Abstract
Although naloxone is an antagonist of the opioid µ receptor, its effect on the peripheral sympathetic nerve function in the blood vessels has not yet been definitively elucidated. Therefore, we examined the effects of naloxone on vasoconstriction of the vascular smooth muscle of rats. Isolated rat mesenteric vascular-intestinal loop preparations were treated with either endogenous or exogenous α1 adrenoceptor agonists followed by prazosin, a selective antagonist of the α1 adrenoceptor, or naloxone, and noradrenaline overflow was measured. Vasoconstriction caused by peri-arterial nerve stimulation (PNS) and phenylephrine, an exogenous agonist of the α1 adrenoceptor, was abolished by prazosin. However, prazosin did not affect PNS-induced endogenous noradrenaline overflow. Naloxone did not affect either PNS-induced endogenous noradrenaline overflow or vasoconstriction. However, naloxone did inhibit phenylephrine-induced vasoconstriction. In addition, naloxone did not affect the angiotensin II-induced vasoconstriction. These results demonstrate that naloxone selectively inhibits vasoconstriction caused by phenylephrine, but not vasoconstriction caused by endogenous noradrenaline released from sympathetic nerve cells in the rat mesenteric vasculature.
Keywords: α1 adrenoceptor, mesenteric vasculature, naloxone, noradrenaline, vasoconstriction
Introduction
Naloxone is an opioid µ receptor antagonist that is widely administered to rapidly reverse opioid overdose (1). It directly affects the central nervous system and improves the respiratory depressant effects of morphine (1, 2). Naloxone is an overall safe medication, although it may rarely be fatal. In addition, some reports of life-threatening or lethal responses to naloxone administration do exist (1). In particular, severe but rarely reported side effects of opioid withdrawal include pulmonary edema, cardiac arrhythmias, profound hyper- or hypotension, and cardiac arrest (1, 3). Therefore, a comprehensive understanding of the mechanisms underlying the effects of naloxone on the whole body is warranted.
Some reports have revealed the effects of naloxone on the peripheral nervous system, vascular system, and central nervous system (4, 5). Sasaki et al. (6) found that naloxone (300 µmol/l; at a high concentration) induced non-specific vasodilation, and that naloxone (0.3, 3, and 30 µmol/l; at a lower concentration) inhibited the vasoconstrictor effects of noradrenaline without changing KCl-, serotonin-, or hemoglobin-induced constrictions in ring preparations of canine basilar arteries. Naloxone (30 µmol/l) suppressed feline basilar artery constriction induced by high concentrations of noradrenaline, but it failed to change the constriction induced by lower noradrenaline concentrations (7). Naloxone (3 and 30 µmol/l) did not alter the constrictor response of the monkey basilar artery to noradrenaline. Moreover, naloxone (30 µmol/l) enhances adrenaline-induced constriction of the mesenteric arterial rings obtained from both the feline and monkey mesenteric arteries at adrenaline concentrations of 100 nmol/l to 10 µmol/l (7). Conversely, regarding the release of noradrenaline from the sympathetic nerve endings, Bucher et al. (8) revealed that the prejunctional opioid µ receptor interacts with the prejunctional α2 adrenoceptors and modulates the release of noradrenaline in the sympathetic nerve endings of the rat tail artery. Naloxone increased both noradrenaline overflow and left ventricular pressure during stimulation and prevented or significantly delayed the gradual decline in overflow observed in cardiac nerve-stimulated controls in anesthetized dogs (9). These results indicate that naloxone directly or indirectly affects the sympathetic nerve function and vascular smooth muscle function; however, their effects are controversial. Furthermore, the association between naloxone, sympathetic nerves, and the small mesenteric artery function remains unknown.
Noradrenaline neurotransmission mainly controls the changes in mesenteric artery tension and vascular resistance (10, 11). The determination of endogenous noradrenaline release and the associated vascular tone in the mesenteric arterial bed is quite difficult because the sympathetic nerves are tightly innervated (12) and many mesenteric vessels are extremely small. However, we revealed that isolated rat mesenteric vascular-intestinal loop preparations enable the measurement of endogenous noradrenaline release from the sympathetic nerve endings and measured the mesenteric total vascular tone as perfusion pressure (13, 14). Mesenteric circulation plays a crucial role in controlling systemic blood pressure, and naloxone may affect blood pressure modulation by altering the mesenteric arterial function. Therefore, our study aimed to evaluate whether naloxone modulates the sympathetic nerve function and vasoconstriction in rat mesenteric arterial beds using an isolated rat mesenteric vascular-intestinal loop preparation system.
Materials and Methods
Chemicals
Naloxone, prazosin, phenylephrine, and angiotensin II were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pure naloxone, prazosin, phenylephrine, and angiotensin II were dissolved in distilled water (DW). Distilled water was prepared using Millipore filters (Merck Co. Inc., Kenilworth, NJ, USA). Alumina was purchased from Fujifilm Wako Pure Chemical Industries (Osaka, Japan). All the chemicals and solvents used were of the highest commercially available grade.
Animals
Male Sprague–Dawley rats (10–11-weeks-old) obtained from Kyudo Co. Ltd. (Tosu, Saga, Japan) were used in this study. All animals were housed under a 12 h/12 h light/dark cycle with food and water available ad libitum. The animals were allowed to acclimatize for at least one week before starting the experimental procedures. They were euthanised by decapitation under anaesthesia with the i.p. injection of medetomidine (0.15 mg/kg), midazolam (2.0 mg/kg), and butorphanol (2.5 mg/kg). All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals and the International Guiding Principles for Biomedical Research Involving Animals. All efforts were made to minimize suffering. In addition, the operating procedures involved in this study met the requirements of animal welfare, followed the 3R principle, and were approved by the Laboratory Animal Use and Management Committee of the Institute of Kyusyu University of Medical Science (formerly Kyushu University of Health and Welfare) (approval number 4-1-18).
Perfusion of mesenteric vascular bed with intestinal tract
A mesenteric vascular bed with the intestinal tract was constructed and perfusion experiments were performed as previously described (13). Briefly, the superior mesenteric artery was cannulated at its origin from the abdominal aorta. The mesenteric vascular bed of the intestinal tract was isolated. After the mesenteric vascular bed and intestinal tract were washed, the preparation was placed in an organ bath maintained at 37°C and perfused at a constant flow rate of 5 ml/min using a peristaltic roller pump (model SJ-1215, Atto, Tokyo, Japan) with modified Krebs-Ringer bicarbonate solution (122 mmol/l NaCl, 26 mmol/l NaHCO3, 5 mmol/l KCl, 2.4 mmol/l CaCl2, 1 mmol/l MgSO4, 0.03 mmol/l EDTA, 11 mmol/l glucose, 20 mg/l ascorbic acid, pH 7.4) at 37°C. The perfusate was aerated using a mixture of 95% oxygen and 5% carbon dioxide. Perfusion pressure changes were recorded using a pressure transducer (model MPU-0.5A; Nihon Kohden Co., Tokyo, Japan).
PNS and infusion of phenylephrine or angiotensin II
After stabilization for 30 min, the mesenteric vascular bed along with the intestinal tract was treated with peri-arterial nerve stimulation (PNS), phenylephrine, or angiotensin II. PNS was added at 4 min intervals via platinum electrodes; one side was placed in the upper part of the superior mesenteric artery, and the other side was placed at the end of the intestine. Two-millisecond rectangular pulses (50 V) were applied for 30 s at 4, 8, 12, and 16 Hz using an electrical stimulator (model SEN-3301; Nihon Kohden Co.). The neural basis and noradrenaline basis of the vasoconstrictor response elicited by PNS have been confirmed previously in studies reporting the abolition of the response after perfusion with 100 nmol/l tetrodotoxin (13,14,15), 10 µmol/l guanethidine (13,14,15), or 30 nmol/l prazosin (15,16,17). Fifteen minutes after PNS at 16 Hz, phenylephrine or angiotensin II was directly added to the perfusate for 10 s at 4 min intervals using an infusion pump (model 975; Harvard Apparatus, Holliston, MA, USA). The concentrations of phenylephrine used were 7.5, 15, 30, and 60 nmol. The amount of added angiotensin II was 400 pmol. The addition of 30 nmol/l prazosin or 1 µmol/l naloxone was started 20 min before the first PNS and continued throughout the experiments. The prazosin concentrations were based on those described in our previous reports (13, 15). The naloxone concentration was based on a rat experiment performed by Ngai et al. (18). The serum concentration of naloxone following i.v. injection of 5 mg/kg, a generally used dose in rat models, was more than 1 µg/ml. Therefore, we adopted a naloxone concentration of 1 µmol/l in this study.
Determining the degree of noradrenaline overflow in the perfusate
The noradrenaline content of the perfusate was measured using an HPLC system (13). The perfusate was collected 4 min before the first PNS to measure spontaneous noradrenaline overflow. The perfusate was collected for 4 min from each PNS to measure PNS-induced total noradrenaline overflow. Noradrenaline in the perfusate was absorbed onto alumina and then eluted with 0.1 N perchloric acid. The samples were analyzed by HPLC (model LC3A; Shimadzu Co., Tokyo, Japan) with an electrochemical detector (model 5100; ESA, Bedford, MA, USA). The amount of noradrenaline was calculated using the height ratio of 3,4-dihydroxybenzylamine as an internal standard. PNS-induced noradrenaline overflow was defined as the difference between the spontaneous noradrenaline overflow and total PNS-induced overflow.
Statistical analysis
Data are expressed as the mean ± S.E.M. of five experiments. Statistical comparisons between two groups were performed using unpaired Student’s t-test (GraphPad Prism ver. 8.4; GraphPad Software Inc., San Diego, CA, USA). Significance was analyzed using repeated-measures two-way analysis of variance, followed by Tukey’s multiple comparison test to compare the full concentration-response curves (GraphPad Prism). Statistical significance was set at P<0.05.
Results
Pressor responses to PNS
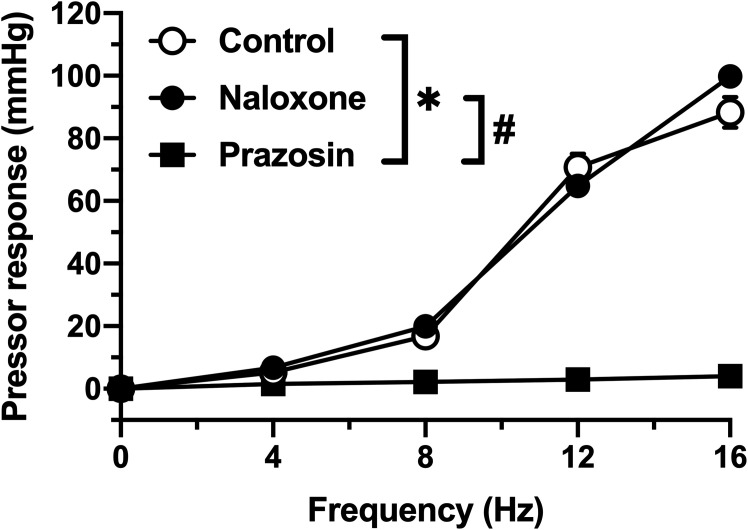
As shown in Fig. 1, the PNS increased the perfusion pressure in a frequency-dependent manner. Prazosin pretreatment abolished PNS-induced pressor responses. In contrast, naloxone pretreatment did not affect the PNS-induced pressor responses.
Fig. 1.
Perfusion pressure changes in isolated mesenteric vascular beds with the intestinal tract induced by periarterial nerve stimulation (PNS). PNS at 4, 8, 12, and 16 Hz was applied to the vascular bed in the absence (Control) or presence of 30 nmol/l prazosin (Prazosin) or 1 µmol/l naloxone (Naloxone). Each point and bar represents the mean and standard error of the mean (S.E.M.) of five rats. *P<0.05, control vs. prazosin. #P<0.05, naloxone versus prazosin. Repeated-measures two-way ANOVA and Tukey’s multiple comparison test.
Noradrenaline overflow induced by PNS
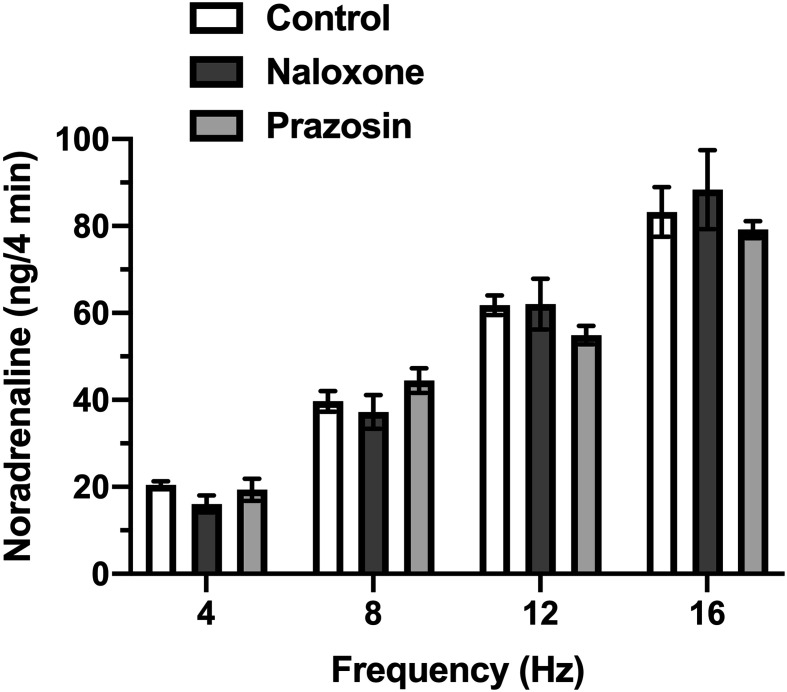
As shown in Fig. 2, the noradrenaline content in the perfusate was increased by the PNS in a frequency-dependent manner. Neither naloxone (1 µmol/l) nor prazosin (30 nmol/l) pre-treatment affected the PNS-induced noradrenaline overflow.
Fig. 2.
The noradrenaline overflow in perfusate induced by periarterial nerve stimulation (PNS). PNS at 4, 8, 12, and 16 Hz was applied to isolated mesenteric vascular beds with an intestinal tract in the absence (Control) or presence of 30 nmol/l prazosin (Prazosin) or 1 µmol/l naloxone (Naloxone). Data are shown as the mean ± S.E.M. of five rats.
Pressor responses to phenylephrine or angiotensin II
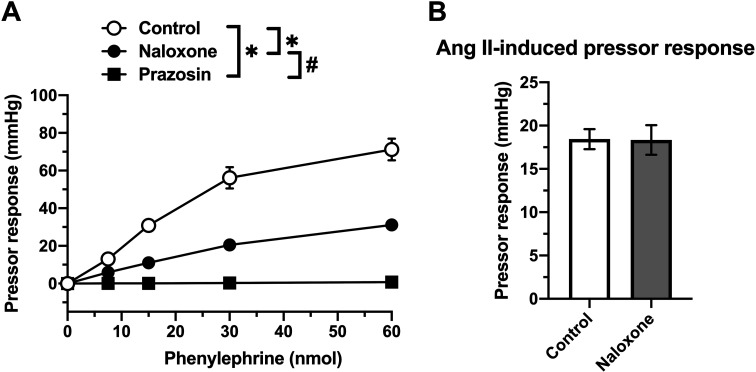
Phenylephrine increased the perfusion pressure in a dose-dependent manner (Fig. 3A). Phenylephrine-induced pressor responses were abolished by pre-treatment with 30 nmol/l prazosin. Notably, phenylephrine-induced pressor responses were significantly inhibited by pre-treatment with 1 µmol/l naloxone (vs. control). In contrast, the angiotensin II (400 pmol)-induced pressor response was not altered by naloxone pretreatment (Fig. 3B).
Fig. 3.
The perfusion pressure changes in isolated mesenteric vascular beds with an intestinal tract, induced by phenylephrine or angiotensin II. (A) Phenylephrine at doses of 7.5 to 60 nmol was applied to the vascular bed in the absence (Control) or presence of 30 nmol/l prazosin (Prazosin) or 1 µmol/l naloxone (Naloxone). (B) Angiotensin II (Ang II; 400 pmol)-induced contraction in the absence (Control) or presence of 1 µmol/l naloxone (Naloxone). Data are shown as the mean ± S.E.M. of five rats. *P<0.05, vs. Control. #P<0.05, Naloxone vs. Prazosin. Repeated-measures two-way ANOVA, Tukey’s multiple comparison test.
Discussion
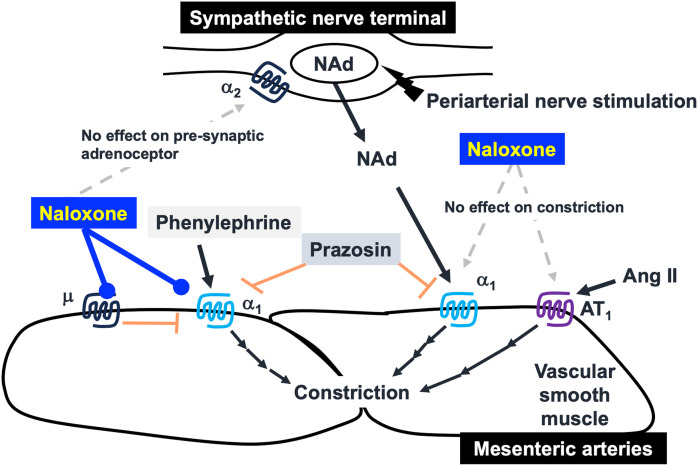
Intriguingly, a potentially important finding of this study was that naloxone did not affect either the PNS-induced endogenous noradrenaline overflow or vasoconstriction, and that naloxone inhibited exogenous phenylephrine-induced vasoconstriction without affecting angiotensin II-induced vasoconstriction in rat mesenteric arterial beds. A schematic summary of the data is shown in Fig. 4.
Fig. 4.
A schematic summary based on the present study. The suppressive mechanism of naloxone on vasoconstrictor responses around the sympathetic nerve terminals of blood vessels. NAd: noradrenaline, Ang II: angiotensin II.
In the isolated rat mesenteric vascular-intestinal loop system, naloxone did not modify vasoconstriction during PNS. It is likely that both noradrenaline and ATP are involved in PNS-induced vasoconstriction, because ATP is released as a co-transmitter together with noradrenaline (19). However, in the rat mesenteric vasculature, no functionally significant amount of ATP was co-released by PNS in the presence of 30 nmol/l prazosin, which blocked only post-synaptic α1 adrenoceptors (16, 17). Moreover, in the same vasculature, moderate cooling (24°C) or pre-treatment with 1 µmol/l prazosin revealed that PNS could induce the release of functionally significant amounts of ATP; however, the PNS-induced ATP release caused only slight vasoconstrictor responses and occurred in a frequency-independent manner (16, 17). Moreover, in isolated rat mesenteric vascular-intestinal loop preparations, the pressor responses induced by nerve stimulation or noradrenaline were blocked by prazosin alone (15,16,17). Regarding the release of noradrenaline from sympathetic nerve endings, one of the important physiological modulators of noradrenergic nerve function appears to be the neuronally released noradrenaline, which inhibits the exocytotic release of noradrenaline through the action of prejunctional α2 adrenoceptors (20, 21). We previously reported that treatment with 100 nmol/l yohimbine, a selective α2 adrenoceptor antagonist, increased PNS-induced noradrenaline overflow in isolated rat mesenteric vascular-intestinal loop preparations (13). Bucher et al. (8) revealed that the prejunctional opioid µ receptor interacts with prejunctional α2 adrenoceptors and modulates the release of noradrenaline in the sympathetic nerve endings of the rat tail artery. In the present study, we found that naloxone did not change the release of noradrenaline or vasoconstrictions induced by PNS, and that PNS-induced vasoconstriction was completely blocked by prazosin. These results and relevant evidence suggest that naloxone does not modulate endogenous sympathetic nerve-mediated vasoconstrictions, including presynaptic α2 receptors, post-synaptic adrenoceptors, and purinoceptors.
In our experiments, prazosin blocked both endogenous and exogenous α1 adrenoceptor agonist-induced pressor response. However, although naloxone did not affect endogenous α1 adrenoceptor agonist-induced pressor responses, it inhibited those induced by exogenous α1 adrenoceptor agonist. In addition, naloxone did not affect the pressor response induced by angiotensin II, another G protein-coupled receptor ligand [i.e., AT1 receptor) (22)]. Three subtypes (α1A, α1B, α1D) of the α1 adrenoceptor exist in mammalian tissues (23, 24), and they have differences in their binding affinities for the α1 adrenoceptor blocker (25). Moreover, in rat vas deferens, synaptically released noradrenaline and exogenous α1 adrenoceptor agonist activate different subtypes of the α1 adrenoceptor (26). These reports demonstrated that intrasynaptic and extrasynaptic receptors have different subtypes in some tissues. Our results indicate that naloxone may exert a stronger suppressive effect on the α1 adrenoceptor subtype stimulated by exogenous α1 adrenoceptor agonist than on the α1 adrenoceptor subtypes stimulated by synaptically released noradrenaline. In other words, the difference in the effects of prazosin and naloxone may be attributed to the difference in sensitivity to α1 adrenoceptors inside and outside of the sympathetic synaptic cleft. However, no data exist regarding naloxone specificity for the α1 adrenoceptor subtype. The mechanisms underlying the specific suppression of exogenous α1 agonist-induced vasoconstriction in rat mesenteric arterial beds are currently unknown. Based on the present findings, naloxone may affect α1 adrenoceptor-mediated signalings in the receptor itself rather than downstream pathways or affecting other proteins such as potassium channels; if naloxone suppressed vasoconstriction by modulating the intracellular signaling pathway in smooth muscle cells, it should also suppress vasoconstriction induced by PNS and other Gq-coupled AT1 receptor stimulation. Although it is possible that naloxone directly binds α1 receptor, it indirectly affects α1 receptor function after naloxone binding opioid µ receptor (e.g., heterodimerization between opioid µ receptor and α1 receptor). Further investigations will be required on these points, including cellular mechanisms in mesenteric smooth muscle cells.
The present study is associated with some limitations. Affinity and efficacy may differ between noradrenaline and phenylephrine in the rat mesenteric vascular-intestinal loop system. The present study used phenylephrine to rule out uptake at the sympathetic nerve ending in order to investigate the effect of naloxone on vasoconstriction caused by an exogenous α1 adrenoceptor agonist. Currently, we cannot completely rule out the possibility that the suppressive effect of naloxone on phenylephrine-induced constriction is caused by a compound-specific effect because there is no evidence of any direct chemical interaction between naloxone and phenylephrine before binding to their receptors. Further investigation is required on the effect of naloxone on other specific α1 adrenoceptor agonists in the rat mesenteric vascular-intestinal loop system. The exogenous application of noradrenaline in the rat mesenteric vascular-intestinal loop system has several possibilities for noradrenaline motion, including binding to post-junctional adrenoceptors on mesenteric arterial smooth muscle, prejunctional α2 adrenoceptors, and prejunctional uptake by transporters. Furness and Marshall (27) revealed a gradual increase in sensitivity to topically applied noradrenaline from larger arteries toward the capillary mesenteric bed of rats. The histochemical assessment of these same vessels revealed that the density of adrenergic innervation gradually reduced from the principal arteries to the terminal arterioles and that the precapillary arterioles demonstrated no adrenergic innervation. Marshall (28) demonstrated that cocaine and chronic denervation increased the sensitivity of the innervated rat mesenteric arterial vessels to topically applied noradrenaline in direct proportion to the density of their adrenergic innervation, and the dose-response curves of the principal arteries were always shifted more than those of the small arteries and terminal arterioles. In contrast, neither cocaine nor chronic denervation affects the sensitivity of non-innervated precapillary arterioles (28). Therefore, the interpretation of the effect of noradrenaline on perfusion pressure in rat mesenteric arterial beds may be challenging to support our hypothesis because of the above possibilities. In the present study, the total amount of noradrenaline released by the PNS (i.e., from the overall mesenteric arterial beds) did not differ between the control and naloxone groups. Unfortunately, we could not identify the effects of naloxone on the uptake of noradrenaline throughout and at each site of the nerve endings in the rat mesenteric arterial beds. Therefore, elucidating the mechanisms underlying the effects of naloxone on vasoconstriction induced by exogenous noradrenaline in this system is difficult. We assessed vasoconstrictions as increased perfusion pressures in whole mesenteric arterial beds, including principal arteries and terminal arterioles, which are different innervation of sympathetic nerves. Thus, the difference in the effect of naloxone on naïve and experimental stimuli may be caused by different contributions to constrictions in each region. Future experiments are therefore required to further our understanding of the effects of naloxone on different regions of the mesenteric arterial beds.
Conclusion
In conclusion, our results suggest that naloxone specifically suppresses vasoconstrictions induced by exogenous but not endogenous agonist of α1 adrenoceptor in rat mesenteric arterial beds. We believe that our findings will stimulate further interest in the relationship between naloxone treatment and mesenteric circulation in (patho)physiological states.
Data Availability
The data are available from the corresponding author upon reasonable request.
Author Contribution
Conceptualization: RY
Data curation: MH, TM, TN, RY, NTT
Formal analysis: MH, TM, NTT
Investigation: RY, NTT
Methodology: RY, NTT
Project administration: RY, NTT
Resources: RY, NTT
Visualization: TM, MH, TN, NTT
Writing-original draft: TM, MH, NTT
Writing-review & editing: MH, TM, TN, RY, NTT
Funding
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Rzasa Lynn R, Galinkin JL. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther Adv Drug Saf. 2018; 9(1): 63–88. doi: 10.1177/2042098617744161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gairola RL, Gupta PK, Pandley K. Antagonists of morphine-induced respiratory depression. A study in postoperative patients. Anaesthesia. 1980; 35(1): 17–21. doi: 10.1111/j.1365-2044.1980.tb03714.x [DOI] [PubMed] [Google Scholar]
- 3.Narcan Nasal Spray (full prescribing information), www.accessdata.fda.gov/drugsatfda_docs/label/2015/208411lbl.pdf (Accessed March 21, 2017).
- 4.Cohen RA, Coffman JD. Naloxone reversal of morphine-induced peripheral vasodilatation. Clin Pharmacol Ther. 1980; 28(4): 541–4. doi: 10.1038/clpt.1980.200 [DOI] [PubMed] [Google Scholar]
- 5.Turner DM, Kassell NF, Sasaki T, Comair YG, Beck DO, Boarini DJ. High dose naloxone produces cerebral vasodilation. Neurosurgery. 1984; 15(2): 192–7. doi: 10.1227/00006123-198408000-00008 [DOI] [PubMed] [Google Scholar]
- 6.Sasaki T, Kassell NF, Turner DM, Maixner W, Torner JC, Coester HC. Effects of naloxone on canine cerebral vascular smooth muscle. J Cereb Blood Flow Metab. 1984; 4(2): 166–72. doi: 10.1038/jcbfm.1984.24 [DOI] [PubMed] [Google Scholar]
- 7.Sasaki T, Kassell NF, Turner DM, Coester HC. Comparison of the effect of naloxone on cerebral versus mesenteric arterial smooth muscle in feline and primate species. Stroke. 1984; 15(6): 1025–8. doi: 10.1161/01.STR.15.6.1025 [DOI] [PubMed] [Google Scholar]
- 8.Bucher B, Corriu C, Stoclet JC. Prejunctional opioid mu-receptors and adenosine A1-receptors on the sympathetic nerve endings of the rat tail artery interact with the alpha 2-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1992; 345(1): 37–43. doi: 10.1007/BF00175467 [DOI] [PubMed] [Google Scholar]
- 9.Gu H, Barron BA, Gaugl JF, Caffrey JL. Dynorphin, naloxone, and overflow of norepinephrine during cardiac nerve stimulation in dogs. Am J Physiol. 1992; 263(1 Pt 2): H153–61. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto R, Wada A, Asada Y, Yuhi T, Yanagita T, Niina H, et al. Functional relation between nitric oxide and noradrenaline for the modulation of vascular tone in rat mesenteric vasculature. Naunyn Schmiedebergs Arch Pharmacol. 1994; 349(4): 362–6. doi: 10.1007/BF00170881 [DOI] [PubMed] [Google Scholar]
- 11.Shimamura K, Sekiguchi F, Matsuda K, Yamamoto K, Tanaka S, Sunano S, et al. Membrane potential of mesenteric artery from carvedilol-treated spontaneously hypertensive rats. Eur J Pharmacol. 1998; 344(2-3): 161–8. doi: 10.1016/S0014-2999(97)01573-2 [DOI] [PubMed] [Google Scholar]
- 12.Park J, Galligan JJ, Fink GD, Swain GM. Differences in sympathetic neuroeffector transmission to rat mesenteric arteries and veins as probed by in vitro continuous amperometry and video imaging. J Physiol. 2007; 584(Pt 3): 819–34. doi: 10.1113/jphysiol.2007.134338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto R, Cline WH, Jr. Effects of propranolol and yohimbine on periarterial nerve stimulation-induced release of endogenous norepinephrine from the mesenteric vasculature of Wistar Kyoto and spontaneously hypertensive rats. J Pharmacol Exp Ther. 1988; 244(3): 905–11. [PubMed] [Google Scholar]
- 14.Yamamoto R, Cline WH, Jr. Release of endogenous NE from the mesenteric vasculature of WKY and SHR in response to PNS. J Pharmacol Exp Ther. 1987; 241(3): 826–32. [PubMed] [Google Scholar]
- 15.Komidori H, Yamamoto R, Nickols GA, Takasaki K. Characterization of the isolated rat mesenteric vascular-intestinal loop preparation. J Pharmacol Toxicol Methods. 1992; 27(1): 59–65. doi: 10.1016/1056-8719(92)90022-S [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto R, Cline WH, Jr, Takasaki K. Reassessment of the blocking activity of prazosin at low and high concentrations on sympathetic neurotransmission in the isolated mesenteric vasculature of rats. J Auton Pharmacol. 1988; 8(4): 303–9. doi: 10.1111/j.1474-8673.1988.tb00573.x [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto R, Takasaki K, Nickols GA. Purinergic vasoconstrictor component revealed by moderate cooling in the isolated mesenteric vasculature of Sprague-Dawley rats. J Pharmacol Exp Ther. 1992; 262(3): 1133–8. [PubMed] [Google Scholar]
- 18.Ngai SH, Berkowitz BA, Yang JC, Hempstead J, Spector S. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976; 44(5): 398–401. doi: 10.1097/00000542-197605000-00008 [DOI] [PubMed] [Google Scholar]
- 19.Burnstock G. Purinergic signaling in the cardiovascular system. Circ Res. 2017; 120(1): 207–28. doi: 10.1161/CIRCRESAHA.116.309726 [DOI] [PubMed] [Google Scholar]
- 20.Langer SZ. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980; 32(4): 337–62. [PubMed] [Google Scholar]
- 21.Westfall TC. Neuroeffector mechanisms. Annu Rev Physiol. 1980; 42: 383–97. doi: 10.1146/annurev.ph.42.030180.002123 [DOI] [PubMed] [Google Scholar]
- 22.Soares de Moura R, Resende AC, Emiliano AF, Tano T, Mendes-Ribeiro AC, Correia ML, et al. The role of bradykinin, AT2 and angiotensin 1-7 receptors in the EDRF-dependent vasodilator effect of angiotensin II on the isolated mesenteric vascular bed of the rat. Br J Pharmacol. 2004; 141(5): 860–6. doi: 10.1038/sj.bjp.0705669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Take H, Shibata K, Awaji T, Hirasawa A, Ikegaki I, Asano T, et al. Vascular alpha1-adrenoceptor subtype selectivity and alpha1-blocker-induced orthostatic hypotension. Jpn J Pharmacol. 1998; 77(1): 61–70. doi: 10.1254/jjp.77.61 [DOI] [PubMed] [Google Scholar]
- 24.Wenzel K, Wallukat G, Qadri F, Hubner N, Schulz H, Hummel O, et al. Alpha1A-adrenergic receptor-directed autoimmunity induces left ventricular damage and diastolic dysfunction in rats. PLoS One. 2010; 5(2): e9409. doi: 10.1371/journal.pone.0009409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proudman RGW, Pupo AS, Baker JG. The affinity and selectivity of α-adrenoceptor antagonists, antidepressants, and antipsychotics for the human α1A, α1B, and α1D-adrenoceptors. Pharmacol Res Perspect. 2020; 8(4): e00602. doi: 10.1002/prp2.602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honner V, Docherty JR. Investigation of the subtypes of alpha1-adrenoceptor mediating contractions of rat vas deferens. Br J Pharmacol. 1999; 128(6): 1323–31. doi: 10.1038/sj.bjp.0702913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furness JB, Marshall JM. Correlation of the directly observed responses of mesenteric vessles of the rat to nerve stimulation and noradrenaline with the distribution of adrenergic nerves. J Physiol. 1974; 239(1): 75–88. doi: 10.1113/jphysiol.1974.sp010556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall JM. The effect of uptake by adrenergic nerve terminals on the sensitivity of arterial vessels to topically applied noradrenaline. Br J Pharmacol. 1977; 61(3): 429–32. doi: 10.1111/j.1476-5381.1977.tb08436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.