Abstract
Vaginal infections in women occur due to the downregulation of lactobacilli and the upregulation of Gardnerella vaginalis (GV), leading to bacterial vaginosis (BV). While certain practices are recognized as risk factors for contracting GV infections, this scoping review highlights the severity and importance of other lesser-known risk factors, such as smoking, ethnicity, socioeconomic status (SES), genetics, and anatomy, which can be used to develop an updated point-based screening tool for clinicians. A total of 438 articles were gathered from Embase, Ovid MEDLINE, and Web of Science, and after screening, 31 articles were included. There was a positive association with the presence of GV in those who were sexually active, practiced sexual penetrative vaginal acts, had frequent vaginal and/or receptive oral sexual activity, had unprotected sex, and used insertive contraception (intrauterine device, vaginal rings, and condoms). Women with primary school education levels showed a higher GV colonization increase compared to those with secondary or university education levels, and girls from the highest SES reported the lowest incidence. GV was the predominant bacteria found among sub-Saharan, South African, African Surinamese, Ghanaian, Tanzanian, and Kenyan women. In the USA, self-identified “black” women had a higher prevalence of GV in their vaginal microbiome compared to self-identified “white” women; however, this was the opposite in pregnant women. Significant data show that nicotine use has a strong correlation with increased incidence of GV. Other factors that were found to be associated with GV infections were the increase of sialidase A gene in GV, short cervix (<25mm), and women who performed vaginal douching. Timely screening of GV is vital, especially in high-risk populations, such as pregnant and immunocompromised patients, who may present with more severe and exaggerated symptoms if they contract BV. This paper proposes a numerical scale for evaluating patients' likelihood of contracting a GV infection during their hospital visit.
Keywords: bacterial vaginosis, ethnicity, gardnerella vaginalis, haemophilus haemolyticus vaginalis, hygiene practices, risk factors, screening criteria, sexual practices, smoking, socioeconomic status
Introduction and background
The vaginal microbiota is partially responsible for maintaining the cervicovaginal environment that contributes to the state of reproductive health in women. Imbalances in the vaginal microbiota lead to diseased states of reproductive health such as bacterial vaginosis (BV). The optimal cervicovaginal microbiome is dominated by Lactobacillus bacteria, which confer protection against infections in the reproductive tract [1]. The most common vaginal infection in women is caused by the downregulation of lactobacilli and the upregulation of Gardnerella, which leads to BV [1,2]. Gardnerella vaginalis (GV) is a predominant anaerobic, gram-variable Coccobacillus bacterium currently accepted as the prevailing etiological agent of BV, with an occurrence rate ranging between 5% and 70% among countries [3]. Research has identified GV to be a component of normal vaginal flora in over 50% of asymptomatic women; however, GV has been determined to play a pivotal role in both asymptomatic and symptomatic BV [4]. Many cases of BV often go undiagnosed, as only 50% of affected women present with symptoms like vaginal discharge, odor, itching, and burning during urination. The virulence factors of GV, namely, hemolysin and mucus-degrading sialidases, allow the bacteria to produce biofilms and attach to epithelial cells, which displaces pre-coated lactobacilli and thus weakens protection against infection [1,2]. The overgrowth of GV as a result of the reduction of lactobacilli leads to a nonoptimal cervicovaginal environment that predisposes women to BV [1]. The prevalence rate highlights the importance of clarifying causative factors, screening guidelines, and access to treatment.
The long-term pregnancy-related complications of GV affect both the mother and the fetus, which include premature labor, premature rupture of membranes, chorioamnionitis, neonatal meningitis, postpartum endometriosis, and low birth weight. Despite these devastating adverse events, routine screening for BV during pregnancy is yet to be mandated, therefore making appropriate screening of at-risk individuals and timely treatment of symptomatic patients all the more imperative [5]. Complications outside of pregnancy include an increased risk of developing endometriosis, postsurgical infections, pelvic inflammatory disease, and increased susceptibility to contracting sexually transmitted infections, including HIV [5,6].
BV is diagnosed using Amsel criteria and the Nugent scoring system. An elevated pH level is recognized as the most sensitive but least specific criterion and is significantly associated with BV [7]. Differential diagnoses for BV include atrophic vaginitis, candidiasis, cervicitis, chlamydia, desquamative inflammatory vaginitis, gonorrhea, herpes simplex, and trichomoniasis, which can be ruled out by pelvic examination, speculum exam, wet mount, and cervical swab cultures [6,8]. Treatment is not necessary for asymptomatic patients, and 30% of BV cases resolve on their own; however, if the patient experiences distress (unfavorable social, emotional, and sexual impacts or economic strain), treatment regimens may be started, including oral and topical metronidazole and topical clindamycin [8].
While certain sexual practices are recognized as risk factors for contracting GV infections, this scoping review highlights the severity and importance of other lesser-known risk factors, which can be used to develop an updated point-based screening tool for clinicians.
Review
Methods
An initial literature search was conducted on Google Scholar to gain a brief overview of the topic, after which certain keywords were sought from relevant articles. As GV was the pathogenic organism in question, the keyword “Gardnerella vaginalis” was searched across abstracts, keywords, and titles, alongside second-level keywords, including “Corynebacterium vaginale” OR “corynebacterium vaginalis” OR “Haemophilus haemolyticus vaginalis” OR “Haemophilus vaginalis.” Next, Boolean terms “Gardnerella vaginalis” AND “risk factors” OR “Smoking” were used. Due to smoking being linked to numerous comorbidities, the term was searched across abstracts, titles, and keywords to explore its association, if any, with GV. Due to the review focusing specifically on risk factors, no search terms that singled out a population based on geographic region or age group, for example, were included. The search strategy was replicated across three databases, namely, Embase, Ovid MEDLINE, and Web of Science, which yielded 58 articles. Articles were excluded due to the following: publication date being outside the established timeframe for this review and articles being irrelevant to the specific research question.
Before the article screening and selection processes, duplicates were removed, leaving 438 articles to be analyzed. After the initial title and abstract screening, 379 articles were excluded, leaving 59 articles. Twenty-eight more articles were excluded after full-text screening, with 31 articles included in the final review. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart details the study’s article screening and selection processes (Figure 1) [9].
Figure 1. PRISMA flowchart of the selection procedure .
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses, GV: Gardnerella vaginalis.
Source: [9].
Results
Data were extrapolated from the final 31 papers and charted independently by each of the three reviewers. The variables utilized in the form included aspects of the study design, risk factor category, and number of subjects.
Table 1 shows the 31 articles analyzed for the study design, risk factor category, and number of subjects in selected studies.
Table 1. Analysis of selected studies.
| Study | Study design | Risk factor category | Number of subjects |
| Beamer et al. [10] | Clinical trial | Sexual practices | 440 |
| Borgdorff et al. [11] | Cross-sectional study | Sexual practices, ethnicity | 610 |
| Datcu et al. [12] | Clinical trial | Sexual practices | 196 |
| Plummer et al. [13] | Longitudinal study | Sexual practices | 100 |
| Plummer et al. [14] | Longitudinal study | Sexual practices, smoking | 100 |
| Payne et al. [15] | Cohort study | Sexual practices, hygiene practices | 1001 |
| Jespers et al. [16] | Longitudinal study | Sexual practices | 110 |
| Jespers et al. [17] | Clinical trial | Sexual practices, ethnicity | 430 |
| Mitchell et al. [18] | Cohort study | Sexual practices | 97 |
| Mitchell et al. [19] | Cross-sectional study | Sexual practices | 320 |
| Fethers et al. [20] | Cohort study | Sexual practices, smoking | 339 |
| Balashov et al. [21] | Cohort Study | Sexual practices | 60 |
| Shihab et al. [22] | Interventional prospective study | Sexual practices | 100 |
| Achilles et al. [23] | Cohort study | Sexual practices | 266 |
| Huang et al. [24] | Cohort study | Sexual practices | 120 |
| Francis et al. [25] | Cohort study | Socioeconomic status, genetics | 25 |
| Machado et al. [26] | Cross-sectional study | Socioeconomic status, ethnicity | 206 |
| Desseauve et al. [27] | Cross-sectional study | Socioeconomic status, smoking | 14,193 |
| Wang et al. [28] | Clinical trial | Ethnicity | 688 |
| Lennard et al. [29] | Cohort study | Ethnicity | 298 |
| Balkus et al. [30] | Clinical trial | Ethnicity | 234 |
| Tossas et al. [31] | Cohort study | Ethnicity | 4851 |
| Fettweis et al. [32] | Cohort study | Ethnicity, smoking | 1684 |
| Muzny et al. [33] | Longitudinal study | Ethnicity | 164 |
| Salinas et al. [34] | Cross-sectional study | Ethnicity | 95 |
| Nelson et al. [35] | Cross-sectional study | Smoking | 36 |
| Tužil et al. [36] | Cross-sectional study | Smoking | 250 |
| Vallejo et al. [37] | Retrospective cohort study | Smoking | 4752 |
| Santos-Greatti et al. [38] | Cross-sectional study | Smoking | 526 |
| Ugur et al. [39] | Cross-sectional study | Smoking | 114 |
| Silvano et al. [40] | Cohort study | Anatomy | 97 |
| Total | 32502 |
After analyzing the findings of the articles, commonalities were found regarding sexual practices, SES, ethnicity, smoking, the virulence of GV, anatomic variation, and hygiene practices.
Sexual Practices
Data extracted from several studies point to sexual intercourse as a risk factor for GV infection, due to alterations in the cervicovaginal Lactobacillus-to-GV ratio introduced through sexual practices [10-14]. Single, nonvirgin women are more likely to be infected by GV than women who have not had sexual intercourse [15]. Studies done on women aged 18-22 and girls aged 14-19 demonstrate a significant increase in the occurrence of GV in those who have experienced sexual debut compared to those who have not engaged in sexual intercourse [16-18]. In terms of sexual intercourse, penetrative vaginal acts aside from penile-vaginal sex, such as digital-vaginal, have also demonstrated an increased risk for GV [19]. Additionally, having more than one sexual partner over a three-month period, greater than 10 lifetime sexual partners, and frequent vaginal and/or receptive oral sexual activity were shown to be positively associated with the presence of GV [16,17,20,21].
Furthermore, the lack of contraception has been shown to be related to the incidence of GV, while condoms were the least associated contraceptive method demonstrating some level of protection against GV infection [15,20]. Natural contraceptive methods rendered an increased incidence of GV compared to oral contraception [22]. Insertive methods of contraception, such as copper intrauterine devices (IUDs) and vaginal rings, demonstrated a high level of association between GV and vaginal cultures [23-24]. Aside from contraceptives, penetrative toys were also found to be related to a higher likelihood of GV colonization. In a cross-sectional study of 320 women, women reporting greater than 10 acts of toy-vaginal sex were 70% more likely to be colonized [18].
SES
Three papers highlighted SES in their assessment of Gardnerella prevalence in the population [25-27]. While each of the three papers used their own parameters to define sociodemographic and SES, all three studies found an inverse relationship between SES and GV colonization [25-27]. Women with primary school education levels showed higher rates of GV colonization compared to those with secondary or university education levels [26,27]. Francis et al. looked solely at Tanzanian girls enrolled in a secondary school with SES being defined by material possessions owned by a household member, with owning a car being the highest status and not owning a cellphone, car, or television being the lowest [25]. Following the pattern, girls deemed as being in the highest SES, with a household member in possession of a car, had the lowest reported incidence of reproductive tract infections [25].
These articles not only highlighted the different aspects of SES, but their classifications of each subdivision of SES, such as wealth, were specific to that region and consequently may not be applicable elsewhere [25-27].
Ethnicity
The colonization of GV in the vaginal microbiota varies between different ethnic groups. Several studies have been conducted on GV prevalence in specific ethnic groups; however, very few have compared their prevalence between ethnic groups.
Compared to women of Dutch ethnicity, the vaginal microbiota of African Surinamese and Ghanaian (sub-Saharan African descent) women were significantly more likely to contain a polybacterial GV-containing VMB [11]. Furthermore, GV was the predominant bacteria found in women from sub-Saharan Africa, South Africa, Tanzania, Kenya, and Rwanda [16,25,28-30]. In the USA, women who self-identified as “black” or “African American” had a higher prevalence of GV in their VMB compared to women who self-identified as “white” [31-33]. In an Ecuadorian study, GV was found in more than 93% of pregnant teenagers [34]. Interestingly, in the context of preterm births, pregnant women who self-identified as “white” were more likely to be colonized by GV in their VMB and amniotic fluid compared to pregnant women who self-identified as “black” [32].
Smoking
Multiple studies have been conducted aiming to find a correlation between smoking and an altered VBM, which can increase the predilection for urogenital disease pathology. While the exact pathogenesis for the increase in GV species is still unknown, studies have unanimously found a correlation between GV and smoking, including both cigarette and marijuana products. These studies have shown a markedly increased presence of GV in relation to Lactobacillus in smokers versus nonsmokers [14,20,27,32,35-39]. When looking at metabolic profiles, other studies have concluded the breakdown products of the chemicals in cigarette smokes may be the ultimate culprits that lead to the dysregulation of the vaginal microbiota that in turn create a more favorable environment for the overgrowth of “bad” bacteria [35]. In the aforementioned study, nonsmokers were more likely to have a 39-fold increase in Lactobacillus, while smokers were more likely to be Lactobacillus-depleted [35].
While there have been established studies on smoking metabolites, the by-products of marijuana are lesser known. A study conducted with marijuana users found a statistically significant correlation between marijuana use and recurrent BV infections caused primarily by GV [37].
Genetics
Few studies have shown that variations in bacterial genetic factors may be associated with the prevalence of GV in certain populations. The main gene of interest in studying GV is the sialidase A gene, an important virulence factor, which is higher among women with detectable GV [25].
Anatomical Variations
A single study analyzed the association of cervix length with the risk of GV infection. It was noted that there is a fourfold increase in the abundance of GV in women with short cervixes (<25mm) compared to those with longer cervixes (>25mm) [40].
Hygiene Practices
Hygiene practices may also be correlated with the increased incidence of GV. One study demonstrated an increased risk of GV in women who performed vaginal douching as part of their customary personal hygiene practices [15].
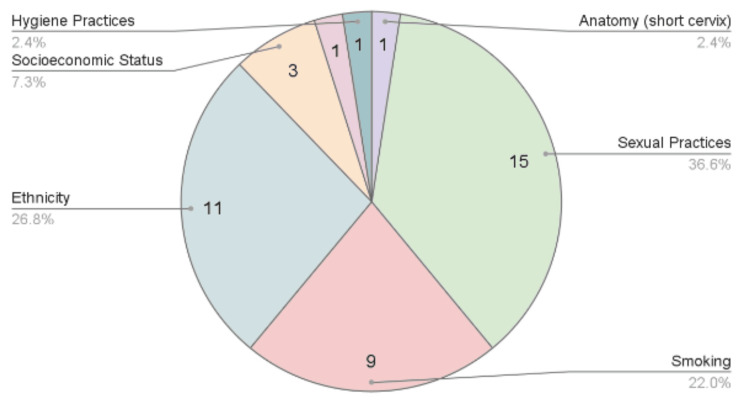
Figure 2 shows a pie chart of the most prevalent evidence-based risk factors of GV based on the number of papers found in this scoping review of recent literature: sexual practices with 15 papers, followed by ethnicity with 11, smoking with nine, SES with three, and genetics, hygiene practices, and cervix length with one each.
Figure 2. Pie chart of Gardnerella risk factors.
This pie chart summarizes the findings of the studies recorded in Table 1 and groups the GV risk factors based on the number of papers identified in this review [10-40].
Table 2 shows all nine risk factors from this study, including contraception use, number of sexual partners in three months, type of intercourse, ethnicity, smoking, SES, hygiene practices, genetics, and anatomical variations. Each risk factor was further divided into point values that represented tier-relative risk profiles inferred from the analysis of the included articles. No contraception use was found to be the most risky for GV infections, so it was given a point value of 3, followed by IUDs/vaginal rings given 2, and oral contraceptive pills given 1. Greater than one sexual partner in the past three months was considered riskier and is given a point value of 2, and one or fewer sexual partners were given 1. Penile-vaginal sex was given 3 points, followed by digital-vaginal and penetrative toy-vaginal that were both given 2. Oral-vaginal was considered the least risky, which is given 1 point. Being of African descent was given a risk point value of 5, followed by pregnant women of European or South American descent given 4, pregnant women of African descent given 3, European or South American descent given 2, and finally all other ethnicities given 1. Smoking more than 20 cigarettes per day was given 4 points, 10-20 cigarettes per day was given 3, less than 10 cigarettes per day was given 2, and nonsmokers were given 1. Low SES was given a risk point value of 3, middle SES was given 2, and high SES was given 1. The vaginal douching practice was given a value of 2, while no vaginal douching was given 1. Sialidase A gene-positive individuals were given 2 points, while negative individuals were given 1. Those with cervixes less than 25mm (about 0.98in) were given 2, and those with cervixes greater than or equal to 25mm were given a point value of 1. To determine the value ranges for high, moderate, and low risk, we first calculated the highest and lowest scores possible, which were 33 and 8, respectively. The difference between these total scores was found (25) and subsequently divided into three (8.33). After splitting each risk category into containing around 8 points each, we finally determined that a total sum of all risk factors equivalent to 26 and above was defined as high risk for GV infection, a total between 17 and 25 was moderate risk, and a total of 16 and under was considered low risk (Table 3).
Table 2. Risk factor screening tool for Gardnerella infection.
IUDs: intrauterine devices.
This table categorizes GV risk factors based on severity. Source: [10-40].
| Risk factors for Gardnerella infection | Points | |
| Contraception use | No contraception | 3 |
| IUDs/vaginal rings | 2 | |
| Oral contraceptive pills | 1 | |
| Number of sexual partners in three months | >1 | 2 |
| ≤1 | 1 | |
| Type of Intercourse | Penile-vaginal | 3 |
| Digital-vaginal | 2 | |
| Penetrative toy-vaginal | 2 | |
| Oral-vaginal | 1 | |
| Ethnicity | African descent | 5 |
| European or South American descent, pregnant | 4 | |
| African descent, pregnant | 3 | |
| European or South American descent | 2 | |
| Other | 1 | |
| Smoking | Heavy (20+ cigarettes/day) | 4 |
| Moderate (10-20 cigarettes/day) | 3 | |
| Light (<10 cigarettes/day) | 2 | |
| Never | 1 | |
| Socioeconomic status | Low | 3 |
| Middle | 2 | |
| High | 1 | |
| Hygiene practices | Vaginal douching | 2 |
| No vaginal douching | 1 | |
| Genetics | Sialidase A gene positive | 2 |
| Sialidase A gene negative | 1 | |
| Anatomical variation | Short cervix (<25mm) | 2 |
| Long cervix (>25mm) | 1 | |
Table 3. Screening criteria tool key.
GV: Gardnerella vaginalis.
This table proposes a point-based system to categorize patients into low risk, moderate risk, and high risk for GV infections.
| Total | Risk |
| 26-33 | High risk for GV infection |
| 17-25 | Moderate risk for GV infection |
| 8-17 | Low risk for GV infection |
Discussion
BV is known as a condition caused by the disruption of normal vaginal flora. As a result of the overgrowth of GV in proportion to the protective, Lactobacillus species, the GV-to-Lactobacillus ratio is increased in BV. BV is commonly misunderstood as a sexually transmitted disease due to its most commonly associated risk factor of risky sexual practices [41]. Given this misunderstanding, public health campaigns can be tailored to improve awareness and education about BV, including the distribution of pamphlets and flyers in obstetrics and gynecology (OB-GYN) and primary care offices, presentations in school health classes, and spreading awareness through social media by medical professionals. Based on the data from this review, risky sexual behaviors include factors such as completion of sexual debut, the presence of multiple sexual partners, lack of condom usage during intercourse, increased penetrative forms of sexual intercourse, insertive sex toys, and vaginal douching [15-21,23,24]. This information suggests that the introduction of foreign materials may lead to mechanical vaginal tissue trauma, disrupting Lactobacillus species’ protective defenses of the natural vaginal environment and increasing susceptibility to GV colonization [42]. This mechanism of infection also explains why some data from this review point toward insertive forms of contraception, such as copper IUDs and vaginal rings, leading to an increased risk of GV [23,24]. However, since contraception is beneficial for preventing unwanted pregnancies and treating hormonal imbalances, it may be useful for those predisposed to GV to consider non-insertive forms of contraception, thereby decreasing the potential for vaginal tissue trauma. Furthermore, considering that vaginal douching is a common hygiene practice with known associations with vaginal infections, it is imperative that physicians educate women to utilize alternative low-risk hygiene practices, such as regular cleaning of the genital area with mild soap and warm water, use of breathable cotton underwear, and avoidance of irritants.
Based on the analysis of the findings, risky sexual behaviors hold a higher risk of GV colonization than SES. However, SES is an important factor to consider, as the aforementioned risky sexual practices have shown increased prevalence in populations of lower SES [43]. This is due to reasons such as increased rates of high school dropout , lack of access to resources such as childcare and safe environments, and earlier sexual debut associated with populations of lower SES [44]. Therefore, educational programs targeting these specific populations may help mitigate the incidence of GV. This happens by tailoring resources and being both mindful and considerate of the cultural, traditional, and spiritual components of education on sexual practices. In addition, independent of SES, sexual practices may be seen as taboo in certain cultures, potentially leading to a lack of sexual health education and inaccessibility to sexual healthcare [45]. Consequently, it is imperative for healthcare providers to educate both themselves and high-risk patient populations and provide culturally sensitive educational resources and timely access to care.
Geographic location and ethnicity can alter the microbial composition of the VMB, potentially due to differences in access to healthcare and cultural practices regarding vaginal care, as well as genetic factors. At baseline, black women have a higher probability of having a GV-predominant VMB [11]. Additionally, compared to the VMB of women classified as white, the VMB of women classified as black has a significantly higher probability of GV colonization after exposure to risk factors [11]. While culturally varied diets and hygiene practices could account for this variability, the possibility of a genetic component cannot be ignored. After controlling for factors that are known to increase GV prevalence, such as sociodemographic, sexual risk behaviors, vaginal cleansing practices, and hormonal contraceptive use, women of sub-Saharan African descent were significantly more likely to have a GV-containing VMB than Dutch women, who were more likely to have a Lactobacillus-dominant VMB, which is commonly accepted as the healthier VMB [11]. Similar results were noted in a study where external factors, such as smoking and multiple sexual partners, were not statistically significant, suggesting variability in the VMB in healthy women among ethnicities [28]. From these results, it can be interpreted that black women have an increased risk of contracting BV or that the definition of BV must be curated differently depending on the ethnic background and genetic makeup of the patient.
The observation of increased GV colonization in pregnant women interestingly contradicts trends in nonpregnant populations. One hypothesis that may explain why GV prevalence shifts in pregnancy is due to the downregulation of T-helper 1 (Th1)-mediated immunity and upregulation of Th2-mediated immunity during pregnancy to accommodate a growing fetus [1]. The suppression of Th1 responses in pregnancy reduces the ability to control GV populations in the VMB [1]. In addition, the Th2-dominant environment in pregnant females may reduce the ability to control GV virulence factor, sialidase A, facilitating GV colonization [38]. Pregnancy-related hormonal changes may also affect vaginal pH and glycogen content, creating more favorable conditions for GV proliferation [38].
The premise of BMI was based on the work of a Belgian astronomer in the 19th century who sampled a group of high-income, mostly white men, which aimed to represent the typical sizes of the total population and determine the “ideal body weight” [44]. This BMI continues to be used in medicine today, even though new studies have shown some people in the “overweight” BMI category, typically mislabeled due to varying ethnic body compositions, have a lower risk of death from heart-related causes than those with a “normal” BMI [44]. This shows that ethnicity must be considered a factor when deciding screening and treatment plans for patients with BV. Changing the way providers view certain risk factors of disease, such as BMI depending on ethnicity, is not a new phenomenon, yet an ongoing development of understanding differences between ethnicities during screening and prior to treatment. Future studies on different ethnicities in different geographical locations should be conducted to determine the specific role and relationship of these two variables on GV-to-Lactobacillus ratio.
Since GV prevalence has shown to be higher among certain populations of women, the prevalence of an important GV virulence factor, sialidase A, among different ethnicities can be investigated [25]. The sialidase A gene codes for sialidase, an enzyme that cleaves sialic acid residues from glycans in the cell wall, such as glycoproteins and glycolipids, which in turn aids in the adherence and colonization of GV [25]. This gene is higher among women with detectable GV and must be further explored as a potential diagnostic factor when diagnosing BV. In addition, further GV virulence factors can be investigated for use in clinical practice to potentially predict future adverse complications in patients. BV-associated GV strains demonstrated increased virulence by encoding mucin-degradable protein and biofilm-associated protein genes [46]. While there may be more genes implicated in the contraction of GV infection, the lack of research in this sphere renders genetics a low-risk factor compared to sexual practices, SES, and ethnicity.
Smoking was the second highest risk factor for GV infection after sexual practices. Cigarette smoking is a risk factor that has a predisposition toward GV-predominant VMBs [35]. Women who reported smoking nicotine products, particularly in the last three months, had an increased risk of GV infection [14]. While all studies investigating the relationship between GV infections and nicotine by-products from cigarette smoking have unanimously concluded there is a direct relationship between the two, the exact pathophysiology is still unknown [13,20,24,30-33]. Several studies have hypothesized that smoking metabolites may not necessarily be creating an unfavorable microenvironment for beneficial bacteria such as Lactobacillus, but rather, they are creating a favorable state for pathogenic bacteria, such as GV, to replicate [35,36]. Hippurate, a normal excretory product found in urine, is a well-known substrate utilized by GV, and its abundance increases with exposure to the breakdown products of cigarette smokes [35]. Therefore, similar to the use of VMB biomarkers to detect diseases such as high-risk human papillomavirus infections, hippurate concentrations in the urine may be a useful biomarker for assessing the risk of GV infections in smokers [47].
While there have been established studies on nicotine metabolites, the effects of marijuana use are lesser known. While there was a twofold increase in recurrent BV infections in marijuana users, a metabolic profile was not completed in these patients [33]. Therefore, while it has been established that marijuana affects the VMB similarly to nicotine, there is no definitive data on the pathophysiology by which this is occurring [48]. Future studies should be conducted to determine the breakdown products of marijuana and their respective effects when building up in the vaginal tract or cervical mucus membranes.
Though low risk, anatomical factors also play a role in GV prevalence. The data indicate a higher risk of GV infection in women with shorter cervixes, presumably drawing to the hypothesis that a smaller cervicovaginal surface area allows GV to overpopulate the VMB [40]. However, the exact mechanism behind increased GV prevalence in women with shorter cervixes continues to remain unknown [40]. The abovementioned possible correlation highlights the need for further research to be conducted, to explore a possible direct correlation between variations in anatomical length and GV infection. Furthermore, in regard to age, after further evaluation of all articles chosen for the scoping review, no consensus was reached on the association of age with the presence of Gardnerella.
Regarding limitations, the following paragraph outlines the ones in this scoping review. In regard to SES, the classifications of each subdivision, such as wealth, were specific to the geographic region being studied and consequently may not be applicable in other locations. Therefore, not all factors are as universal as education, and reproducibility utilizing the same criteria in different geographical locations may be challenging. Furthermore, SES and education may differ in their influence and hence importance on GV in different geographical locations. In addition, investigating the genetic component of bacterial variations among ethnicities in the VMB is imperative to determine whether or not those variances describe a pathological process or are healthy for a certain population. Furthermore, it is important to note that this review may not include all the subsets of one risk factor. For example, in regard to smoking, there are many other nicotine and non-nicotine alternatives outside of cigarettes and marijuana including but not limited to electronic cigarettes, nicotine patches, vapes, and chewing tobacco. While the mechanism by which these products damage the vaginal and genital tract may be similar, further studies are needed to elucidate the pathogenicity of each when compared against one another. Furthermore, there are confounding variables in many of these studies, such as ethnicity, sexual practices, age, and variances in the VMB with the typical menstrual cycle, and therefore each of the highlighted risk factors cannot be assessed independently. The screening criteria were created with the information acquired through the analysis of this topic; it should act as a template that can be altered ,as further information continues to be understood regarding the risk factors leading to BV.
Conclusions
Based on the various risk factors identified in this review, it can be inferred that a patient who presents with a greater number of independent risk factors confers a higher risk of developing a GV infection. Timely screening of GV is vital, especially in high-risk populations, such as pregnant and immunocompromised patients, who may present with more severe and exaggerated symptoms if they contract BV. Educating physicians and healthcare providers on such risk factors would allow them to better identify these at-risk populations.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Afrida Sara, Apurva Ramanujam, Dhiya Ram, Marc M. Kesselman
Acquisition, analysis, or interpretation of data: Afrida Sara, Apurva Ramanujam, Dhiya Ram, Marc M. Kesselman, Kelley L. Davis, Stephanie Nagy
Drafting of the manuscript: Afrida Sara, Apurva Ramanujam, Dhiya Ram
Critical review of the manuscript for important intellectual content: Afrida Sara, Apurva Ramanujam, Dhiya Ram, Marc M. Kesselman, Kelley L. Davis, Stephanie Nagy
Supervision: Marc M. Kesselman
References
- 1.Gardnerella revisited: species heterogeneity, virulence factors, mucosal immune responses, and contributions to bacterial vaginosis. Shvartsman E, Hill JE, Sandstrom P, MacDonald KS. Infect Immun. 2023;91:0. doi: 10.1128/iai.00390-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardnerella and vaginal health: the truth is out there. Rosca AS, Castro J, Sousa LG, Cerca N. FEMS Microbiol Rev. 2020;44:73–105. doi: 10.1093/femsre/fuz027. [DOI] [PubMed] [Google Scholar]
- 3.Prevalence and distribution of Gardnerella vaginalis subgroups in women with and without bacterial vaginosis. Janulaitiene M, Paliulyte V, Grinceviciene S, Zakareviciene J, Vladisauskiene A, Marcinkute A, Pleckaityte M. BMC Infect Dis. 2017;17:394. doi: 10.1186/s12879-017-2501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacterial vaginosis: what do we currently know? Chacra LA, Fenollar F, Diop K. Front Cell Infect Microbiol. 2022;11:672429. doi: 10.3389/fcimb.2021.672429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardnerella vaginalis in infections of reproductive organs. Kasprowicz A, Białecka A. https://europepmc.org/article/med/8309297. Med Dosw Mikrobiol. 1993;45:199–203. [PubMed] [Google Scholar]
- 6.Gardnerella vaginalis is associated with other sexually transmittable microorganisms in the male urethra. Elsner P, Hartmann AA, Wecker I, STD-Study Group. https://www.sciencedirect.com/science/article/abs/pii/S0176672488800841. Zbl Bakt Hyg A. 1988;269:56–63. doi: 10.1016/s0176-6724(88)80084-1. [DOI] [PubMed] [Google Scholar]
- 7.Role of Gardnerella vaginalis as an etiological agent of bacterial vaginosis. Baruah FK, Sharma A, Das C, Hazarika NK, Hussain JH. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4411427/ Iran J Microbiol. 2014;6:409–414. [PMC free article] [PubMed] [Google Scholar]
- 8.Gardnerella vaginalis infection. Clinical aspects, diagnosis and therapy. Hartmann AA. https://pubmed.ncbi.nlm.nih.gov/3318083/ Urologe A. 1987;26:252–255. [PubMed] [Google Scholar]
- 9.PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Tricco AC, Lillie E, Zarin W, et al. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 10.Microbial risk factors for acquisition of symptomatic bacterial vaginosis (BV) Beamer M, Meyn L, Petrina M, et al. https://sti.bmj.com/content/95/Suppl_1/A185.1 Sex Transm Infect. 2019;95:365. [Google Scholar]
- 11.The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. Borgdorff H, van der Veer C, van Houdt R, et al. PLoS One. 2017;12:0. doi: 10.1371/journal.pone.0181135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaginal microbiome in women from Greenland assessed by microscopy and quantitative PCR. Datcu R, Gesink D, Mulvad G, et al. BMC Infect Dis. 2013;13:480. doi: 10.1186/1471-2334-13-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sexual practices have a significant impact on the vaginal microbiota of women who have sex with women. Plummer EL, Vodstrcil LA, Fairley CK, et al. Sci Rep. 2019;9:19749. doi: 10.1038/s41598-019-55929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardnerella vaginalis clade distribution is associated with behavioral practices and Nugent score in women who have sex with women. Plummer EL, Vodstrcil LA, Murray GL, et al. J Infect Dis. 2020;221:454–463. doi: 10.1093/infdis/jiz474. [DOI] [PubMed] [Google Scholar]
- 15.Risk factors associated with prevalence of Candida albicans, Gardnerella vaginalis, and Trichomonas vaginalis among women at the district hospital of Dschang, West Region, Cameroon. Payne VK, Cécile TT, Cedric Y, Nadia NA, José O. Int J Microbiol. 2020;2020:8841709. doi: 10.1155/2020/8841709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Association of sexual debut in adolescents with microbiota and inflammatory markers. Jespers V, Hardy L, Buyze J, Loos J, Buvé A, Crucitti T. Obstet Gynecol. 2016;128:22–31. doi: 10.1097/AOG.0000000000001468. [DOI] [PubMed] [Google Scholar]
- 17.The significance of Lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women. Jespers V, van de Wijgert J, Cools P, et al. BMC Infect Dis. 2015;15:115. doi: 10.1186/s12879-015-0825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Effect of sexual debut on vaginal microbiota in a cohort of young women. Mitchell CM, Fredricks DN, Winer RL, Koutsky L. Obstet Gynecol. 2012;120:1306–1313. doi: 10.1097/aog.0b013e31827075ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Effect of sexual activity on vaginal colonization with hydrogen peroxide-producing lactobacilli and Gardnerella vaginalis. Mitchell C, Manhart LE, Thomas KK, Agnew K, Marrazzo JM. Sex Transm Dis. 2011;38:1137–1144. doi: 10.1097/OLQ.0b013e31822e6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacterial vaginosis (BV) candidate bacteria: associations with BV and behavioural practices in sexually-experienced and inexperienced women. Fethers K, Twin J, Fairley CK, et al. PLoS One. 2012;7:0. doi: 10.1371/journal.pone.0030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Identification, quantification and subtyping of Gardnerella vaginalis in noncultured clinical vaginal samples by quantitative PCR. Balashov SV, Mordechai E, Adelson ME, Gygax SE. J Med Microbiol. 2014;63:162–175. doi: 10.1099/jmm.0.066407-0. [DOI] [PubMed] [Google Scholar]
- 22.The story of oral contraceptive users and bacterial vaginosis…. Is it real? Shihab EM, Ahmed HN, Ahmed IT, Abood MN. https://openurl.ebsco.com/EPDB%3Agcd%3A11%3A5402427/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A155797284&crl=f Int J Pharm Res. 2020:1809. [Google Scholar]
- 23.Impact of contraceptive initiation on vaginal microbiota. Achilles SL, Austin MN, Meyn LA, Mhlanga F, Chirenje ZM, Hillier SL. Am J Obstet Gynecol. 2018;218:622. doi: 10.1016/j.ajog.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Effects of a one year reusable contraceptive vaginal ring on vaginal microflora and the risk of vaginal infection: an open-label prospective evaluation. Huang Y, Merkatz RB, Hillier SL, Roberts K, Blithe DL, Sitruk-Ware R, Creinin MD. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The vaginal microbiota among adolescent girls in Tanzania around the time of sexual debut. Francis SC, Crucitti T, Smekens T, et al. Front Cell Infect Microbiol. 2020;10:305. doi: 10.3389/fcimb.2020.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prevalence of bacterial vaginosis in Portuguese pregnant women and vaginal colonization by Gardnerella vaginalis. Machado D, Castro J, Martinez-de-Oliveira J, Nogueira-Silva C, Cerca N. PeerJ. 2017;5:0. doi: 10.7717/peerj.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prevalence and risk factors of bacterial vaginosis during the first trimester of pregnancy in a large French population-based study. Desseauve D, Chantrel J, Fruchart A, Khoshnood B, Brabant G, Ancel PY, Subtil D. Eur J Obstet Gynecol Reprod Biol. 2012;163:30–34. doi: 10.1016/j.ejogrb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Non-Lactobacillus-dominant and polymicrobial vaginal microbiomes are more common in younger South African women and predictive of increased risk of human immunodeficiency virus acquisition. Wang Y, Noël-Romas L, Perner M, et al. Clin Infect Dis. 2023;76:1372–1381. doi: 10.1093/cid/ciac938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in South African adolescent females. Lennard K, Dabee S, Barnabas SL, et al. Infect Immun. 2018;86:0–17. doi: 10.1128/IAI.00410-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Impact of periodic presumptive treatment for bacterial vaginosis on the vaginal microbiome among women participating in the preventing vaginal infections trial. Balkus JE, Srinivasan S, Anzala O, et al. J Infect Dis. 2017;215:723–731. doi: 10.1093/infdis/jiw622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Does the vaginal microbiome operate differently by race to influence risk of precervical cancer? Tossas KY, Zhu B, Perera RA, et al. https://pubmed.ncbi.nlm.nih.gov/36897755/ J Womens Health (Larchmt) 2023;32:553–560. doi: 10.1089/jwh.2022.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Differences in vaginal microbiome in African American women versus women of European ancestry. Fettweis JM, Brooks JP, Serrano MG, et al. Microbiology (Reading) 2014;160:2272–2282. doi: 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Risk factors for incident bacterial vaginosis among heterosexual women. Muzny C, Aaron K, Pontius A, Aycock C, Schwebke J. https://www.researchgate.net/publication/341246707_P375_Risk_factors_for_incident_bacterial_vaginosis_among_heterosexual_women. Sex Transm Infect. 2019;95:375. doi: 10.1136/sextrans-2018-053824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacterial identification of the vaginal microbiota in Ecuadorian pregnant teenagers: an exploratory analysis. Salinas AM, Osorio VG, Endara PF, Salazar ER, Vasco GP, Vivero SG, Machado A. PeerJ. 2018;6:0. doi: 10.7717/peerj.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Nelson TM, Borgogna JC, Michalek RD, et al. Sci Rep. 2018;8:852. doi: 10.1038/s41598-017-14943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smoking in women with chronic vaginal discomfort is not associated with decreased abundance of Lactobacillus spp. but promotes Mobiluncus and Gardnerella spp. overgrowth - secondary analysis of trial data including microbio-me analysis. Tužil J, Filková B, Malina J, Kerestes J, Doležal T. Ceska Gynekol. 2021;86:22–29. doi: 10.48095/cccg202122. [DOI] [PubMed] [Google Scholar]
- 37.Marijuana use in women of reproductive age and recurrent bacterial vaginosis: a retrospective chart review. Vallejo V, Shan W, Ilagan J, Goldberg G. https://www.authorea.com/users/728352/articles/709518-marijuana-use-in-women-of-reproductive-age-and-recurrent-bacterial-vaginosis-a-retrospective-chart-review Authorea (Authorea) Preprints. 2024 [Google Scholar]
- 38.Cervicovaginal cytokines, sialidase activity and bacterial load in reproductive-aged women with intermediate vaginal flora. Santos-Greatti MM, da Silva MG, Ferreira CS, Marconi C. J Reprod Immunol. 2016;118:36–41. doi: 10.1016/j.jri.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Identification of bacterial vaginosis-associated bacteria in male urethra: co-occurrence of Atopobium vaginae and Gardnerella vaginalis. Ugur A, Tuncer EI, Findik D. https://api.semanticscholar.org/CorpusID:165158417 Malays J Microbiol. 2019;15:69–75. [Google Scholar]
- 40.Vaginal microbiome in pregnant women with and without short cervix. Silvano A, Meriggi N, Renzi S, Seravalli V, Torcia MG, Cavalieri D, Di Tommaso M. Nutrients. 2023;15:2173. doi: 10.3390/nu15092173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Relationship between bacterial vaginosis and sexually transmitted infections: coincidence, consequence or co-transmission? Abou Chacra L, Ly C, Hammoud A, Iwaza R, Mediannikov O, Bretelle F, Fenollar F. Microorganisms. 2023;11:2470. doi: 10.3390/microorganisms11102470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lactobacillus crispatus CCFM1339 inhibits vaginal epithelial barrier injury induced by Gardnerella vaginalis in Mice. Huang X, Lin R, Mao B, Tang X, Zhao J, Zhang Q, Cui S. Biomolecules. 2024;14:240. doi: 10.3390/biom14020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Socioeconomic variations in risky sexual behavior among adolescents in 14 sub-Saharan Africa countries who report ever having had sex. Ali MM, Merdad L, Bellizzi S. Int J Equity Health. 2021;20:11. doi: 10.1186/s12939-020-01352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Body mass index: obesity, BMI, and health: a critical review. Nuttall FQ. Nutr Today. 2015;50:117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Socio-cultural challenges to sexual health education for female adolescents in Iran. Roudsari RL, Javadnoori M, Hasanpour M, Hazavehei SM, Taghipour A. https://pubmed.ncbi.nlm.nih.gov/24639734/ Iran J Reprod Med. 2013;11:101–110. [PMC free article] [PubMed] [Google Scholar]
- 46.Gardnerella vaginalis in perinatology: an overview of the clinicopathological correlation. Wong YP, Tan GC, Wong KK, Anushia S, Cheah FC. https://pubmed.ncbi.nlm.nih.gov/30580358/ Malays J Pathol. 2018;40:267–286. [PubMed] [Google Scholar]
- 47.Research of the potential biomarkers in vaginal microbiome for persistent high-risk human papillomavirus infection. Chao X, Sun T, Wang S, et al. Ann Transl Med. 2020;8:100. doi: 10.21037/atm.2019.12.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannabis and microbiome: a systematic review and meta-analysis. Thu MS, Ondee T, Hall SR, Nopsopon T, Jagota A, Hirankarn N, Pongpirul K. https://www.researchgate.net/publication/366859886_Cannabis_and_Microbiome_A_Systematic_Review_and_Meta-Analysis MedRxiv. 2023;3:2022–2012. [Google Scholar]