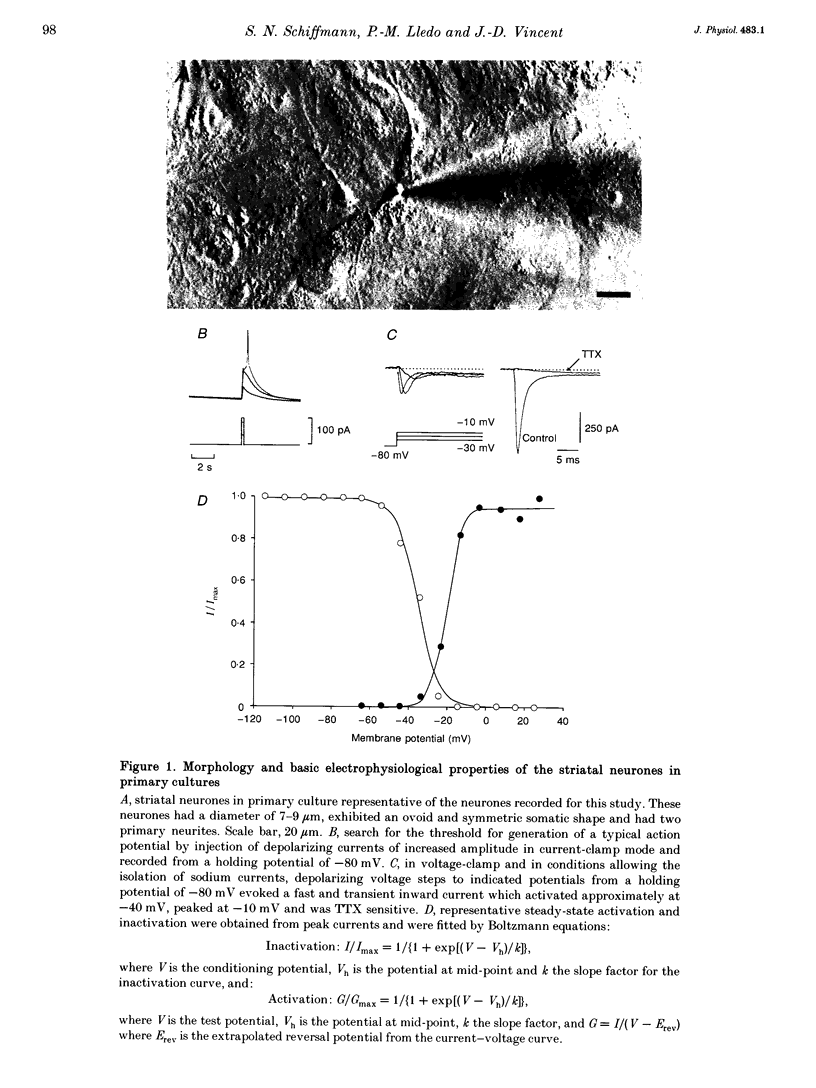
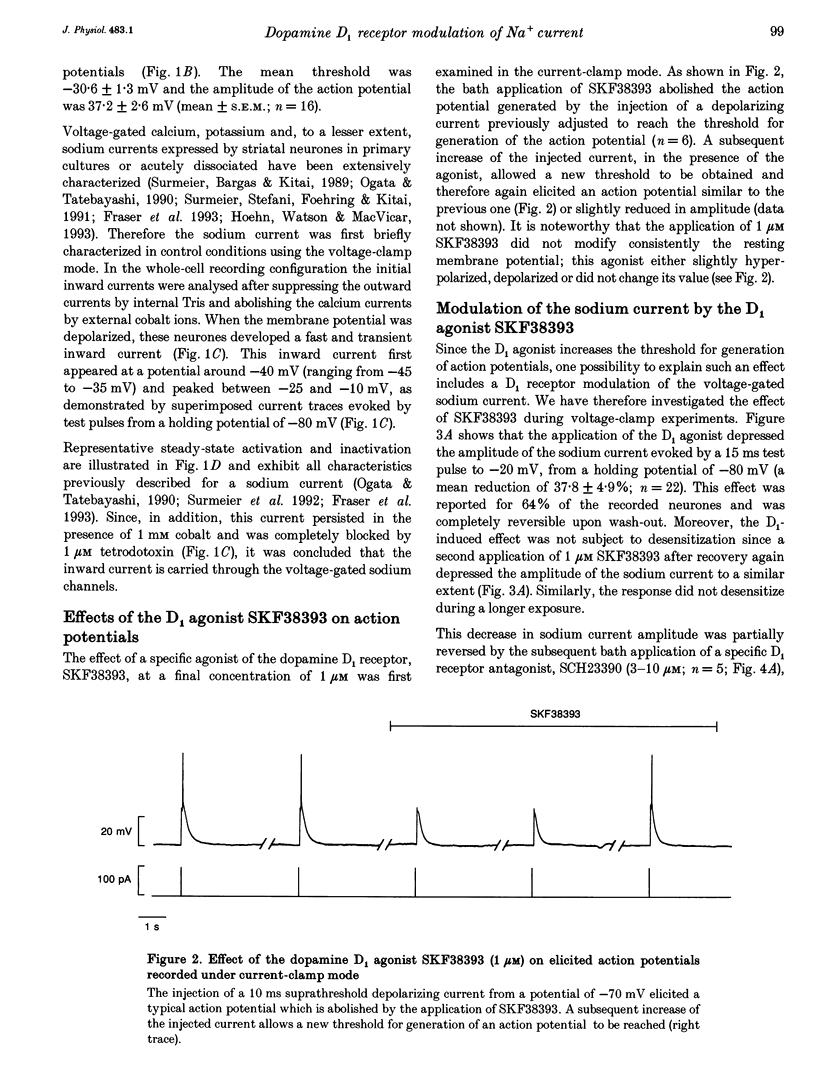
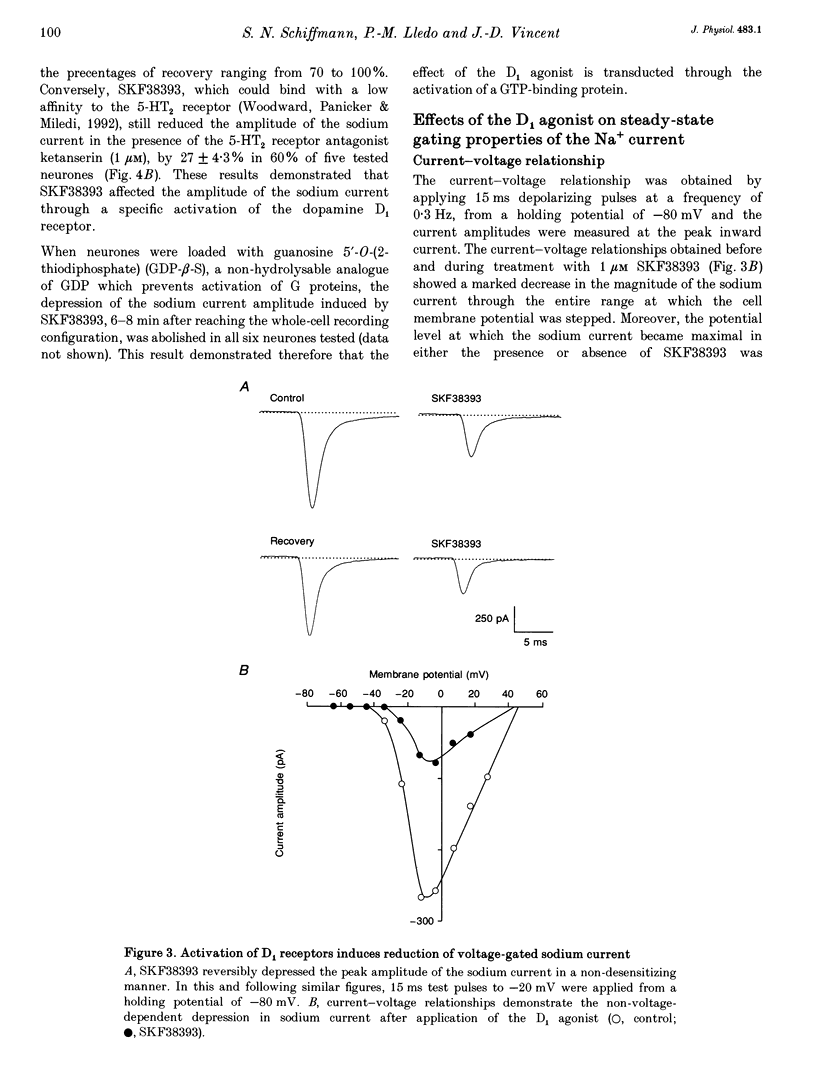
Abstract
1. Whole-cell recordings were made from striatal neurones obtained from neonatal rats and maintained in primary cultures. The effects of dopamine D1 receptor activation were studied on the voltage-gated sodium current. 2. Bath application of a specific D1 agonist, SKF38393 (1 microM), reduced the neuronal excitability recorded in current-clamp by increasing the threshold for generation of action potentials. 3. In voltage-clamp recordings, SKF38393 (1 microM) reversibly reduced the peak amplitude of the sodium current by 37.8 +/- 4.95%. This effect was reversed by the D1 antagonist SCH23390 and was blocked by the intracellular loading of GDP-beta-S (2 mM) suggesting GTP-binding protein involvement. 4. The D1 agonist reduced the peak amplitude of the sodium current without significantly affecting (i) the voltage dependence of the current-voltage relationship, (ii) the voltage dependence of the steady-state activation and inactivation, (iii) the kinetics of the time-dependent inactivation, and (iv) the kinetics of recovery from inactivation. 5. The peak amplitude of the sodium current was progressively reduced by intracellular loading of cyclic AMP-dependent protein kinase (100 U ml-1). 6. Diffusion of a specific peptide inhibitor of the cyclic AMP-dependent protein kinase (PKI; 10 microM) into the cytosol of neurones blocked the effect of the D1 agonist on the sodium current amplitude. 7. These results demonstrate that dopamine acting at the D1 receptor reduces the amplitude of the sodium current without modifying its voltage- and time-dependent properties. This effect involves activation of the cyclic AMP-dependent protein kinase and results in a depression of the striatal neuronal excitability by increasing the threshold for generation of action potentials.
Full text
PDF


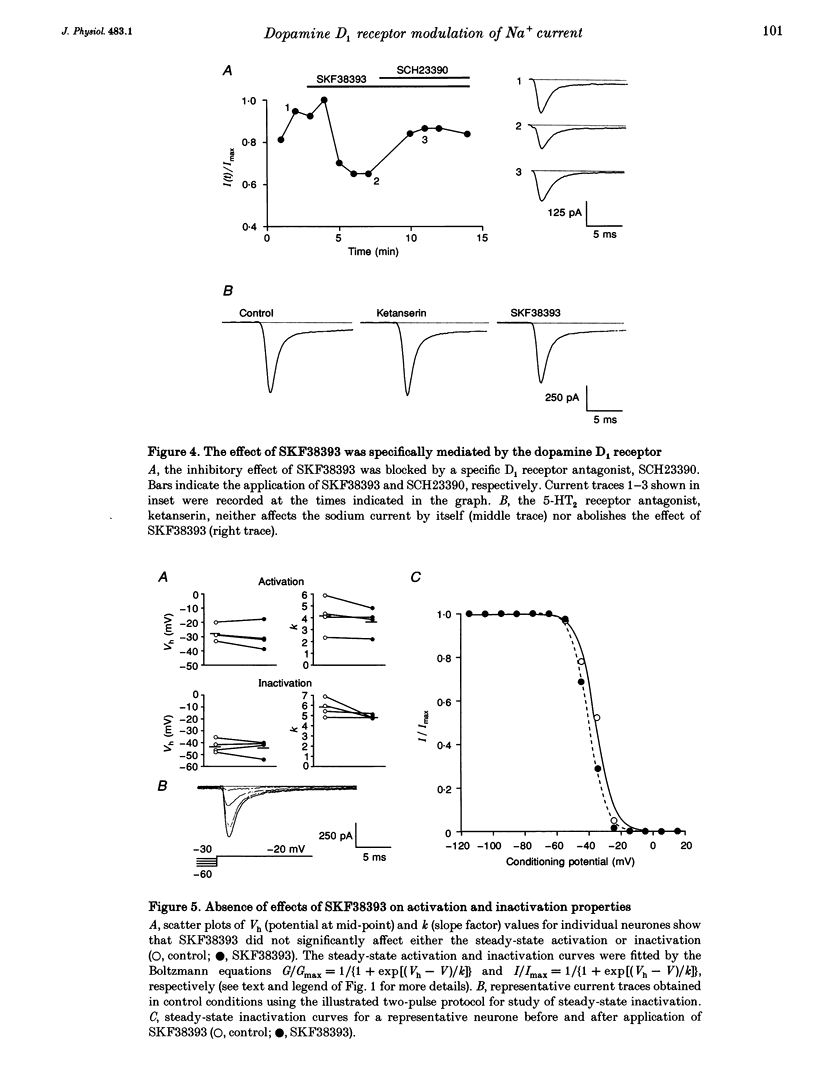
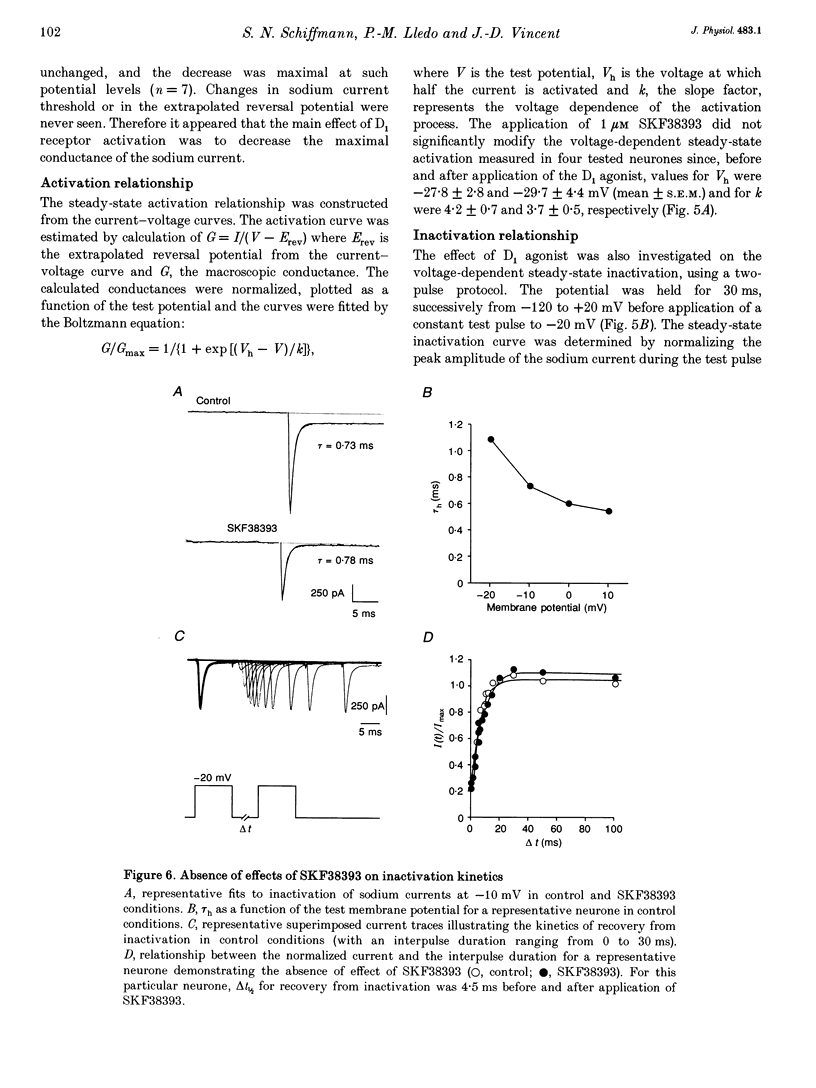
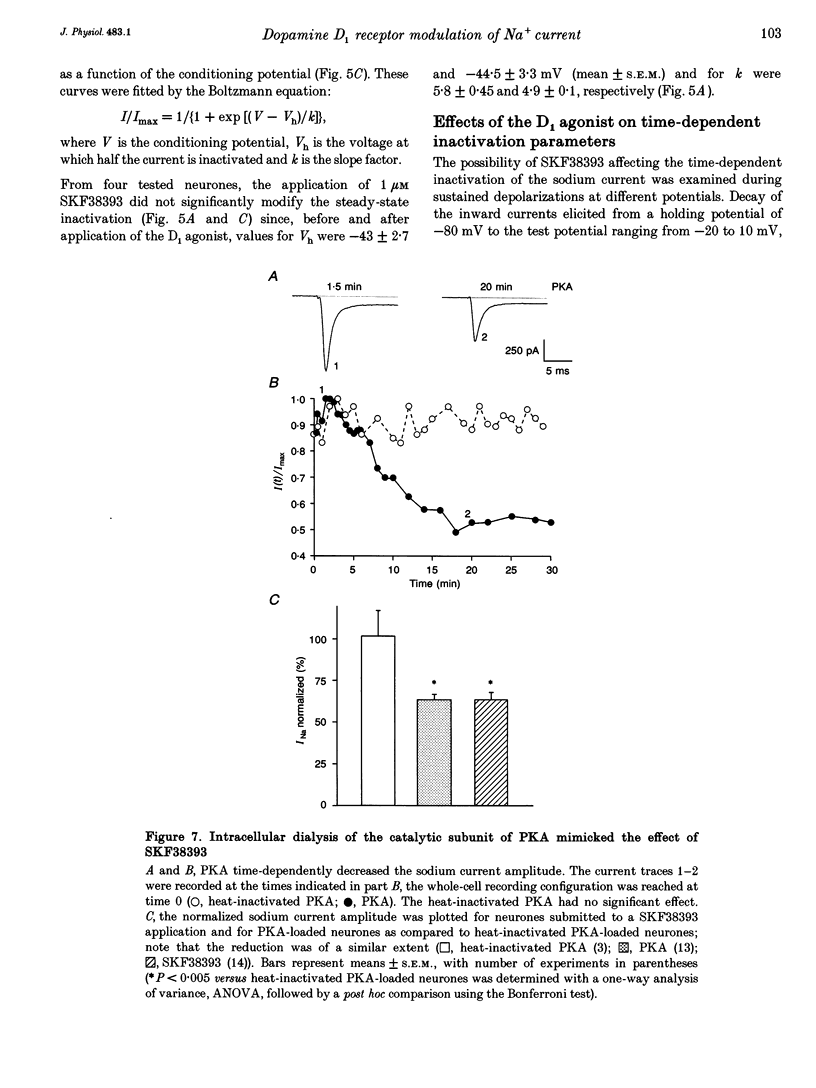
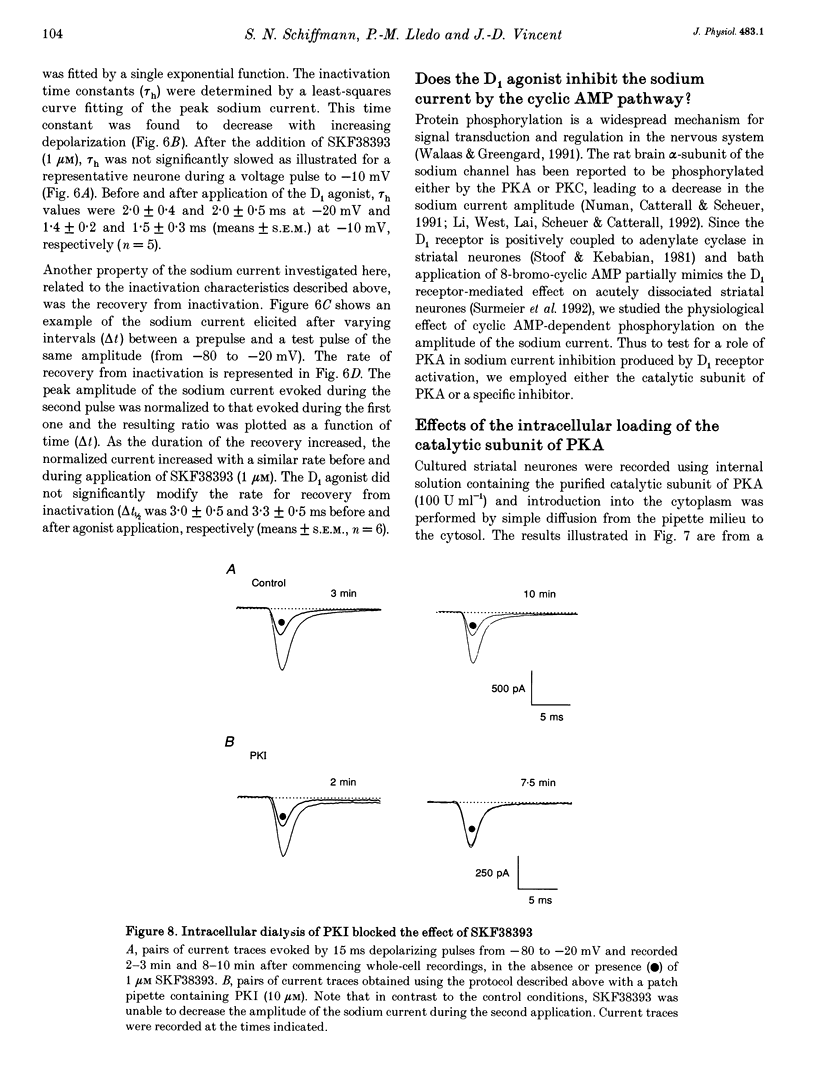



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R. L., Young A. B., Penney J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989 Oct;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Brussaard A. B., Lodder J. C., ter Maat A., de Vlieger T. A., Kits K. S. Inhibitory modulation by FMRFamide of the voltage-gated sodium current in identified neurones in Lymnaea stagnalis. J Physiol. 1991 Sep;441:385–404. doi: 10.1113/jphysiol.1991.sp018757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Benedetti M., Mercuri N. B., Bernardi G. Endogenous dopamine and dopaminergic agonists modulate synaptic excitation in neostriatum: intracellular studies from naive and catecholamine-depleted rats. Neuroscience. 1988 Oct;27(1):145–157. doi: 10.1016/0306-4522(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N., Stanzione P., Stefani A., Bernardi G. Intracellular studies on the dopamine-induced firing inhibition of neostriatal neurons in vitro: evidence for D1 receptor involvement. Neuroscience. 1987 Mar;20(3):757–771. doi: 10.1016/0306-4522(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992 Oct;72(4 Suppl):S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Cepeda C., Buchwald N. A., Levine M. S. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser D. D., Hoehn K., Weiss S., MacVicar B. A. Arachidonic acid inhibits sodium currents and synaptic transmission in cultured striatal neurons. Neuron. 1993 Oct;11(4):633–644. doi: 10.1016/0896-6273(93)90075-3. [DOI] [PubMed] [Google Scholar]
- Freedman J. E., Weight F. F. Single K+ channels activated by D2 dopamine receptors in acutely dissociated neurons from rat corpus striatum. Proc Natl Acad Sci U S A. 1988 May;85(10):3618–3622. doi: 10.1073/pnas.85.10.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoehn K., Watson T. W., MacVicar B. A. Multiple types of calcium channels in acutely isolated rat neostriatal neurons. J Neurosci. 1993 Mar;13(3):1244–1257. doi: 10.1523/JNEUROSCI.13-03-01244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. T., Wang R. Y. Comparison of effects of D-1 and D-2 dopamine receptor agonists on neurons in the rat caudate putamen: an electrophysiological study. J Neurosci. 1988 Nov;8(11):4340–4348. doi: 10.1523/JNEUROSCI.08-11-04340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H. Voltage- and time-dependent inhibition of neuronal calcium channels by a GTP-binding protein in a mammalian cell line. J Physiol. 1992 Mar;448:189–209. doi: 10.1113/jphysiol.1992.sp019036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleuss C., Hescheler J., Ewel C., Rosenthal W., Schultz G., Wittig B. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991 Sep 5;353(6339):43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- Li M., West J. W., Lai Y., Scheuer T., Catterall W. A. Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron. 1992 Jun;8(6):1151–1159. doi: 10.1016/0896-6273(92)90135-z. [DOI] [PubMed] [Google Scholar]
- Li M., West J. W., Numann R., Murphy B. J., Scheuer T., Catterall W. A. Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993 Sep 10;261(5127):1439–1442. doi: 10.1126/science.8396273. [DOI] [PubMed] [Google Scholar]
- Lledo P. M., Homburger V., Bockaert J., Vincent J. D. Differential G protein-mediated coupling of D2 dopamine receptors to K+ and Ca2+ currents in rat anterior pituitary cells. Neuron. 1992 Mar;8(3):455–463. doi: 10.1016/0896-6273(92)90273-g. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., Castellano A., Toledo-Aral J. Thyrotropin-releasing-hormone (TRH) and its physiological metabolite TRH-OH inhibit Na+ channel activity in mammalian septal neurons. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8150–8154. doi: 10.1073/pnas.87.20.8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Numann R., Catterall W. A., Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991 Oct 4;254(5028):115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F. Structures of dopamine receptors. J Neurochem. 1993 Mar;60(3):804–816. doi: 10.1111/j.1471-4159.1993.tb03224.x. [DOI] [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Sodium current kinetics in freshly isolated neostriatal neurones of the adult guinea pig. Pflugers Arch. 1990 Jul;416(5):594–603. doi: 10.1007/BF00382695. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Pilon C., Giros B., Sokoloff P., Martres M. P., Schwartz J. C. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991 Sep 12;353(6340):164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- Rossie S., Catterall W. A. Cyclic-AMP-dependent phosphorylation of voltage-sensitive sodium channels in primary cultures of rat brain neurons. J Biol Chem. 1987 Sep 15;262(26):12735–12744. [PubMed] [Google Scholar]
- Schubert B., VanDongen A. M., Kirsch G. E., Brown A. M. Beta-adrenergic inhibition of cardiac sodium channels by dual G-protein pathways. Science. 1989 Aug 4;245(4917):516–519. doi: 10.1126/science.2547248. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Monsma F. J., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992 Feb;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Stack J., Surprenant A. Dopamine actions on calcium currents, potassium currents and hormone release in rat melanotrophs. J Physiol. 1991 Aug;439:37–58. doi: 10.1113/jphysiol.1991.sp018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof J. C., Kebabian J. W. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature. 1981 Nov 26;294(5839):366–368. doi: 10.1038/294366a0. [DOI] [PubMed] [Google Scholar]
- Strange P. G. New insights into dopamine receptors in the central nervous system. Neurochem Int. 1993 Mar;22(3):223–236. doi: 10.1016/0197-0186(93)90050-f. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Bargas J., Kitai S. T. Two types of A-current differing in voltage-dependence are expressed by neurons of the rat neostriatum. Neurosci Lett. 1989 Sep 11;103(3):331–337. doi: 10.1016/0304-3940(89)90122-5. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Eberwine J., Wilson C. J., Cao Y., Stefani A., Kitai S. T. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Kita H., Kitai S. T. The expression of gamma-aminobutyric acid and Leu-enkephalin immunoreactivity in primary monolayer cultures of rat striatum. Brain Res. 1988 Aug 1;470(2):265–282. doi: 10.1016/0165-3806(88)90245-3. [DOI] [PubMed] [Google Scholar]
- Surmeier D. J., Stefani A., Foehring R. C., Kitai S. T. Developmental regulation of a slowly-inactivating potassium conductance in rat neostriatal neurons. Neurosci Lett. 1991 Jan 14;122(1):41–46. doi: 10.1016/0304-3940(91)90188-y. [DOI] [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev. 1991 Sep;43(3):299–349. [PubMed] [Google Scholar]
- Woodward R. M., Panicker M. M., Miledi R. Actions of dopamine and dopaminergic drugs on cloned serotonin receptors expressed in Xenopus oocytes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4708–4712. doi: 10.1073/pnas.89.10.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarowsky P. J., Krueger B. K., Olson C. E., Clevinger E. C., Koos R. D. Brain and heart sodium channel subtype mRNA expression in rat cerebral cortex. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9453–9457. doi: 10.1073/pnas.88.21.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]