Abstract
DeSanto-Shinawi (DESSH) syndrome is a rare autosomal dominant condition caused by pathogenic variants in the WAC gene. DESSH syndrome was first identified in 2015 in six patients, but has since been diagnosed in more than 200 individuals worldwide. Patients exhibit a variable degree of developmental delay (DD), intellectual disability (ID), hypotonia, gastrointestinal and eye abnormalities, epilepsy, behavioral difficulties, and recognizable facial features. In order to educate families and address the complex medical needs of the increasing number of patients with DESSH syndrome, we established a new multidisciplinary clinic at Washington University in St. Louis. The first clinic was held in September 2022 and attended by 15 patients and their families. Herein, we report the structure of the clinic and present the main clinical findings of these patients. This pilot experience highlights the utility of a multidisciplinary approach to evaluating individuals with rare genetic diseases and the value of collaborating with family support groups to establish multidisciplinary clinics for these disorders, and provides guidance for future clinic planning.
Introduction
In 2015, six individuals with heterozygous loss-of-function pathogenic variants in the WW domain containing adaptor coiled coil (WAC) gene were identified by whole exome sequencing.1 The individuals in the initial cohort exhibited developmental delay, hypotonia, behavioral problems, eye abnormalities, GI problems including constipation and feeding difficulties, seizures, and sleep problems.1 Characteristic facial features such as broad/prominent forehead, synophrys and/or bushy eyebrows, depressed nasal bridge and bulbous nasal tip were also observed among all individuals. The recognizable facial gestalt and the constellation of findings associated with pathogenic variants in WAC highly suggested a new syndromic intellectual disability condition which later was named DeSanto-Shinawi (DESSH) syndrome (OMIM #616708) or WAC-related intellectual disability.2
Since DESSH syndrome was first reported,1 39 additional individuals have been reported.3–13 However, it is estimated that more than 200 individuals worldwide have been diagnosed with this condition. Additional reports describing DESSH syndrome have allowed for further characterization of individuals with this condition. It became clear that children with DESSH syndrome exhibit varying degrees of developmental delay and intellectual disability. Although almost all children display speech or motor delay and the majority of individuals have intellectual disability, affected individuals may have low normal range cognitive abilities.1,5 Children often have a variety of behavioral difficulties including anxiety, ADHD, and aggression. Autism and autistic features are also common features in affected individuals. Epilepsy has been reported in individuals with DESSH syndrome;10,14 the seizures often occur during sleep and may be associated with focal epileptiform discharges and continuous spike-wave on EEG.4,6,7 Structural brain anomalies (e.g., hydrocephalus, corpus callosum hypoplasia, pineal cyst and Chiari malformation type 1, etc.) have been reported.4 Eye problems including myopia, hyperopia and strabismus have also been reported.1,8 Frequent respiratory infections, skin infections, and hypogammaglobulinemia have been detected in DESSH individuals.5,8,9
The WAC gene (OMIM *615049) maps to the 10p12.1 region and encodes 647 amino acid protein called WW domain-containing adapter protein with coiled-coil.15,16 Most disease-causing variants in WAC are predicted to cause loss of function and haploinsufficiency; however, a missense variant has been reported in one individual with DESSH syndrome.3 There are individuals with microdeletions encompassing the short arm of chromosome 10 at 10p12p11 who also exhibit similar manifestations to individuals with DESSH syndrome secondary to single nucleotide variants, though larger deletions are typically associated with more severe clinical and developmental findings. 6,8,17
The WAC protein is mainly located in the nucleus but also found in Golgi18 and is involved in several cellular functions and pathways. It is expressed in all adult and fetal tissues but highest expression is found in the cerebellum. It regulates transcription elongation through histone H2B monoubiquitination and in vitro studies showed that loss of WAC function results in decreased H2B ubiquitination and subsequently inhibition of gene transcription.19 WAC also regulates p53-dependent cell-cycle checkpoint activation after DNA damage. While, this may implicate DESSH patients are at a higher risk of malignancy, this has not been reported.2,3 In addition, WAC is involved in microtubule generation, Golgi apparatus reformation, autophagy regulation, autophagosome formation, and mTOR signaling pathway.20,21 Given the multiple roles of the WAC protein and its ubiquitous expression in the body, it is not surprising that dysfunction of WAC results in disease affecting several organ systems.
Here, we describe our experience building a multidisciplinary clinic for individuals with DESSH syndrome and present a detailed clinical characterization of 15 individuals with this condition who attended our inaugural clinic in 2022.
Methods
To address the complex medical needs of patients with DESSH syndrome and to further explore the DESSH phenotype, in September 2022, Washington University School of Medicine (WUSM) and St. Louis Children’s Hospital in collaboration with the DESSH Foundation (https://www.dessh.org/) hosted the first DESSH clinic in St. Louis, Missouri. A multidisciplinary team including genetics, neurology, ophthalmology, and neuropsychology evaluated each individual. Of the 15 individuals who traveled to the clinic, 13 patients came from USA, one from Germany, and one from Australia. The guardians of the individuals signed consents form to participate in research approved by Washington University Institutional Review Board. Biospecimens were obtained for research purposes. At the conclusion of the clinic, families were invited to stay for an additional day where the DESSH Foundation hosted the DESSH Family Conference at WUSM, where experts presented educational materials and discussed the condition with families and answered their questions. Demographic, clinical and neurological data were pulled from each of the specialty note visits to determine the prevalence of various aspects of the DESSH phenotype.
Results
Each individual was examined and assessed by all four specialty teams. The duration of each specialty visit ranged from 20 to 60 minutes, and the clinic lasted for 2 days. The genetics team counseled the parents about the genetic results, recurrence risk, and the phenotypes associated with DESSH syndrome. Other specialty teams discussed their recommendations with families and issued clinic notes to be shared with each individual’s local providers. The mean age of individuals who attended the clinic was 9.27 +/−4.26 years (Range 3 y 1 month, 18 y 4 months). Eight of the individuals were females and seven were males. All individuals were previously diagnosed by their medical teams with DESSH syndrome at a mean age of 5 y 7 mo (range 3mo-16 yo) based on clinical exome sequencing (n=13), neurodevelopmental expanded panel (n=1), and chromosomal microarray analysis (CMA) (n=1). For 12 individuals, parental testing has been completed; the pathogenic variant was de novo in 11 and maternally inherited in one individual. All patients had heterozygous loss of function pathogenic variants in WAC. Eight of these variants were frameshift, four nonsense, and two splicing. In addition, one female individual had a 715 Kb deletion encompassing the WAC gene on 10p12.1 (28,637,557–29,352,961; hg19).
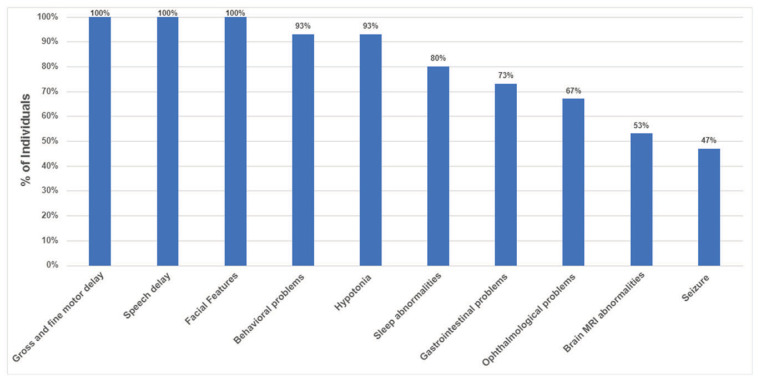
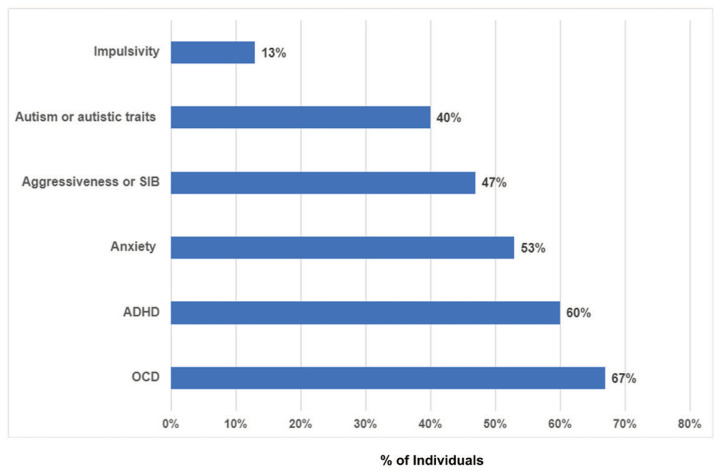
All patients experienced gross motor, fine motor, and speech delay (15/15) (Figure 1) as well as characteristic facial features (Figure 2). Nine patients had formal cognitive testing based on which they displayed mild to moderate intellectual disability (mean Intelligence Quotient=66; range:51–81; n=7; 2 additional patients diagnosed with moderate ID using other tools). Expressive language delay, within the realm of a speech apraxia, in early childhood and fine motor delays were particularly common. Hypotonia was also a common finding in infancy and early childhood, occurring in 14 out of the 15 patients in this cohort. Ninety-three percent of patients experienced behavioral problems (Figure 3) including obsessive compulsive disorder (OCD) (67%), attention deficit hyperactivity disorder (ADHD) (60%), autism or autistic traits (40%), anxiety, aggressive behaviors and impulsivity. Eighty percent of children in this cohort experienced sleep disturbances including difficulty falling asleep, multiple awakenings, and sleep apnea. Gastrointestinal problems were reported in 73% of the patients including constipation, gastroesophageal reflux, and feeding difficulties. Eye findings were also common and detected in 67% of patients and included strabismus (53%), astigmatism (13%), and cortical visual impairment (13%). Forty-seven percent of individuals experienced seizures and seven out of the 13 individuals who underwent brain imaging (53%) had nonspecific MRI findings. Interestingly, five out of the seven individuals with epilepsy had brain abnormalities; this correlation may suggest a higher seizure risk among individuals with structural brain abnormalities.
Figure 1.
Frequency of most common clinical findings among individuals with DESSH syndrome.
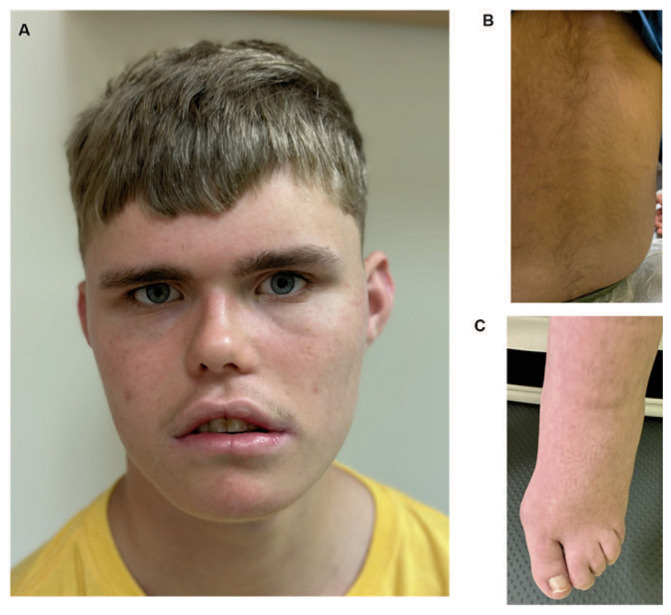
Figure 2.
A, B, Physical findings in individuals with DESSH syndrome. A. A 16-year-old boy with facial differences that are typical for DESSH syndrome including broad forehead, squared face, deep set eyes, and think and straight eyebrow. B. Hirsutism is a common feature in DESSH syndrome as illustrated here on the back of a 6-year-old girl. C. Foot and toe anomalies are being increasingly recognized as a common findings; show her hallus valgus deformity and 2-3 toe syndactyly.
Figure 3.
Prevalence of behavioral problems in individuals with DESSH syndrome. ADHD- attention deficit hyperactivity disorder; OCD-obsessive compulsive disorder; SIB- self injurious behavior.
All patients displayed recognizable facial and physical features. Common findings included deep set eyes (100%), hirsutism and/or low hairline (100%), thickened eyebrows (80%), broad forehead (60%), depressed nasal bridge (53%), synophrys (53%), wide mouth and dental anomalies (47% each).
At the conclusion of the multidisciplinary clinic, families were invited to stay for the DESSH Family Conference hosted by the DESSH Foundation. Expert speakers gave talks to families attending the event regarding the genetic aspects of DESSH syndrome, ongoing molecular research utilizing animal and in vitro models, and the neurological manifestations, ophthalmological findings, and psychiatric/psychological difficulties associated with this syndrome (Figure 4). This conference provided families with the opportunity to speak with experts and ask questions about interventions and management options, and it enabled experts to engage with each other to discuss research opportunities to advance the molecular understanding of this disorder. Overall, feedback from families indicated the success of this multidisciplinary model.
Figure 4.
Dr. Margaret Reynolds presented a talk at the DESSH Family Conference about the ophthalmological findings in individuals with DESSH syndrome.
Discussion
DESSH syndrome is a rare autosomal dominant syndromic intellectual disability condition caused by pathogenic variants in the WAC gene resulting in characteristic facial features, variable degrees of developmental delay/intellectual disability, hypotonia, and behavioral problems. We also show high prevalence in our cohort of gastrointestinal problems, ophthalmological findings, nonspecific brain MRI abnormalities and epilepsy.
We show that the complex problems and needs of individuals with DESSH syndrome can be evaluated successfully in a multidisciplinary setting. Such multidisciplinary clinics for rare diseases will help pave the way for medical professionals to develop expertise in rare conditions. In addition, this pilot experience highlights the value of collaborating with family support groups and provides guidance for future DESSH clinic planning. Furthermore, the collection of biospecimens will serve as a platform for the establishment of a sizeable repository that will benefit researchers looking into this condition.
The limitations of this study included a relatively small sample size, which limited the power of our statistical analysis. Although all individuals had a uniform assessment by same medical professional, a formal cognitive assessment has not been conducted due to time constrains in the clinic. There may have been a bias in the selection of individuals attending the clinic based on the severity of their condition or epilepsy.
We plan to study larger cohorts of individuals with DESSH syndrome in subsequent clinics to understand the full spectrum of manifestations and investigate potential genotype phenotype correlation. In the future, questionnaires distributed to parents will be used to assess utility of this clinic from the viewpoint of the families. Formal neuropsychological evaluation, either as part of the multidisciplinary clinic or through an evaluation collaborative, can help to further characterize the cognitive phenotype beyond diagnoses of intellectual disability, as well as better delineating the specific behavioral phenotype of DESSH syndrome, as has been the case for other intellectual disability syndromes.22, 23 It is important also to evaluate adult individuals with DESSH syndrome to understand the natural history of this condition focusing on signs or symptoms of developmental regression or neurodegeneration. There is a great need to study the effect of early therapeutic interventions on ameliorating the neurobehavioral abnormalities and medical treatments (e.g., medications for ADHD and anxiety) on improving neurocognitive outcomes. To accomplish these objectives, it is crucial to research in depth the functions of WAC and the pathophysiology of DESSH syndrome in model organisms and in vitro systems. We anticipate that the utilization of available resources, including the DESSH clinic, and opportunities in science, which will be enhanced by the biorepository collection, will advance our efforts toward the long-term goal to find a cure for DESSH syndrome.
Acknowledgment
We would like to thank the families of the individuals presented in this article for agreeing to participate in the study. This project was made possible by the generosity of many medical professionals (Nino Kerashvili, MD, Jennifer Heeley, MD, Angela Lee, MD, Rodrigo Tzovenos Starosta, MD, Nicholas DeKorver, MD, and Kathleen Sisco, PNP) and the DESSH Foundation. Special thanks to Patricia Dickson, MD, who supported all stages of establishing this clinic. We also thank Yolonda Phillips, Becky Kolb, Dana Kiley, Shelby Cripe, and Sophia Couteranis for their planning and logistic support of the clinic.
Footnotes
Margaret Reynolds, MD, is in the Department of Ophthalmology and Visual Sciences; Judith Weisenberg, MD, is in the Department of Neurology, Division of Pediatric Neurology; and Marwan Shinawi, MD, (pictured), is Division of Genetics and Genomic Medicine; all are at the Washington University School of Medicine, St. Louis, Missouri. Rachel Jensen, PhD, is in Department of Pediatrics, Division of Neurology, Children’s Mercy/ University of Missouri Kansas City, Kansas City, Missouri.
Disclosure: No financial disclosures reported. Artificial intelligence was not used in the study, research, preparation, or writing of this manuscript.
References
- 1.De Santo C, D’Aco K, Araujo GC, et al. WAC loss-of-function mutations cause a recognisable syndrome characterised by dysmorphic features, developmental delay and hypotonia and recapitulate 10p11.23 microdeletion syndrome. J Med Genet. 2015;52(11) doi: 10.1136/jmedgenet-2015-103069. [DOI] [PubMed] [Google Scholar]
- 2.Lugtenberg D, Reijnders MRF, Fenckova M, et al. De novo loss-of-function mutations in WAC cause a recognizable intellectual disability syndrome and learning deficits in Drosophila. European Journal of Human Genetics. 2016;24(8) doi: 10.1038/ejhg.2015.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquali D, Torella A, Grandone A, et al. Patients with DeSanto–Shinawi syndrome: Further extension of phenotype from Italy. Am J Med Genet A. 2023;191(3) doi: 10.1002/ajmg.a.63061. [DOI] [PubMed] [Google Scholar]
- 4.Morales JA, Valenzuela I, Cuscó I, et al. Clinical and molecular characterization of five new individuals with WAC-related intellectual disability: Evidence of pathogenicity for a novel splicing variant. Am J Med Genet A. 2022;188(5) doi: 10.1002/ajmg.a.62648. [DOI] [PubMed] [Google Scholar]
- 5.Leonardi E, Bellini M, Aspromonte MC, et al. A novel WAC loss of function mutation in an individual presenting with encephalopathy related to status epilepticus during sleep (ESES) Genes (Basel) 2020;11(3) doi: 10.3390/genes11030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolat H, Derin H, Ünsel-Bolat G. Phenotypic and Brain Imaging Findings Associated with a 10p Proximal Deletion Including the WAC Gene: Case Report and Literature Review. Cognitive and Behavioral Neurology. 2022;35(3) doi: 10.1097/WNN.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 7.Branco J, Amorim M, Conde M. A novel variant of DeSanto-Shinawi Syndrome with joint manifestations. Eur J Med Genet. 2022;65(7) doi: 10.1016/j.ejmg.2022.104534. [DOI] [PubMed] [Google Scholar]
- 8.Toledo-Gotor C, García-Muro C, García-Oguiza A, Poch-Olivé ML, Ruiz-del Prado MY, Domínguez-Garrido E. Phenotypic comparison of patients affected with DeSanto-Shinawi syndrome: Point mutations in WAC gene versus a 10p12.1 microdeletion including WAC. Mol Genet Genomic Med. 2022;10(5) doi: 10.1002/mgg3.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uehara T, Ishige T, Hattori S, et al. Three patients with DeSanto-Shinawi syndrome: Further phenotypic delineation. Am J Med Genet A. 2018;176(6) doi: 10.1002/ajmg.a.38703. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Yao P, Zhou YF, et al. [WAC gene pathogenic variation cause DeSanto-Shinawi syndrome with electrical status epilepticus during sleep]. Zhonghua Er Ke Za Zhi. 2019;57(10):802–804. doi: 10.3760/cma.j.issn.0578-1310.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Ho S, Luk HM, Lo IFM. Extending the phenotype of DeSanto-Shinawi syndrome: A case report and literature review. Am J Med Genet A. 2022;188(3) doi: 10.1002/ajmg.a.62571. [DOI] [PubMed] [Google Scholar]
- 12.Vanegas S, Ramirez-Montanõ D, Candelo E, Shinawi M, Pachajoa H. DeSanto-shinawi syndrome: First case in South America. Mol Syndromol. 2018;9(3) doi: 10.1159/000488815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsahlawi Z, Jailani M, Alaradi H, AlAbbad A. A Case of DeSanto-Shinawi Syndrome in Bahrain with a Novel Mutation. Case Rep Pediatr. 2020:2020. doi: 10.1155/2020/8820966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alawadhi A, Morgan AT, Mucha BE, Scheffer IE, Myers KA. Self-limited focal epilepsy and childhood apraxia of speech with WAC pathogenic variants. European Journal of Paediatric Neurology. 2021:30. doi: 10.1016/j.ejpn.2020.12.010. [DOI] [PubMed] [Google Scholar]
- 15.David-Morrison G, Xu Z, Rui YN, et al. WAC Regulates mTOR Activity by Acting as an Adaptor for the TTT and Pontin/Reptin Complexes. Dev Cell. 2016;36(2) doi: 10.1016/j.devcel.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joachim J, Jefferies HBJ, Razi M, et al. Activation of ULK Kinase and Autophagy by GABARAP Trafficking from the Centrosome Is Regulated by WAC and GM130. Mol Cell. 2015;60(6) doi: 10.1016/j.molcel.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto N, Hayashi S, Masui A, et al. Deletion at chromosome 10p11.23-p12.1 defines characteristic phenotypes with marked midface retrusion. J Hum Genet. 2012;57(3) doi: 10.1038/jhg.2011.154. [DOI] [PubMed] [Google Scholar]
- 18.Totsukawa G, Kaneko Y, Uchiyama K, Toh H, Tamura K, Kondo H. VCIP135 deubiquitinase and its binding protein, WAC, in p97ATPase-mediated membrane fusion. EMBO Journal. 2011;30(17) doi: 10.1038/emboj.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F, Yu X. WAC, a Functional Partner of RNF20/40, Regulates Histone H2B Ubiquitination and Gene Transcription. Mol Cell. 2011;41(4) doi: 10.1016/j.molcel.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joachim J, Wirth M, McKnight NC, Tooze SA. Coiling up with SCOC and WAC: Two new regulators of starvation-induced autophagy. Autophagy. 2012;8(9) doi: 10.4161/auto.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKnight NC, Jefferies HBJ, Alemu EA, et al. Genome-wide siRNA screen reveals amino acid starvation-induced autophagy requires SCOC and WAC. EMBO Journal. 2012;31(8) doi: 10.1038/emboj.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mervis CB, John AE. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2. Vol. 154. Hoboken: Wiley Subscription Services, Inc., A Wiley Company.; 2010. May, Cognitive and behavioral characteristics of children with Williams syndrome: implications for intervention approaches; pp. 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grieco J, Pulsifer M, Seligsohn K, Skotko B, Schwartz A. Down syndrome: Cognitive and behavioral functioning across the lifespan. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2015 June;169(2):135–149. doi: 10.1002/ajmg.c.31439. [DOI] [PubMed] [Google Scholar]