Abstract
Protein kinase adenosine monophosphate-activated non-catalytic subunit gamma 2 (PRKAG2) cardiac syndrome is a rare genetic disorder characterized by hypertrophic cardiomyopathy and heart rhythm disturbances caused by mutations in the PRKAG2 gene. Reports on PRKAG2 cardiac syndrome associated with refractory chylous effusion are extremely limited. Here, we present a neonatal case involving severe hypertrophic obstructive cardiomyopathy accompanied by chylous ascites and lymphatic malformations. The patient was diagnosed prenatally with hypertrophic cardiomyopathy. After birth, she developed severe respiratory failure, along with refractory chylous and pericardial effusions. Lymphoscintigraphy revealed lymphatic malformations in the right inguinal region. Prednisolone and sirolimus were administered to manage the chylous ascites and lymphatic malformations. Unfortunately, the patient succumbed to sepsis at two months of age. A de novo c.1592G>A (p.Arg531Gln) heterozygous variant of PRKAG2 has also been identified. The association between PRKAG2, chylous effusion, and lymphatic malformations remains unclear. Further research is required to assess the effects and safety of prednisolone and sirolimus on chylous ascites in patients with PRKAG2 cardiac syndrome.
Keywords: chylous ascites, hypertrophic cardiomyopathy (hcm), lymphatic malformations, mtor inhibitors, prednisolone, prkag2, sirolimus
Introduction
The protein kinase adenosine monophosphate-activated non-catalytic subunit gamma 2 (PRKAG2) gene encodes the γ2 subunit of adenosine monophosphate-activated protein kinase (AMPK), regulating cellular energy homeostasis [1]. Mutations in the PRKAG2 gene cause chronic activation of AMPK, leading to glycogen accumulation in cells [2]. PRKAG2 cardiac syndrome is a rare autosomal dominant genetic disorder characterized by ventricular preexcitation, supraventricular arrhythmias, and cardiac hypertrophy due to glycogen accumulation in the myocardium [1,2]. In severe cases, patients show fetal or neonatal hypertrophic cardiomyopathy with a lethal prognosis [3]. However, there are very few reports of PRKAG2 cardiac syndrome associated with refractory chylous effusion. Here, we report a rare neonatal case of severe hypertrophic cardiomyopathy caused by PRKAG2 cardiac syndrome, presenting with refractory chylous ascites and lymphatic malformations.
Case presentation
The patient's mother was a healthy 27-year-old Asian female, gravida 4 para 3, with no family history of cardiac disease. Fetal ultrasonography performed at 25 weeks of gestation revealed biventricular hypertrophy, pericardial effusion, and bradycardia. Pulmonary hypoplasia was suspected due to severe cardiomegaly and abdominal distension. Fetal hydrops and severe myocardial hypertrophy were worsening.
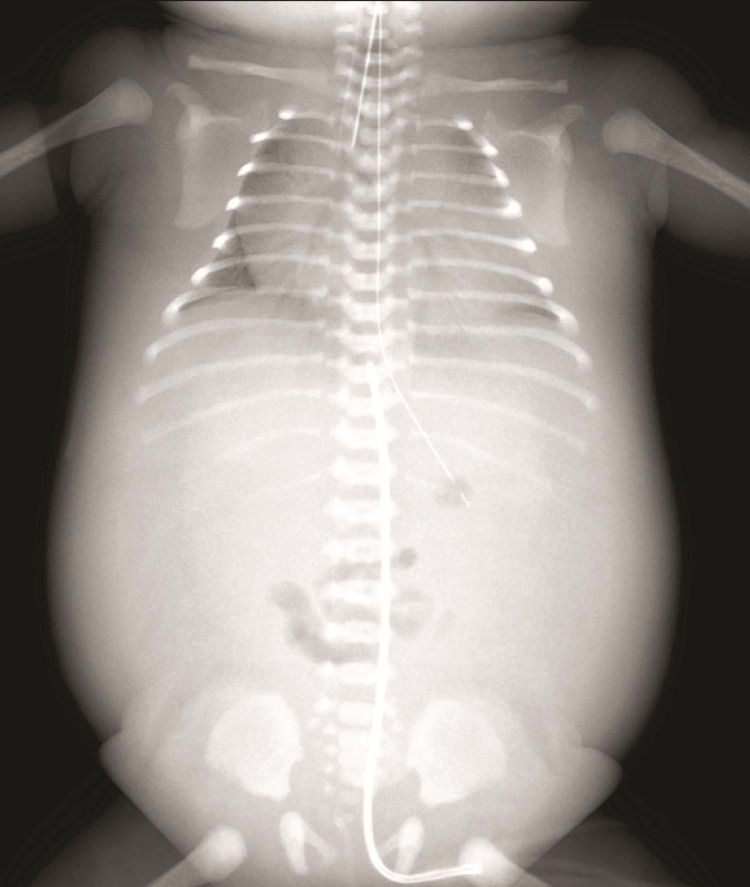
A female infant was delivered vaginally, at 34 weeks gestation, weighing 2,500 grams at birth. Soon after birth, surfactant therapy and mechanical ventilation were required, and inhaled nitric oxide therapy was initiated for persistent pulmonary hypertension of the newborn due to pulmonary hypoplasia (Figure 1).
Figure 1. Chest and abdominal radiograph.
Chest and abdominal radiography performed on the first day of life revealed cardiomegaly, pulmonary hypoplasia, and marked systemic and pulmonary edema.
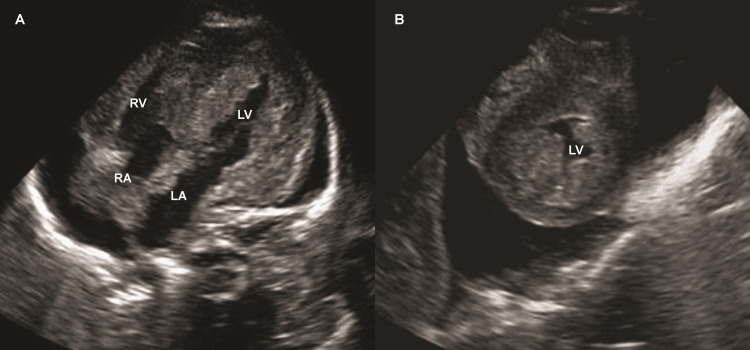
Echocardiography showed asymmetric myocardial hypertrophy, with a ventricular septal thickness of 13.0 mm (+22.2 SD) and a left ventricular posterior wall thickness of 7.7 mm (+10.6 SD). Left ventricular outflow tract blood flow was accelerated (2.2 m/s), leading to a diagnosis of hypertrophic obstructive cardiomyopathy (Figure 2). Electrocardiography revealed normal sinus rhythm, bradycardia, and a short PR interval.
Figure 2. Transthoracic echocardiography.
Echocardiography in the apical four-chamber (A) and short-axis (B) views revealed severe ventricular hypertrophy and pericardial effusion.
RV: right ventricle; RA: right atrium; LV: left ventricle; LA: left atrium
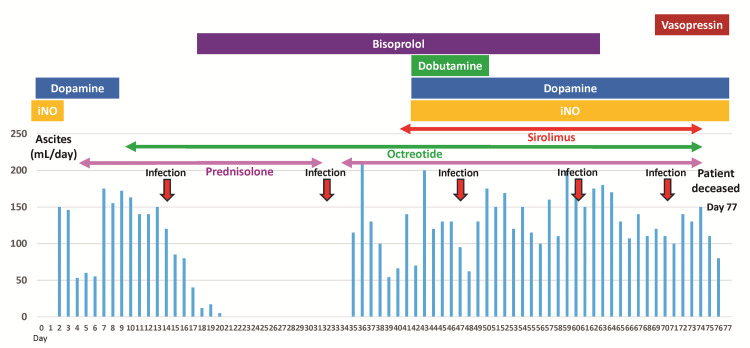
Abdominal tube drainage was required from the first day of age, and ascitic fluid with lymphocytosis (lymphocytes, 92%) was diagnosed as chylous ascites. Despite total parenteral nutrition, the ascites persisted at approximately 50 mL/kg/day. After the initiation of prednisolone and octreotide, the ascites temporarily decreased, and abdominal tube drainage was paused at 21 days of age. However, ascites reaccumulated after the discontinuation of prednisolone at 31 days of age, necessitating re-drainage at 35 days of age (Figure 3).
Figure 3. Clinical course.
The blue bar graph shows the daily volume of ascitic fluid in the abdominal drainage tube. Abdominal drainage was temporarily discontinued at 21 days of age. Ascites reaccumulated after the discontinuation of prednisolone at 31 days of age. The patient repeatedly developed bacterial infections (red arrow). Bisoprolol is also used to treat hypertrophic obstructive cardiomyopathy. At 42 days of age, dopamine, dobutamine, and inhaled nitric oxide were initiated for hypotension and hypoxemia due to worsening ascites and pericardial effusion.
iNO: inhaled nitric oxide
G-band chromosome analysis of the patient revealed a normal karyotype. Although both hypertrophic cardiomyopathy and chylothorax are common in Noonan's syndrome [4], no variants have been identified in the genes related to Noonan syndrome (PTPN11, SOS1, RAF1, RIT1, KRAS, NRAS, SHOC2, CBL, BRAF, SOS2, MRAS, RRAS, LZTR1, RRAS2, HRAS, MAP2K1, MAP2K2, and PPP1CB) by a targeted next-generation sequencing panel testing. Pompe, Fabry, Gaucher, and Mucopolysaccharidosis may be the underlying diseases of hypertrophic cardiomyopathy [5], but the activity of each lysosomal enzyme is normal.
A large amount of chylous ascites of 50 to 200 mL/kg/day persisted, thus daily blood product transfusions were required for hypoalbuminemia and coagulopathy. Pericardial effusion gradually worsened. Lymphoscintigraphy performed at 40 days of age showed abnormal accumulation in the right inguinal region, leading to a diagnosis of lymphatic malformation. Sirolimus, a mechanistic target of rapamycin (mTOR) inhibitor, was initiated for refractory chylous ascites associated with lymphatic malformations at 41 days of age. After initiating sirolimus, the pericardial effusion significantly decreased. However, the chylous ascites and generalized edema did not improve. She developed severe hypoxemia and hypotension due to sepsis at 70 days of age and eventually died at 77 days of age. Next-generation whole exome sequencing of the patient and her parents revealed a pathogenic de novo c.1592G>A (p.Arg531Gln) heterozygous variant in the PRKAG2 gene.
Discussion
We present a rare case of severe hypertrophic cardiomyopathy with refractory chylous ascites that eventually identified a pathogenic mutation in the PRKAG2 gene. To our knowledge, this is the first reported case of PRKAG2 cardiac syndrome with refractory chylous ascites and lymphatic malformations. Additionally, although prednisolone and sirolimus were partially effective in the management of hydrops in this case, these drugs may be related to the occurrence of sepsis, due to their immunosuppressive effects.
PRKAG2 gene encodes the γ2-subunit of AMPK, regulating cellular energy homeostasis [6]. Certain mutations in PRKAG2 cause the continuous activation of AMPK, excessive glucose uptake, and glycogen synthesis, leading to non-lysosomal glycogen storage in cardiac muscle cells [7]. The phenotypes of PRKAG2 cardiac syndrome vary, typically with ventricular pre-excitation and cardiac hypertrophy in adolescence and adulthood, and rarely with fetal- and infantile-onset severe hypertrophic cardiomyopathy [8].
In previous studies, p.Arg531Gln was reported to be a mutation in PRKAG2 associated with lethal hypertrophic cardiomyopathy [9]. We systematically searched fetal and infantile cases in PubMed using following search strategy: ("Fetus"[Mesh] OR "Infant, Newborn"[Mesh] OR "Infant"[Mesh] OR fetus [TIAB] OR fetal [TIAB] OR neonat* [TIAB] OR newborn [TIAB] OR infant* [TIAB] OR baby [TIAB] OR babies [TIAB]) AND (PRKAG2 [TIAB] OR "AMP-Activated Protein Kinases"[Mesh] OR AMP-activated protein kinase [TIAB]) AND ("glycogen storage disease"[Mesh] OR "Cardiomyopathies"[Mesh] OR "Cardiomyopathy, Hypertrophic"[Mesh] OR cardiomyopathy [TIAB] OR hypertrophic cardiomyopathy [TIAB]). The initial database search identified 80 reports, and 70 reports were excluded after reviewing the titles and abstracts: 64 reports were basic science investigations, and six reports involved children and adults beyond the infantile period. Of these, 14 fetal or infantile cases from 10 reports were identified (Table 1) [3,6,7,9-15].
Table 1. Infantile-onset cardiomyopathy with PRKAG2 mutation.
*Same variant with our case
HCM: hypertrophic cardiomyopathy; DCM: dilated cardiomyopathy; IVS: interventricular septum; PRKAG2: protein kinase adenosine monophosphate-activated non-catalytic subunit gamma 2
| Author | Year | Sex | Gestational age | Presenting age | Clinical findings | Outcome | Mutation |
| Burwinkel et al. [10] | 2005 | Female | 31 weeks | Fetus | Cardiomegaly, bradycardia, ventricular fibrillation | Died (2 months) | Arg531Gln (1592G>A)* |
| Female | 37 weeks | 1st week of life | Fetal bradycardia, cardiac failure, severe hypertrophic cardiomyopathy | Died (34 days) | Arg531Gln (1592G>A)* | ||
| Male | 28 weeks | At birth | Bradycardia, biventricular hypertrophy, pericardial and pleural effusions, ascites | Died (21 days) | Arg531Gln (1592G>A)* | ||
| Akman et al. [3] | 2007 | Female | NA | 10 weeks of life | Severe biventricular hypertrophy | Died (5 months) | Arg384Thr (1151G>C) |
| Kelly et al. [11] | 2009 | Male | NA | 6 months of life | Systolic ejection murmur, left ventricular hypertrophy, HCM | Alive | Glu506Gln (NA) |
| Austin et al. [7] | 2017 | Male | 38 weeks | 2.5 months of life | Hypotonia, areflexia, feeding difficulties, mild hypertrophy of the IVS | Alive | Gly100Ser (298G>A) |
| Torok et al. [12] | 2017 | Female | Term | Fetus (27 weeks) | Mild hypertrophy of the IVS, HCM developmental delay | Alive | Lys475Glu (1423A>G) |
| Female | Term | 5 weeks of life | Severe HCM, ventricular ectopy, progressive cardiopulmonary failure | Died (4 months) | Arg531Gln (1592G>A)* | ||
| Male | Term | 2 months of life | Hypotonia, areflexia, mild hypertrophy of the IVS | Alive | Gly100Ser (298G>A) | ||
| Xu et al. [6] | 2017 | Female | NA | Fetus (27 weeks) | Modest HCM | Alive | Lys475Glu (1423A>G) |
| Gorla et al. [9] | 2018 | Male | 36 weeks | Fetus (28 weeks) | Fetal hydrops, severe HCM small pericardial effusion | Died (7 weeks) | Arg531Gln (1592G>A)* |
| Beyzaei et al. [13] | 2021 | Female | NA | 1 month of life | Pulmonary hypertension, short stature mitral, and tricuspid regurgitation | Alive | Met198Leu (592A>T) |
| Gong et al. [14] | 2022 | Female | NA | 9 months of life | DCM with heart failure | Alive | Thr142Ile (425C>T) |
| White-Brown et al. [15] | 2024 | Male | 35 weeks | Fetus (32 weeks) | Biventricular hypertrophy, bradycardia, large pleural effusion | Died (17 hours) | Arg384Gly (1150A>G) |
| Present case | 2024 | Female | 34 weeks | Fetus (25 weeks) | Fetal hydrops, severe HCM refractory ascites, pericardial effusion | Died (77 days) | Arg531Gln (1592G>A)* |
Of these, five cases showed a heterozygous c.1592G>A (p.Arg531Gln) mutation in PRKAG2, and all of them died within several months of age. The mutation of p.Arg531Gln is associated with the lethal phenotype of PRKAG2 cardiac syndrome. The poor clinical prognosis of the present case with p.Arg531Gln is consistent with that of previous reports.
However, the association between the p.Arg531Gln mutation, refractory chylous ascites, and lymphatic malformations remains unclear. To the best of our knowledge, this is the first reported case of PRKAG2 cardiac syndrome with refractory chylous ascites and lymphatic malformations. Further studies are required to assess the relationship between PRKAG2, chylous ascites, and lymphatic malformations.
Generally, the management of neonatal chylous effusion includes medium-chain triglyceride (MCT) formulas, total parenteral nutrition, and octreotide [16]. Prednisolone is a useful pharmacological treatment for chylous effusions [17,18]. In the present case, chylous ascites significantly worsened after the discontinuation of prednisolone; thus, we assessed whether prednisolone was effective in our patient. Recent studies have reported the effectiveness of sirolimus in treating chylous effusions caused by lymphatic malformations [19]. In the present case, although the pericardial effusion improved after the initiation of sirolimus, the chylous ascites did not improve. Moreover, due to their immunosuppressive effects, prednisolone and sirolimus may be associated with the occurrence of sepsis.
Conclusions
PRKAG2 cardiac syndrome caused by the p.Arg531Gln mutation may present a lethal prognosis. Further studies are required to investigate the pathophysiological association between chylous effusions and lymphatic malformations and to determine the effects and safety of immunosuppressive therapies for chylous effusions in patients with PRKAG2 cardiac syndrome.
Acknowledgments
We thank the patient and her parents for their contribution to this study. We also thank all the doctors and medical staff involved in the treatment of this infant. Exome sequencing was performed at the National Center for Child Health and Development under the Initiative on Rare and Undiagnosed Diseases in Pediatrics (IRUD-P). We would like to thank Editage (www.editage.jp) for English language editing.
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Yohei Minamitani, Satoshi Masutani
Acquisition, analysis, or interpretation of data: Yohei Minamitani, Ayumi Oshima, Masayo Kanai, Yoichi Iwamoto, Hirotaka Ishido, Satoshi Masutani
Drafting of the manuscript: Yohei Minamitani, Satoshi Masutani
Critical review of the manuscript for important intellectual content: Yohei Minamitani, Ayumi Oshima, Masayo Kanai, Yoichi Iwamoto, Hirotaka Ishido, Satoshi Masutani
References
- 1.Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, Bachinski L, Roberts R. Circulation. 2001;104:3030–3033. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- 2.Glycogen storage diseases presenting as hypertrophic cardiomyopathy. Arad M, Maron BJ, Gorham JM, et al. N Engl J Med. 2005;352:362–372. doi: 10.1056/NEJMoa033349. [DOI] [PubMed] [Google Scholar]
- 3.Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the gamma2-subunit of AMP-activated protein kinase. Akman HO, Sampayo JN, Ross FA, et al. Pediatr Res. 2007;62:499–504. doi: 10.1203/PDR.0b013e3181462b86. [DOI] [PubMed] [Google Scholar]
- 4.Genetic testing for diagnosis of hypertrophic cardiomyopathy mimics: yield and clinical significance. Hoss S, Habib M, Silver J, et al. Circ Genom Precis Med. 2020;13:0. doi: 10.1161/CIRCGEN.119.002748. [DOI] [PubMed] [Google Scholar]
- 5.Hypertrophic cardiomyopathy - a heterogeneous and lifelong disease in the real world. Kitaoka H, Kubo T, Doi YL. Circ J. 2020;84:1218–1226. doi: 10.1253/circj.CJ-20-0524. [DOI] [PubMed] [Google Scholar]
- 6.A novel, de novo mutation in the PRKAG2 gene: infantile-onset phenotype and the signaling pathway involved. Xu Y, Gray A, Hardie DG, et al. Am J Physiol Heart Circ Physiol. 2017;313:283–292. doi: 10.1152/ajpheart.00813.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alglucosidase alfa enzyme replacement therapy as a therapeutic approach for a patient presenting with a PRKAG2 mutation. Austin SL, Chiou A, Sun B, Case LE, Govendrageloo K, Hansen P, Kishnani PS. Mol Genet Metab. 2017;120:96–100. doi: 10.1016/j.ymgme.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Identification, clinical manifestation and structural mechanisms of mutations in AMPK associated cardiac glycogen storage disease. Hu D, Hu D, Liu L, et al. EBioMedicine. 2020;54:102723. doi: 10.1016/j.ebiom.2020.102723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Infantile onset hypertrophic cardiomyopathy secondary to PRKAG2 gene mutation is associated with poor prognosis. Gorla SR, Raja KR, Garg A, Barbouth DS, Rusconi PG. J Pediatr Genet. 2018;7:180–184. doi: 10.1055/s-0038-1657763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatal congenital heart glycogenosis caused by a recurrent activating R531Q mutation in the gamma 2-subunit of AMP-activated protein kinase (PRKAG2), not by phosphorylase kinase deficiency. Burwinkel B, Scott JW, Bührer C, et al. Am J Hum Genet. 2005;76:1034–1049. doi: 10.1086/430840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Severe hypertrophic cardiomyopathy in an infant with a novel PRKAG2 gene mutation: potential differences between infantile and adult onset presentation. Kelly BP, Russell MW, Hennessy JR, Ensing GJ. Pediatr Cardiol. 2009;30:1176–1179. doi: 10.1007/s00246-009-9521-3. [DOI] [PubMed] [Google Scholar]
- 12.PRKAG2 mutations presenting in infancy. Torok RD, Austin SL, Phornphutkul C, et al. J Inherit Metab Dis. 2017;40:823–830. doi: 10.1007/s10545-017-0072-0. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and genetic spectrum of glycogen storage disease in Iranian population using targeted gene sequencing. Beyzaei Z, Ezgu F, Geramizadeh B, et al. Sci Rep. 2021;11:7040. doi: 10.1038/s41598-021-86338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Controversial molecular functions of CBS versus non-CBS domain variants of PRKAG2 in arrhythmia and cardiomyopathy: a case report and literature review. Gong X, Yu P, Wu T, et al. Mol Genet Genomic Med. 2022;10:0. doi: 10.1002/mgg3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PRKAG2‐related lethal congenital glycogen storage disease of the heart as rare cause of fetal hydrops with bradycardia and cardiomyopathy: clinical report and literature review. White-Brown AM, Richard M, Morency AM, Maedler-Kron C, De Bie I. Am J Med Genet A. 2024;2024:0. doi: 10.1002/ajmg.a.63865. [DOI] [PubMed] [Google Scholar]
- 16.Chylothorax and chylous ascites: management and pitfalls. Lopez-Gutierrez JC, Tovar JA. Semin Pediatr Surg. 2014;23:298–302. doi: 10.1053/j.sempedsurg.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Treatment of chronic pleural effusions after the Fontan procedure with prednisone. Rothman A, Mayer JE, Freed MD. Am J Cardiol. 1987;60:408–409. doi: 10.1016/0002-9149(87)90267-0. [DOI] [PubMed] [Google Scholar]
- 18.Spontaneous chylothorax in Noonan syndrome. Treatment with prednisone. Goens MB, Campbell D, Wiggins JW. Am J Dis Child. 1992;146:1453–1456. doi: 10.1001/archpedi.1992.02160240063021. [DOI] [PubMed] [Google Scholar]
- 19.Sirolimus efficacy in the treatment of critically ill infants with congenital primary chylous effusions. Agarwal S, Anderson BK, Mahajan P, Fernandes CJ, Margolin JF, Iacobas I. Pediatr Blood Cancer. 2022;69:0. doi: 10.1002/pbc.29510. [DOI] [PubMed] [Google Scholar]