractures that do not heal | Osteogensis Imperfecta, Skeletal Dysplasia |
| Hearing loss | Stickler Syndrome |
| Hypotonia | Myopathic EDS, Spondylodysplastic EDS, Neuromuscular disorders |
| Congenital hip dislocations | Arthrochalasia type EDS |
| Congenital contractures | Myopathic or Muculocontractural EDS, Congenital Contractural Arachnodactyly |
| Severe, early periodontal disease, tooth loss | Periodontal EDS, Hypophosphatasia |
| Severe progressive cardiovalvular disease | Cardiovalvular EDS |
| Congenital heart disease | Noonan Syndrome, Kabuki Syndrome |
| Seizures | FLNA |
| Dysmorphic features | Multiple |
| Birth defects | Multiple |
| Intellectual disability | Multiple |
>2cm on the volar surface of the non-dominant wrist
Does not include delayed wound healing from trauma expected to break the surface of the skin
Patients with a first degree with TAAD should be screened by echocardiogram. Screening for abdominal aortic aneurysms is based on multiple risk factors [PMID: 37389507]
Distinguished from simple pes planus with pronation by the presence of forefoot abduction
When accompanied by associated skin manifestations. This is not included on most CTD panels
Management
HSD an hEDS are lifelong conditions. Patients require ongoing management of joint pain and associated symptoms, but these can often be managed effectively by primary care providers. There are many detailed papers that describe the management of hypermobility associated symptoms including an excellent overview in the American Family Physician.20 Here we hope to provide you with a basic overview.
Many patients with hypermobility have joint pain. Pain due to hypermobility typically begins in childhood with acute injuries (sprains, subluxations) and progresses to chronic pain in late adolescence or early adulthood.21,22 Pain is typically worse with activity. Joint pain starting in adulthood is not typical of HSD/hEDS and other causes of joint pain should be evaluated. Swelling in the absence of trauma, morning joint pain, or other markers of rheumatologic conditions is not indicative of pain due to hypermobility.
The pain in HSD/hEDS is multifactorial and incompletely understood.23 Pain does not always correlate with degree of hypermobility.24 Management of pain associated with hypermobility is symptomatic and follows the same general principles for treatment of any musculoskeletal pain. Physical activity is strongly encouraged. Kinesiophobia is common but inactivity will exacerbate joint pain.
Some patients with chronic pain require referral to pain management. Pain management referrals should be multidisciplinary and include, at a minimum, physical therapy and psychology. Expectations for therapeutic goals should also be discussed in patients with chronic pain. Complete resolution of pain is unlikely. Instead, treatment should focus on reduction of pain to manageable levels.
Physical therapy is a mainstay of treatment. Therapy should include evaluation of any acute injuries and overall balancing the joints and musculature. The approach should be comprehensive rather than focusing on a single joint whenever possible as pain and injuries in one location often lead to biomechanical stress on other parts of the body, muscular imbalance, and pain at remote sites.
Short-term bracing and taping may be used with the guidance of physical therapy while working to strengthen and stabilize the joint. Manual physical therapy can be helpful for patients with severe pain or comorbidities that limit activity such as postural tachycardia syndrome (POTS). Massage, acupuncture, and myofascial release can also be beneficial in some patients but may cause worsening of pain for 24–48 hour following treatments.
There are no specific recommendations for medications to treat musculoskeletal pain in patients with hypermobility. Over the counter analgesics (acetaminophen) and NSAIDS are first-line but use should be monitored and patients counseled on side effects and effects of medication over-use. Topical analgesics are well tolerated and have limited systemic effects. Muscle tension often develops as a result of joint stability and many patients benefit from use of muscle relaxers. Patients with neuropathic pain may benefit from medications such as SNRIs, TCAs, or gabapentin. Opiates are not recommended and should be used with extreme caution only when necessary.
Mitral valve prolapse and aortic dilation is seen more commonly in patients with hEDS and slightly more common in patients with HSD.25 A screening echocardiogram is recommended during late childhood and in adulthood. Serial echocardiograms are not recommended unless there are abnormalities identified on screening echocardiogram. True aortic aneurysms and arterial dissections are rare and should prompt consideration of an alternate diagnosis.25
POTS and orthostatic intolerance are common in patients with HSD/hEDS. Many patients experience symptom relief by increasing water intake to 2–3L per day, compression stockings, or increasing salt intake to 3–5g/d when there are no medical contraindications. Those with severe symptoms may require referral to a POTS specialist.
There is connective tissue throughout the body and abnormal connective tissue affects nearly every system. Easy bruising and slow wound healing may improve with vitamin C supplementation. Headaches and are common.26 Patients with severe headaches should be referred to a neurologist with low threshold for imaging to evaluate for Chiari malformation which is common in patients with HSD/hEDS. GI symptoms are very common. Many patients have functional GI disorders but there is also an increased incidence of gastroparesis and diverticulosis.27,28 Mental health disorders, particularly anxiety and depression, are also very common in patients with HSD/hEDS and concurrent mental health treatment is recommended in all patients.29,30
Conclusion
Joint hypermobility is common in the population and frequently associated with both musculoskeletal and systemic symptoms. A subset of patients with hypermobility meet clinical diagnostic criteria for HSD or hEDS. HSD/hEDS is a clinical diagnosis and genetic testing should not routinely be recommended. Patients with symptoms of other connective tissue disorders should be referred to a CTD specialist for evaluation. Primary care providers can effectively assess hypermobility and manage most patients with HSD/hEDS. Physical therapy, occupational therapy, and pain management specialists are an integral part of the care team.
Footnotes
Laura White, NP, and Sara S. Procknow, MD, PhD, are in the Department of Pediatrics, Division of Genetics and Genomic Medicine, Washington University School of Medicine, St. Louis, Missouri.
Disclosure: No financial disclosures reported. Artificial intelligence was not used in the study, research, preparation, or writing of this manuscript.
References
- 1.Scheper MC, de Vries JE, Juul-Kristensen B, Nollet F, Engelbert RH. The functional consequences of generalized joint hypermobility: a cross-sectional study. BMC Musculoskelet Disord. 2014 Jul 21;15:243. doi: 10.1186/1471-2474-15-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collinge R, Simmonds JV. Hypermobility, injury rate and rehabilitation in a professional football squad--a preliminary study. Phys Ther Sport. 2009 Aug;10(3):91–6. doi: 10.1016/j.ptsp.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Blajwajs L, Williams J, Timmons W, Sproule J. Hypermobility prevalence, measurements, and outcomes in childhood, adolescence, and emerging adulthood: a systematic review. Rheumatol Int. 2023 Aug;43(8):1423–1444. doi: 10.1007/s00296-023-05338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demmler JC, Atkinson MD, Reinhold EJ, Choy E, Lyons RA, Brophy ST. Diagnosed prevalence of Ehlers-Danlos syndrome and hypermobility spectrum disorder in Wales, UK: a national electronic cohort study and case-control comparison. BMJ Open. 2019 Nov 4;9(11):e031365. doi: 10.1136/bmjopen-2019-031365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damseh N, Dupuis L, O’Connor C, et al. Diagnostic outcomes for molecular genetic testing in children with suspected Ehlers-Danlos syndrome. Am J Med Genet A. 2022 May;188(5):1376–1383. doi: 10.1002/ajmg.a.62672. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Alharbi A, Shan H, et al. TAZ induces lung cancer stem cell properties and tumorigenesis by up-regulating ALDH1A1. Oncotarget. 2017 Jun 13;8(24):38426–38443. doi: 10.18632/oncotarget.16430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017 Mar;175(1):8–26. doi: 10.1002/ajmg.c.31552. [DOI] [PubMed] [Google Scholar]
- 8.Beighton P, de Paepe A, Danks D, et al. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am J Med Genet. 1988 Mar;29(3):581–94. doi: 10.1002/ajmg.1320290316. [DOI] [PubMed] [Google Scholar]
- 9.Tofts LJ, Simmonds J, Schwartz SB, et al. Pediatric joint hypermobility: a diagnostic framework and narrative review. Orphanet J Rare Dis. 2023 May 4;18(1):104. doi: 10.1186/s13023-023-02717-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh H, McKay M, Baldwin J, et al. Beighton scores and cut-offs across the lifespan: cross-sectional study of an Australian population. Rheumatology (Oxford) 2017 Nov 1;56(11):1857–1864. doi: 10.1093/rheumatology/kex043. [DOI] [PubMed] [Google Scholar]
- 11.Hakim AJ, Grahame R. A simple questionnaire to detect hypermobility: an adjunct to the assessment of patients with diffuse musculoskeletal pain. Int J Clin Pract. 2003 Apr;57(3):163–6. [PubMed] [Google Scholar]
- 12.Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A. A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet. 2017 Mar;175(1):148–157. doi: 10.1002/ajmg.c.31539. [DOI] [PubMed] [Google Scholar]
- 13.Remvig L, Jensen DV, Ward RC. Epidemiology of general joint hypermobility and basis for the proposed criteria for benign joint hypermobility syndrome: review of the literature. J Rheumatol. 2007 Apr;34(4):804–9. [PubMed] [Google Scholar]
- 14.Makhnoon S, Shirts BH, Bowen DJ. Patients’ perspectives of variants of uncertain significance and strategies for uncertainty management. J Genet Couns. 2019 Apr;28(2):313–325. doi: 10.1002/jgc4.1075. [DOI] [PubMed] [Google Scholar]
- 15.Mighton C, Shickh S, Uleryk E, Pechlivanoglou P, Bombard Y. Clinical and psychological outcomes of receiving a variant of uncertain significance from multigene panel testing or genomic sequencing: a systematic review and meta-analysis. Genet Med. 2021 Jan;23(1):22–33. doi: 10.1038/s41436-020-00957-2. [DOI] [PubMed] [Google Scholar]
- 16.Gould D, Walker R, Makari-Judson G, Seven M. Experiences of individuals with a variant of uncertain significance on genetic testing for hereditary cancer risks: a mixed method systematic review. J Community Genet. 2022 Aug;13(4):371–379. doi: 10.1007/s12687-022-00600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith TO, Easton V, Bacon H, et al. The relationship between benign joint hypermobility syndrome and psychological distress: a systematic review and meta-analysis. Rheumatology (Oxford) 2014 Jan;53(1):114–22. doi: 10.1093/rheumatology/ket317. [DOI] [PubMed] [Google Scholar]
- 18.Bulbena-Cabre A, Duno L, Almeda S, et al. Joint hypermobility is a marker for anxiety in children. Rev Psiquiatr Salud Ment (Engl Ed) 2019 Apr–Jun;12(2):68–76. doi: 10.1016/j.rpsm.2019.01.004. La hiperlaxitud articular como marcador de ansiedad en ninos. [DOI] [PubMed] [Google Scholar]
- 19.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010 Jul;47(7):476–85. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 20.Yew KS, Kamps-Schmitt KA, Borge R. Hypermobile Ehlers-Danlos Syndrome and Hypermobility Spectrum Disorders. Am Fam Physician. 2021 Apr 15;103(8):481–492. [PubMed] [Google Scholar]
- 21.Chopra P, Tinkle B, Hamonet C, et al. Pain management in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017 Mar;175(1):212–219. doi: 10.1002/ajmg.c.31554. [DOI] [PubMed] [Google Scholar]
- 22.Syx D, De Wandele I, Rombaut L, Malfait F.Hypermobility, the Ehlers-Danlos syndromes and chronic pain Clin Exp Rheumatol Sep-Oct201735Suppl 107(5)116–122. [PubMed] [Google Scholar]
- 23.Malfait F, Colman M, Vroman R, et al. Pain in the Ehlers-Danlos syndromes: Mechanisms, models, and challenges. Am J Med Genet C Semin Med Genet. 2021 Dec;187(4):429–445. doi: 10.1002/ajmg.c.31950. [DOI] [PubMed] [Google Scholar]
- 24.Reuter PR, Fichthorn KR.Prevalence of generalized joint hypermobility, musculoskeletal injuries, and chronic musculoskeletal pain among American university students PeerJ 20197e7625 10.7717/peerj.7625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rashed ER, Ruiz Maya T, Black J, et al. Cardiovascular manifestations of hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorders. Vasc Med. 2022 Jun;27(3):283–289. doi: 10.1177/1358863X211067566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jari M, Alesaeidi S. Correlation between benign joint hypermobility syndrome and headache in children and adolescents. BMC Musculoskelet Disord. 2024 May 2;25(1):347. doi: 10.1186/s12891-024-07473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam C, Amarasinghe G, Zarate-Lopez N, et al. Gastrointestinal symptoms and nutritional issues in patients with hypermobility disorders: assessment, diagnosis and management. Frontline Gastroenterol. 2023;14(1):68–77. doi: 10.1136/flgastro-2022-102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broad JB, Wu Z, Clark TG, et al. Diverticulosis and nine connective tissue disorders: epidemiological support for an association. Connect Tissue Res. 2019 Jul;60(4):389–398. doi: 10.1080/03008207.2019.1570169. [DOI] [PubMed] [Google Scholar]
- 29.Berglund B, Pettersson C, Pigg M, Kristiansson P. Self-reported quality of life, anxiety and depression in individuals with Ehlers-Danlos syndrome (EDS): a questionnaire study. BMC Musculoskelet Disord. 2015 Apr 15;16:89. doi: 10.1186/s12891-015-0549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark NL, Kainth GS, Johnson M, Rangan A, Kottam L, Swainston K. Psychological interventions to improve pain, fatigue, anxiety, depression, and quality of life in children and adults with hypermobility spectrum disorders and Ehlers-Danlos syndrome: a systematic review. Rheumatol Int. 2024 Jan;44(1):41–55. doi: 10.1007/s00296-023-05503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]