Abstract
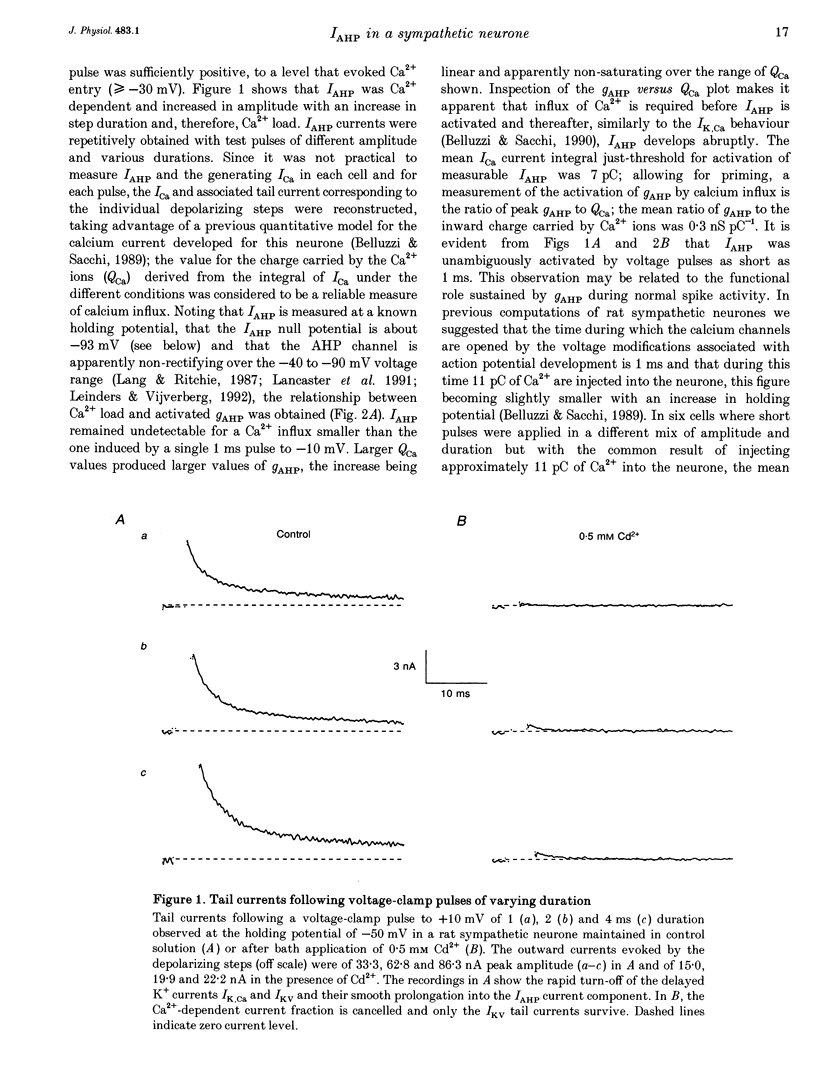
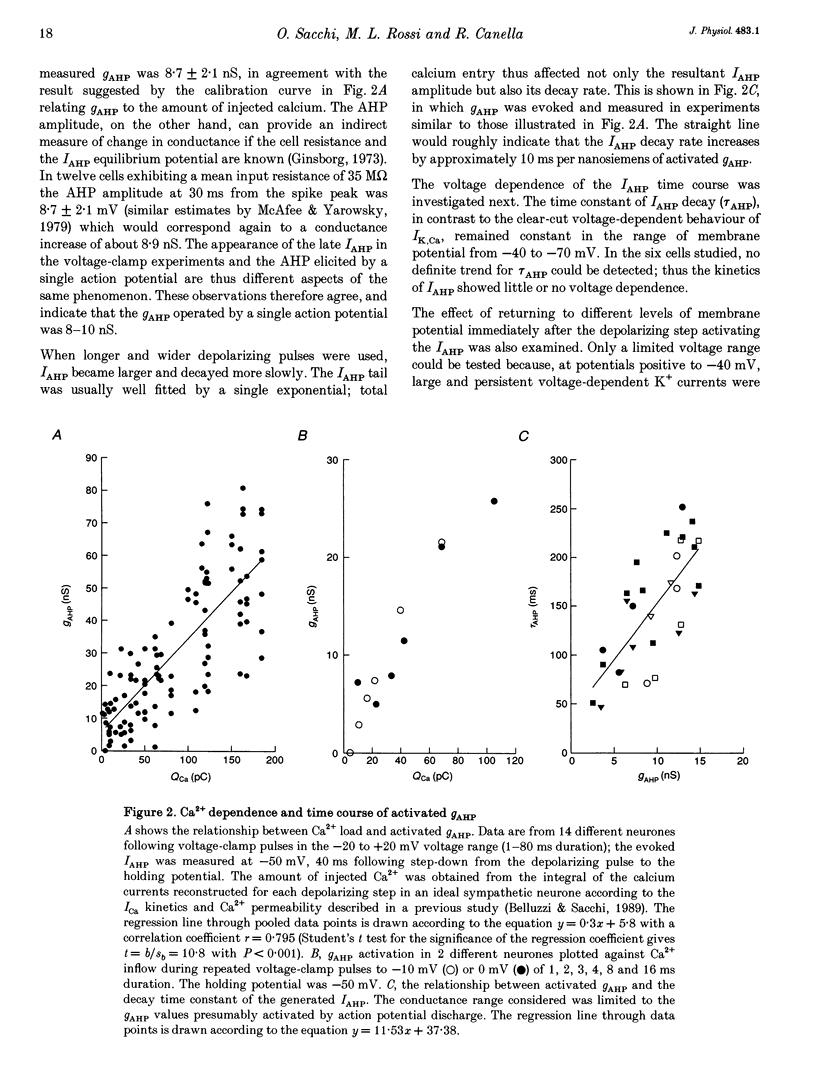
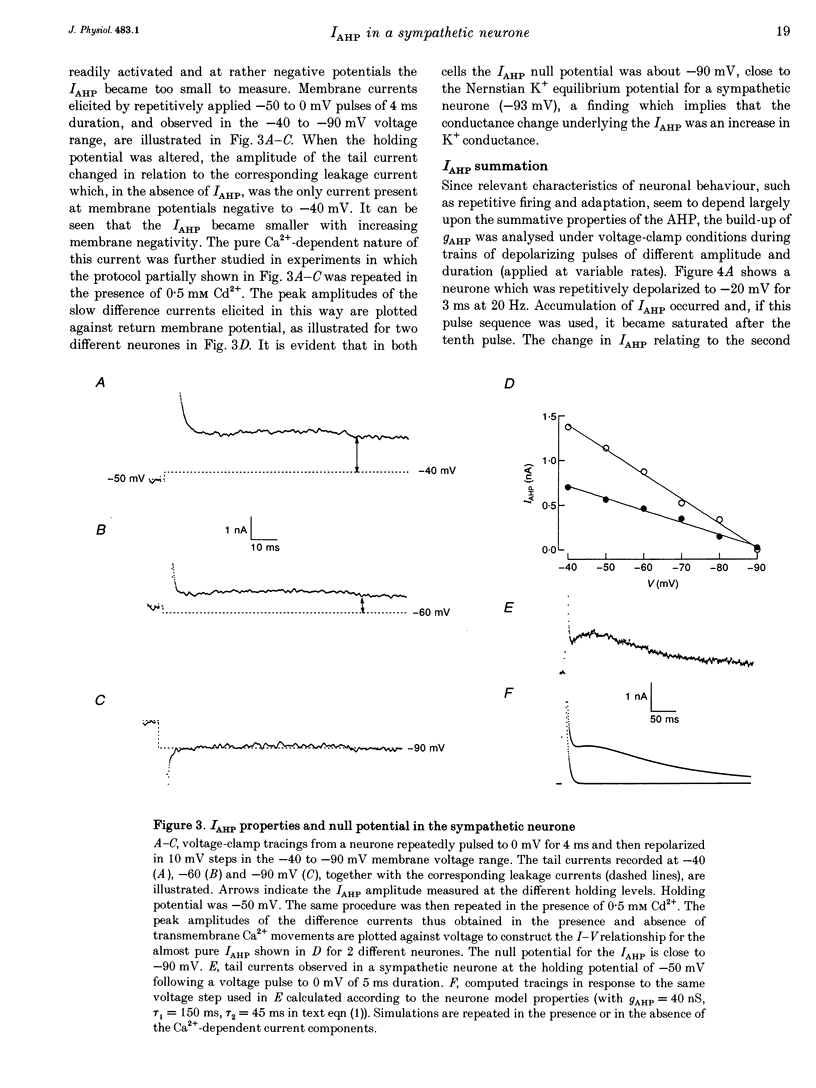
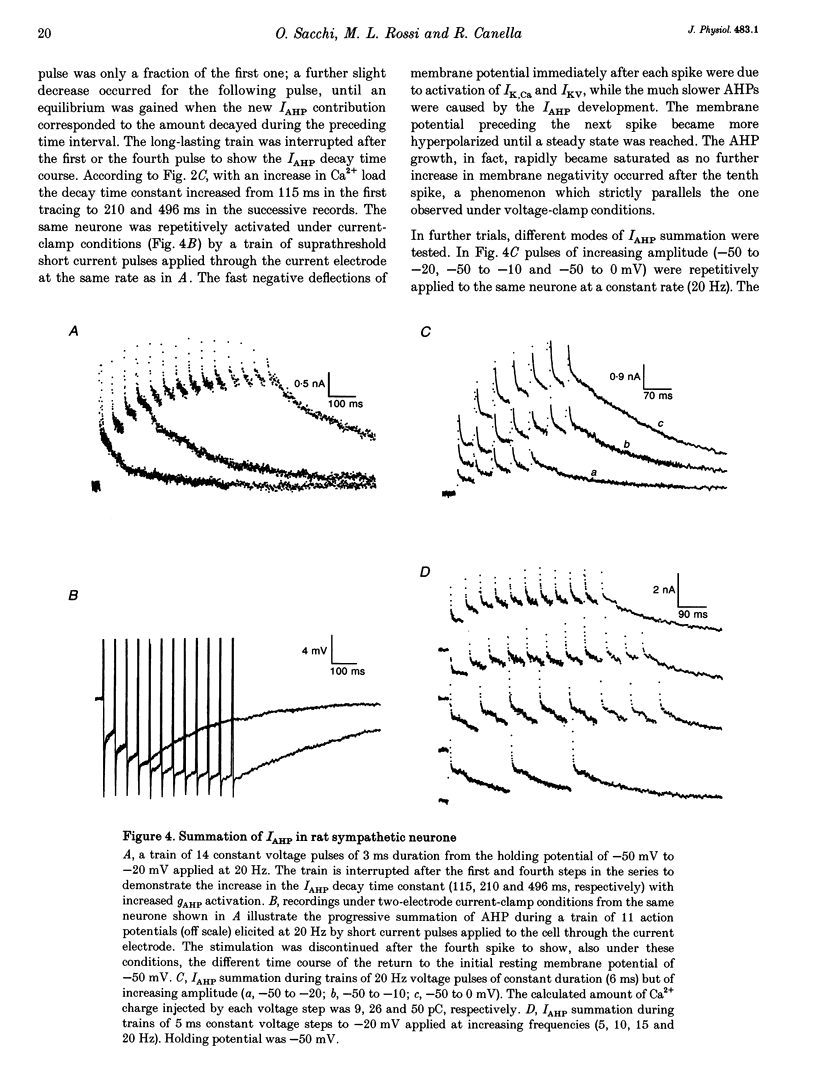
1. Adult and intact sympathetic neurones of the rat superior cervical ganglion maintained in vitro at 37 degrees C were analysed using the two-electrode voltage-clamp technique in order to investigate the slow component of the Ca(2+)-dependent K+ current, IAHP. 2. The relationship between the after-hyperpolarization (AHP) conductance, gAHP, and estimated Ca2+ influx resulting from short-duration calcium currents evoked at various voltages proved to be linear over a wide range of injected Ca2+ charge. An inflow of about 1.7 x 10(7) Ca2+ ions was required before significant activation of gAHP occurred. After priming, the gAHP sensitivity was about 0.3 nS pC-1 of Ca2+ inward charge. 3. IAHP was repeatedly measured at different membrane potentials; its amplitude decreased linearly with membrane hyperpolarization and was mostly abolished close to the K+ reversal potential, EK (-93 mV). The monoexponential decay rate of IAHP was a linear function of total Ca2+ entry and was not significantly altered by membrane potential in the -40 to -80 mV range. 4. Voltage-clamp tracings of IAHP could be modelled as a difference between two exponentials with tau on approximately 5 ms and tau off = 50-250 ms. 5. Sympathetic neurones discharged only once at the onset of a long-lasting depolarizing step. If IAHP was selectively blocked by apamin or D-tubocurarine treatments, accommodation was abolished and an unusual repetitive firing appeared. 6. Summation of IAHP was demonstrated under voltage-clamp conditions when the depolarizing steps were repeated sufficiently close to one another. Under current-clamp conditions the threshold depolarizing charge for action potential discharge significantly increased with progressive pulse numbers in the train, suggesting that an opposing conductance was accumulating with repetitive firing. This frequency-dependent spike firing ability was eliminated by pharmacological inhibition of the slow IAHP. 7. The IAHP was significantly activated by a single action potential; it was turned on cumulatively by Ca2+ load during successive action potential discharge and acted to further limit cell excitability.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artalejo A. R., García A. G., Neher E. Small-conductance Ca(2+)-activated K+ channels in bovine chromaffin cells. Pflugers Arch. 1993 Apr;423(1-2):97–103. doi: 10.1007/BF00374966. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. A five-conductance model of the action potential in the rat sympathetic neurone. Prog Biophys Mol Biol. 1991;55(1):1–30. doi: 10.1016/0079-6107(91)90009-h. [DOI] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. Calcium currents in the normal adult rat sympathetic neurone. J Physiol. 1989 May;412:493–512. doi: 10.1113/jphysiol.1989.sp017628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. The calcium-dependent potassium conductance in rat sympathetic neurones. J Physiol. 1990 Mar;422:561–583. doi: 10.1113/jphysiol.1990.sp018001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Brown D. A. Apamin and d-tubocurarine block the afterhyperpolarization of rat supraoptic neurosecretory neurons. Neurosci Lett. 1987 Nov 23;82(2):185–190. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Cook N. S., Haylett D. G. Effects of apamin, quinine and neuromuscular blockers on calcium-activated potassium channels in guinea-pig hepatocytes. J Physiol. 1985 Jan;358:373–394. doi: 10.1113/jphysiol.1985.sp015556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Goh J. W., Pennefather P. S. Pharmacological and physiological properties of the after-hyperpolarization current of bullfrog ganglion neurones. J Physiol. 1987 Dec;394:315–330. doi: 10.1113/jphysiol.1987.sp016872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Johnson S. M., van Helden D. F. The slow calcium-dependent potassium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985 Apr;361:315–337. doi: 10.1113/jphysiol.1985.sp015648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A., Purves D. Ongoing electrical activity of superior cervical ganglion cells in mammals of different size. J Comp Neurol. 1989 Jun 15;284(3):398–404. doi: 10.1002/cne.902840307. [DOI] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B., Adams P. R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986 Jun;55(6):1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Nicoll R. A., Perkel D. J. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J Neurosci. 1991 Jan;11(1):23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. G., Ritchie A. K. Large and small conductance calcium-activated potassium channels in the GH3 anterior pituitary cell line. Pflugers Arch. 1987 Dec;410(6):614–622. doi: 10.1007/BF00581321. [DOI] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Leinders T., Vijverberg H. P. Ca2+ dependence of small Ca(2+)-activated K+ channels in cultured N1E-115 mouse neuroblastoma cells. Pflugers Arch. 1992 Dec;422(3):223–232. doi: 10.1007/BF00376206. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., Katayama Y. Calcium-dependent slow outward current in visceral primary afferent neurones of the rabbit. Pflugers Arch. 1989 Jun;414(2):171–177. doi: 10.1007/BF00580960. [DOI] [PubMed] [Google Scholar]
- Nohmi M., Kuba K. (+)-Tubocurarine blocks the Ca2+-dependent K+-channel of the bullfrog sympathetic ganglion cell. Brain Res. 1984 May 28;301(1):146–148. doi: 10.1016/0006-8993(84)90412-8. [DOI] [PubMed] [Google Scholar]
- Pennefather P., Lancaster B., Adams P. R., Nicoll R. A. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proc Natl Acad Sci U S A. 1985 May;82(9):3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P., McLachlan E. M. Ca(2+)-activated K+ currents underlying the afterhyperpolarization in guinea pig vagal neurons: a role for Ca(2+)-activated Ca2+ release. Neuron. 1991 Aug;7(2):257–264. doi: 10.1016/0896-6273(91)90264-z. [DOI] [PubMed] [Google Scholar]
- Smart T. G. Single calcium-activated potassium channels recorded from cultured rat sympathetic neurones. J Physiol. 1987 Aug;389:337–360. doi: 10.1113/jphysiol.1987.sp016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Kuba K. The Ca2+-sensitive K+-currents underlying the slow afterhyperpolarization of bullfrog sympathetic neurones. Pflugers Arch. 1987 Oct;410(3):234–242. doi: 10.1007/BF00580271. [DOI] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]


