Abstract
Varicose veins are common lower extremity venous disorders characterized by dilated veins and incompetent valves. Although maintaining the required vein wall tone for adaptive responses depends on a proper contractile function of the human saphenous smooth muscle, the contractile properties of varicose veins are mostly unknown. We investigated the relationship between contractile responses and the internal diameter of human saphenous varicose veins. The absolute contractile forces induced by potassium chloride (KCl, 60 mmol/l), serotonin (5-hydroxytryptamine [5-HT], 10 µmol/l), and noradrenaline (NAd, 10 µmol/l) were similar between normal saphenous veins (control) and varicose veins. When the contractile forces were normalized to the internal diameter in each preparation, the contractile responses to these stimuli were significantly lower in varicose veins than in the control veins. Furthermore, varicose veins were divided into three groups according to their internal diameter (group 1, 3–4.5 mm; group 2, 4.5–6 mm; group 3, >6 mm). The contractile responses induced by KCl, 5-HT, and NAd did not differ between groups 1 and 2 and the control group, while the contractile responses in group 3 were significantly lower than those in the control group. Moreover, the contractions induced by KCl and NAd in Group 3 were smaller than those in group 1 or group 2. This trend was also observed in 5-HT-induced contractions, although the results were not statistically significant. In conclusion, contractile responses in varicose veins may be altered by an increase in internal diameter, although adequate contractile responses are preserved in some diameters.
Keywords: contraction, internal diameter, noradrenaline, serotonin, varicose veins
Introduction
Varicose veins are a common disorder affecting lower-extremity veins, with an estimated prevalence of 5–30% in adults (1). Their severity ranges from the undesirable appearance of telangiectasia to large tortuous varicosities with or without related to cutaneous ulcerations, dermatitis, pigmentation, or swelling (2,3,4).
Varicose veins commonly manifest as dilated veins and incompetent valves (5). Maintaining the required tone in the vein wall during adaptive responses, such as standing and exposure to cold (6), depends on the proper contractile function of human saphenous smooth muscle. Vein wall alterations with disturbances in smooth muscle cells and/or extracellular matrix organization and dysfunctional contraction play important roles in the development of varicose veins (5, 7, 8). The main factor contributing to their development and progression is sustained venous hypertension, which increases the diameter of superficial veins, resulting in further valve incompetence (5). Although the theoretical basis and clinical aspects of varicose veins have been widely discussed and the contractile responses of varicose veins induced by various stimuli have been reported (9,10,11,12,13,14,15,16,17,18), the contractile properties and signaling remain insufficiently understood.
Hence, this study aimed to test the hypothesis that the degree of vasoconstriction is altered in human varicose veins with different internal diameters.
Materials and Methods
Ethics
This study was approved by the ethics committees of both Miyazaki Prefectural Nobeoka Hospital and Kyushu University of Medical Science (formerly Kyushu University of Health and Welfare), with approval number 09-004 (study using human saphenous veins). All patients provided their written informed consent to participate in the study. All experiments conformed to The Code of Ethics of the World Medical Association (Declaration of Helsinki) for Experiments Involving Humans.
Preparation of vessels and functional studies
Human saphenous vein samples were collected from 23 patients who underwent coronary artery bypass graft surgery at Miyazaki Prefectural Nobeoka Hospital (Miyazaki, Japan) and 35 patients who underwent surgical varicose vein treatment at Kuwabara Clinic (Miyazaki, Japan). At Miyazaki Prefectural Nobeoka Hospital, portions of each great saphenous vein graft were sectioned to the desired lengths to bypass the occluded coronary arteries. The remainder were used as the control group. At Kuwabara Clinic, portions of the saphenous vein were sectioned from each patient for surgical treatment of lower-extremity varicose veins. Only the non-distended areas were used for the varicose vein group. Subsequently, we transported the saphenous vein segments and measured their contractile responses as previously described (19,20,21). As previously reported (19,20,21), handling blood vessels during surgical procedures resulted in varying degrees of damage to the endothelial cells; therefore, we used endothelium-denuded rings. Moreover, we obtained as many rings of saphenous veins as possible from each patient to identify the relationship between the internal diameters and contractions. Briefly, we stretched the rings of the saphenous veins to the optimal tension (2.0 g), quickly measured the diameter of each ring using a Vernier caliper, and allowed them to equilibrate for 60 min by washing them with a fresh buffer solution several times. After stabilization, contractile responses to 60 mmol/l potassium chloride (KCl) were obtained, then they were washed again for restabilization. Subsequently, we obtained the contractile response to 60 mmol/l, 10 μmol/l, and 10 μmol/l concentrations of KCl, serotonin (5-hydroxytryptamine [5-HT]), and noradrenaline (NAd). Based on previous studies using human saphenous veins, we selected the concentration of each drug to evaluate adequate contractile responses induced by receptor-independent contractions and two potent vasoconstrictors associated with vasospasm (19,20,21,22,23,24).
Data analysis and statistics
Data are expressed as the mean ± standard error of the mean obtained from different numbers (n) of ring preparations. Contractions are expressed in grams (Fig. 1) or g/internal diameter (mm) (Table 1). To evaluate the relationship between internal diameter and contraction, we further divided the varicose vein groups into three groups according to the diameter obtained (group 1, 3–4.5 mm; group 2, 4.5–6 mm; and group 3, >6 mm). The unpaired Student’s t-test or unpaired Student’s t-test with Welch’s correction was used for two-group comparisons as necessary (GraphPad Prism ver. 8.4; GraphPad Software Inc., San Diego, CA, USA). To compare three or more groups, we used a one-way analysis of variance with Tukey’s multiple comparison test (GraphPad Prism). P-values of <0.05 were considered to indicate statistical significance.
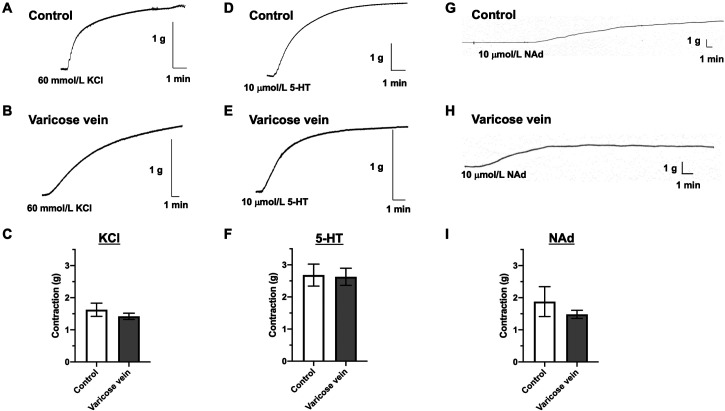
Fig. 1.
Contractile responses induced by KCl, 5-HT, or NAd in endothelium-denuded saphenous veins. Representative traces of KCl (60 mmol/l) (A, B), 5-HT (10 µmol/l) (D, E), or NAd (10 µmol/l) (G, H) in saphenous veins obtained from patients with normal (control) (A, D, G) and varicose veins (B, E, H). (C, F, I) Data represent the mean ± standard error of the mean of the contraction value (g) (n=12–105). KCl, potassium chloride; 5-HT, 5-hydroxytryptamine; NAd, noradrenaline.
Table 1. Contractions induced by KCl, 5-HT, and NAd in the saphenous veins as normalized by the internal diameter.
| KCl (60 mmol/l) | 5-HT (10 µmol/l) | NAd (10 µmol/l) | ||
| Control | 0.50 ± 0.06 (94) | 0.85 ± 0.12 (62) | 0.54 ± 0.13 (12) | |
| Varicose vein | 0.25 ± 0.02 (105)a | 0.55 ± 0.05 (48)a | 0.21 ± 0.02 (82) a | |
| Varicose vein | ||||
| Group 1 (ID: 3–4.5 mm) | 0.41 ± 0.03 (29) | 0.61 ± 0.08 (17) | 0.51 ± 0.08 (10) | |
| Group 2 (ID: 4.5–6 mm) | 0.41 ± 0.06 (22) | 0.69 ± 0.09 (19) | 0.42 ± 0.07 (14) | |
| Group 3 (ID: >6 mm) | 0.09 ± 0.01 (54) b, c, d | 0.24 ± 0.09 (12) b | 0.10 ± 0.01 (58) b, c, d | |
Contractions are expressed as g/internal diameter (mm). Values are presented as the means ± standard error of the mean obtained from different numbers (n) of ring preparations shown within parentheses. aP<0.05 versus control (using the unpaired Student’s t-test with Welchi’s correction). bP<0.05, control versus group 3. cP<0.05, group 1 versus group 3. dP<0.05, group 2 versus group 3 (using a one-way analysis of variance with Tukey’s post hoc test). KCl: potassium chloride; 5-HT: 5-hydroxytryptamine; NAd: noradrenaline; ID: internal diameter.
Results
Contractile responses induced by KCl, 5-HT, and NAd in control saphenous veins and varicose veins
As shown in Fig. 1, the contractions induced by KCl (60 mmol/l), which is a receptor-independent stimulation, cause membrane depolarization and stimulate Ca2+ entry via voltage-gated Ca2+ channels (25), and two potent vasospasm-associated vasoconstrictors (26), including 5-HT (10 µmol/l) and NAd (10 µmol/l), were all similar between the normal saphenous (control) and varicose veins. When the internal diameter normalized contractile forces (Table 1), the contractile responses to KCl, 5-HT, and NAd were all significantly lower in the varicose veins (KCl, n=105 from 27 patients; 5-HT, n=48 from 17 patients; and NAd, n=82 from 18 patients) than those in the control saphenous veins (KCl, n=94 from 23 patients; 5-HT, n=62 from 18 patients; and NAd. n=12 from 5 patients).
To investigate whether the contractile responses induced by these constrictors would alter the internal diameter of varicose veins, varicose veins were divided into three groups according to their internal diameter (Table 1). KCl-, 5-HT-, and NAd-induced contractile responses did not differ between group 1 (KCl, n=29 from 14 patients; 5-HT, n=17 from 9 patients; and NAd, n=10 from 4 patients)/group 2 (KCl, n=22 from 9 patients; 5-HT, n=19 from 5 patients; and NAd, n=14 from 6 patients) and the control group. Conversely, the KCl- and NAd-induced contractile responses in group 3 (KCl, n=54 from 15 patients; and NAd, n=58 from 15 patients) were significantly lower than those in the control group, group 1, and group 2. Moreover, the contractile responses induced by 5-HT were significantly lower in group 3 (n=12 from 8 patients) than in the control group (Table 1).
Discussion
The major findings of this study concern the relationship between the internal diameter and the contractile response of varicose veins. The results showed that when exposed to the three different stimuli, namely, KCl, 5-HT, and NAd, the contractile responses were preserved in varicose veins with an internal diameter of 6 mm but decreased in those with an internal diameter of over 6 mm.
Defective control of venous tone reportedly causes dilatation and subsequent insufficiency in varicose veins (15). Indeed, in clinical observations and experimental studies, varicose veins showed a reduced ability to contract in response to various vasoconstrictors such as α-adrenoceptor agonists, extracellular nucleotides, histamine, 5-HT, and endothelin (12,13,14,15,16,17,18). Asbeutah et al. (17) observed the following: 1) 5-HT and NAd induced concentration-dependent contractions of normal and varicose saphenous vein segments; and 2) the potency of 5-HT and NAd showed no significant differences, but the maximal response normalized to tissue weight was lower in the varicose vein segment. In the present study, although the absolute force development induced by stimuli was similar between the control and varicose veins, the contractile responses normalized by the internal diameter were lower in varicose veins with an internal diameter of >6 mm than in normal saphenous veins induced by both receptor-independent (KCl) and receptor-mediated contractions stimulated by 5-HT and NAd. Reportedly, α1A, α1B, α1C, and α1D adrenoceptors are expressed in human saphenous veins (27,28,29). Moreover, using a pharmacological approach, Yan et al. (29) observed that α1A and α1B adrenergic receptors are the major subtypes linking the contractile functions in the human saphenous vein. Furthermore, the functional post-junctional α2 adrenoceptor mediating contractions of the human saphenous vein closely resembles the human recombinant α2C adrenergic receptor ligand-binding site (30). Conversely, 5-HT2A (20, 22) and 5-HT1B (22, 31) receptors are expressed and function in the human saphenous vein. However, to date, no studies have compared these receptors between normal and varicose veins (and their various diameters) obtained from humans have been conducted. Future investigations are required regarding this point, including the relationship among functional changes, receptor expression, and internal diameter in human saphenous veins with and without varicose veins. In addition, receptor-independent KCl-induced contractions are also decreased in varicose veins with an internal diameter of >6 mm relative to contractions in normal saphenous veins, suggesting that reduced contractile responses in hyperdiastolic veins can be attributed to the contractile apparatus in vascular smooth muscle cells rather than receptor downregulation or to morphological abnormalities in the venous smooth muscle. For example, the RhoA-Rho kinase-dependent smooth muscle function is downregulated in varicose veins, which is correlated with the decreased expression of Rho kinase (7). As the human saphenous venous contractions induced by 5-HT and NAd are partly mediated by the Rho kinase pathway (23), and KCl can also cause Ca2+ sensitization involving Rho kinase translocation and activation (25), Rho kinase may be related to the reduced contraction in hyperdiastolic veins. Another putative mechanism underlying the decrease in contractions of large-diameter varicose veins may be alterations in matrix metalloproteinase (MMP) activity. Several studies have demonstrated alterations in mRNA and/or protein expression levels and proteolytic activities of MMPs have been detected in varicose veins (10, 32, 33). MMPs induce increased proteolysis of different protein substrates in the extracellular matrix, particularly collagen and elastin, which results in weakening of the vein wall and promotes venous dilation via several mechanisms, including activating potassium channels, thereby leading to smooth muscle hyperpolarization and relaxation (10, 32, 33). However, further research is needed to clarify the relationships between smooth muscle content, MMP activities, contractile responses, and their signaling induced by various stimuli and internal diameters in varicose veins. As contractile properties were preserved in varicose veins of smaller internal diameter, this is significant for preventing an increased internal diameter (e.g., remodeling) in varicose veins before the development of hyperdiastolic veins.
Conclusion
Contractile responses in varicose veins may change after an increase in internal diameter, although adequate contractile responses are preserved for certain diameters. These findings may be instrumental in pursuing further studies on varicose vein management.
Data Availability
The data are available from the corresponding author on reasonable request.
Author Contribution
Conceptualization: NTT
Data curation: AY, TM, TN, RY, NTT
Formal analysis: AY, NTT
Investigation: AY, MK, EN, NTT
Methodology: NTT
Project administration: NTT
Resources: AY, MK, EN, RY, NTT
Visualization: TM, TN, NTT
Writing-original draft: TM, NTT
Writing-review and editing: AY, TM, TN, MK, EN, RY, NTT
Funding
This study was supported in part by JSPS KAKENHI, Grant JP17K10772 (to Naoko Tanaka-Totoribe).
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Evans CJ, Fowkes FG, Ruckley CV, Lee AJ. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Community Health. 1999; 53(3): 149–53. doi: 10.1136/jech.53.3.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006; 355(5): 488–98. doi: 10.1056/NEJMra055289 [DOI] [PubMed] [Google Scholar]
- 3.Atta HM. Varicose veins: role of mechanotransduction of venous hypertension. Int J Vasc Med. 2012; 2012: 538627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weingarten MS. State-of-the-art treatment of chronic venous disease. Clin Infect Dis. 2001; 32(6): 949–54. doi: 10.1086/319360 [DOI] [PubMed] [Google Scholar]
- 5.Bradbury AW. Pathophysiology and Principles of Management of Varicose Veins. In: Fitridge R, Thompson M, editors. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]. Adelaide (AU): University of Adelaide Press; 2011. 24. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534256/.
- 6.Flavahan NA, Vanhoutte PM. Sympathetic purinergic vasoconstriction and thermosensitivity in a canine cutaneous vein. J Pharmacol Exp Ther. 1986; 239(3): 784–9. [PubMed] [Google Scholar]
- 7.Cario-Toumaniantz C, Evellin S, Maury S, Baron O, Pacaud P, Loirand G. Role of Rho kinase signalling in healthy and varicose human saphenous veins. Br J Pharmacol. 2002; 137(2): 205–12. doi: 10.1038/sj.bjp.0704849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wali MA, Eid RA. Smooth muscle changes in varicose veins: an ultrastructural study. J Smooth Muscle Res. 2001; 37(5-6): 123–35. doi: 10.1540/jsmr.37.123 [DOI] [PubMed] [Google Scholar]
- 9.Min S, Xing M, Jiang H, Zhang L, Chen C, Ma Y, et al. Exploring causal correlations between inflammatory cytokines and varicose veins: a Mendelian randomization analysis. Int Wound J. 2024; 21(2): e14714. doi: 10.1111/iwj.14714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saberianpour S, Modaghegh MHS, Rahimi H, Kamyar MM. Role of mechanosignaling on pathology of varicose vein. Biophys Rev. 2021; 13(1): 139–45. doi: 10.1007/s12551-021-00783-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lima DC. Varicose veins and occupational health: symptoms, treatment and prevention. Rev Bras Med Trab. 2019; 17(4): 589–93. doi: 10.5327/Z1679443520190460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thulesius O, Said S, Shuhaiber H, Neglen P, Gjores JE. Endothelial mediated enhancement of noradrenaline induced vasoconstriction in normal and varicose veins. Clin Physiol. 1991; 11(2): 153–9. doi: 10.1111/j.1475-097X.1991.tb00108.x [DOI] [PubMed] [Google Scholar]
- 13.Blöchl-Daum B, Schuller-Petrovic S, Wolzt M, Korn A, Böhler K, Eichler HG. Primary defect in alpha-adrenergic responsiveness in patients with varicose veins. Clin Pharmacol Ther. 1991; 49(1): 49–52. doi: 10.1038/clpt.1991.9 [DOI] [PubMed] [Google Scholar]
- 14.Lowell RC, Gloviczki P, Miller VM. In vitro evaluation of endothelial and smooth muscle function of primary varicose veins. J Vasc Surg. 1992; 16(5): 679–86. doi: 10.1016/0741-5214(92)90221-S [DOI] [PubMed] [Google Scholar]
- 15.Brunner F, Hoffmann C, Schuller-Petrovic S. Responsiveness of human varicose saphenous veins to vasoactive agents. Br J Clin Pharmacol. 2001; 51(3): 219–24. doi: 10.1046/j.1365-2125.2001.00334.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziganshin AU, Khaziakhmetov DF, Ziganshina LE, Khaziakhmetova VN, Jourjikiya RK, Ziganshin BA, et al. Varicose disease affects the P2 receptor-mediated responses of human greater saphenous vein. Vascul Pharmacol. 2004; 42(1): 17–21. doi: 10.1016/j.vph.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 17.Asbeutah AM, Asfar SK, Safar H, Oriowo MA, Elhagrassi I, Abu-Assi MA, et al. In vivo and in vitro assessment of human saphenous vein wall changes. Open Cardiovasc Med J. 2007; 1: 15–21. doi: 10.2174/1874192400701010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raffetto JD, Qiao X, Beauregard KG, Tanbe AF, Kumar A, Mam V, et al. Functional adaptation of venous smooth muscle response to vasoconstriction in proximal, distal, and varix segments of varicose veins. J Vasc Surg. 2010; 51(4): 962–71. doi: 10.1016/j.jvs.2009.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokota A, Gamoh S, Tanaka-Totoribe N, Shiba T, Kuwabara M, Nakamura E, et al. Angiotensin II, as well as 5-hydroxytriptamine, is a potent vasospasm inducer of saphenous vein graft for coronary artery bypass grafting in patients with diabetes mellitus. Biochem Biophys Rep. 2016; 6: 82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai T, Kuwabara M, Tanaka-Totoribe N, Nakamura E, Matsuo Y, Gamoh S, et al. Insulin induces internalization of the plasma membrane 5-hydroxytryptamine2A (5-HT2A) receptor in the isolated human endothelium-denuded saphenous vein via the phosphatidylinositol 3-kinase pathway. J Pharmacol Sci. 2012; 118(2): 178–85. doi: 10.1254/jphs.11172FP [DOI] [PubMed] [Google Scholar]
- 21.Matsuo Y, Kuwabara M, Tanaka-Totoribe N, Kanai T, Nakamura E, Gamoh S, et al. The defective protein level of myosin light chain phosphatase (MLCP) in the isolated saphenous vein, as a vascular conduit in coronary artery bypass grafting (CABG), harvested from patients with diabetes mellitus (DM). Biochem Biophys Res Commun. 2011; 412(2): 323–7. doi: 10.1016/j.bbrc.2011.07.097 [DOI] [PubMed] [Google Scholar]
- 22.Nakamura E, Tanaka N, Kuwabara M, Yamashita A, Matsuo Y, Kanai T, et al. Relative contributions of 5-hydroxytryptamine (5-HT) receptor subtypes in 5-HT-induced vasoconstriction of the distended human saphenous vein as a coronary artery bypass graft. Biol Pharm Bull. 2011; 34(1): 82–6. doi: 10.1248/bpb.34.82 [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto T, Nagano T, Yokota A, Nakamura E, Kuwabara M, Yamamoto R, et al. Differential suppressive effects of Rho kinase inhibitor fasudil on serotonin- and noradrenaline-induced contractions of human internal thoracic arteries and saphenous veins. Biol Pharm Bull. 2024; (in press). doi: 10.1248/bpb.b24-00502 [DOI] [PubMed] [Google Scholar]
- 24.Giessler C, Wangemann T, Silber RE, Dhein S, Brodde OE. Noradrenaline-induced contraction of human saphenous vein and human internal mammary artery: involvement of different alpha-adrenoceptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 2002; 366(2): 104–9. doi: 10.1007/s00210-002-0582-6 [DOI] [PubMed] [Google Scholar]
- 25.Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005; 288(4): C769–83. doi: 10.1152/ajpcell.00529.2004 [DOI] [PubMed] [Google Scholar]
- 26.Wanstall JC, O’Donnell SR, Kay CS. Increased relaxation by felodipine on pulmonary artery from rats with monocrotaline-induced pulmonary hypertension does not reflect functional impairment of the endothelium. Pulm Pharmacol. 1991; 4(1): 60–6. doi: 10.1016/0952-0600(91)90041-Z [DOI] [PubMed] [Google Scholar]
- 27.Diehl NL, Shreeve SM. Identification of the alpha 1c-adrenoceptor in rabbit arteries and the human saphenous vein using the polymerase chain reaction. Eur J Pharmacol. 1994; 268(3): 393–8. doi: 10.1016/0922-4106(94)90064-7 [DOI] [PubMed] [Google Scholar]
- 28.Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D’Amico EB, et al. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation. 1999; 100(23): 2336–43. doi: 10.1161/01.CIR.100.23.2336 [DOI] [PubMed] [Google Scholar]
- 29.Yan M, Sun J, Bird PI, Liu DL, Grigg M, Lim YL. Alpha1A- and alpha1B-adrenoceptors are the major subtypes in human saphenous vein. Life Sci. 2001; 68(10): 1191–8. doi: 10.1016/S0024-3205(00)01027-4 [DOI] [PubMed] [Google Scholar]
- 30.Gavin KT, Colgan MP, Moore D, Shanik G, Docherty JR. Alpha 2C-adrenoceptors mediate contractile responses to noradrenaline in the human saphenous vein. Naunyn Schmiedebergs Arch Pharmacol. 1997; 355(3): 406–11. doi: 10.1007/PL00004961 [DOI] [PubMed] [Google Scholar]
- 31.van den Broek RW, Bhalla P, VanDenBrink AM, de Vries R, Sharma HS, Saxena PR. Characterization of sumatriptan-induced contractions in human isolated blood vessels using selective 5-HT(1B) and 5-HT(1D) receptor antagonists and in situ hybridization. Cephalalgia. 2002; 22(2): 83–93. doi: 10.1046/j.1468-2982.2002.00295.x [DOI] [PubMed] [Google Scholar]
- 32.Raffetto JD, Khalil RA. Mechanisms of lower extremity vein dysfunction in chronic venous disease and implications in management of varicose veins. Vessel Plus. 2021; 5: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucukguven A, Khalil RA. Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr Drug Targets. 2013; 14(3): 287–324. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author on reasonable request.