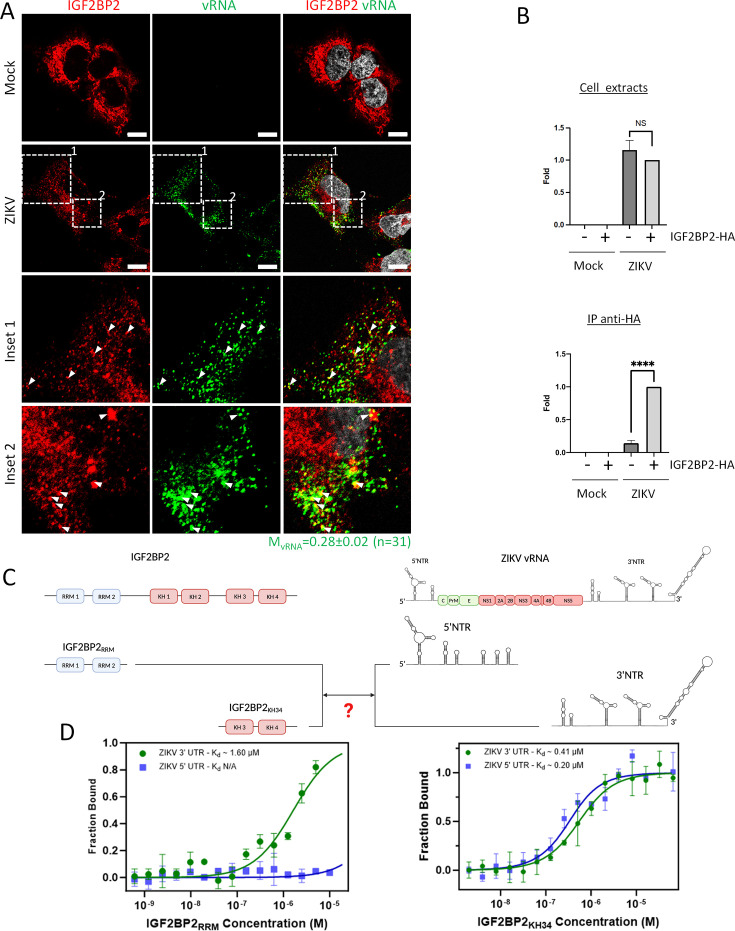
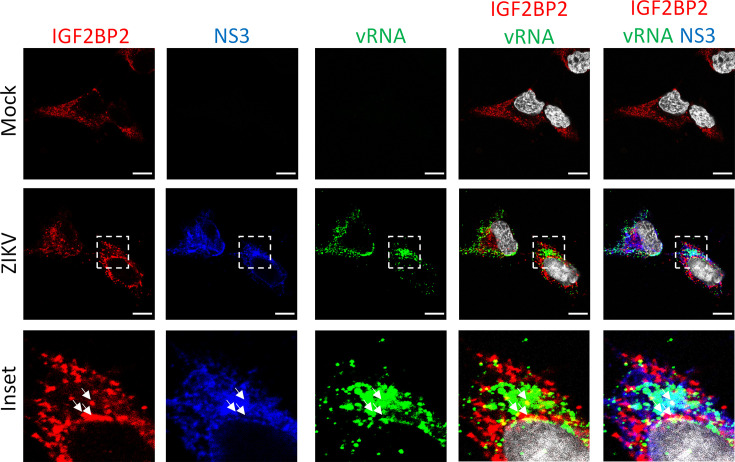
Figure 5. IGF2BP2 interacts with Zika virus (ZIKV) viral RNA (vRNA).
(A) Fluorescence in situ hybridization (FISH) and IGF2BP2 immunostaining were performed using Huh 7.5 cells which were infected for 2 days with ZIKV (MOI = 10) or left uninfected. The Manders’ coefficient (mean ± SEM) representing the fraction of vRNA signal overlapping with IGF2BP2 signal is shown (n=number of cells). Scale bar = 10 µm. (B) Huh7.5 cells expressing IGF2BP2-HA and control cells were infected with ZIKV H/PF/2013 at an MOI of 10, or left uninfected. Two days later, cell extracts were prepared and subjected to anti-HA immunoprecipitations. Extracted vRNA levels were measured by RT-qPCR. Means ± SEM are shown based on three independent experiments. ****: p<0.0001; NS: not significant (unpaired t-test). (C) IGF2BP2 recombinant proteins containing either the two RNA recognition motifs (RRM) or KH3 and KH4 domains were produced in bacteria and purified. In parallel ZIKV 5’ nontranslated region (NTR) and 3’ NTR were synthesized by in vitro transcription. (D) Combination of truncated IGF2BP2 proteins and either ZIKV 5’ NTR (blue squares) or ZIKV 3’ NTR (green circles) were used for in vitro binding assays using microscale thermophoresis.