Abstract
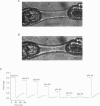
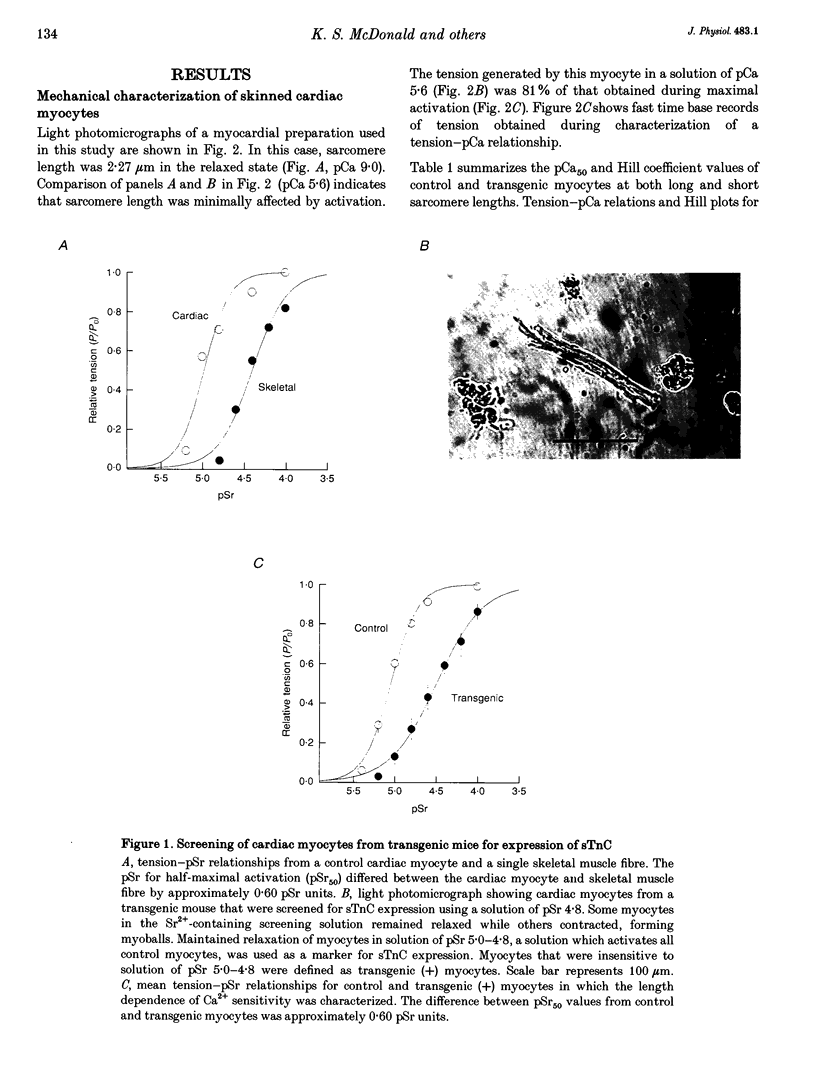
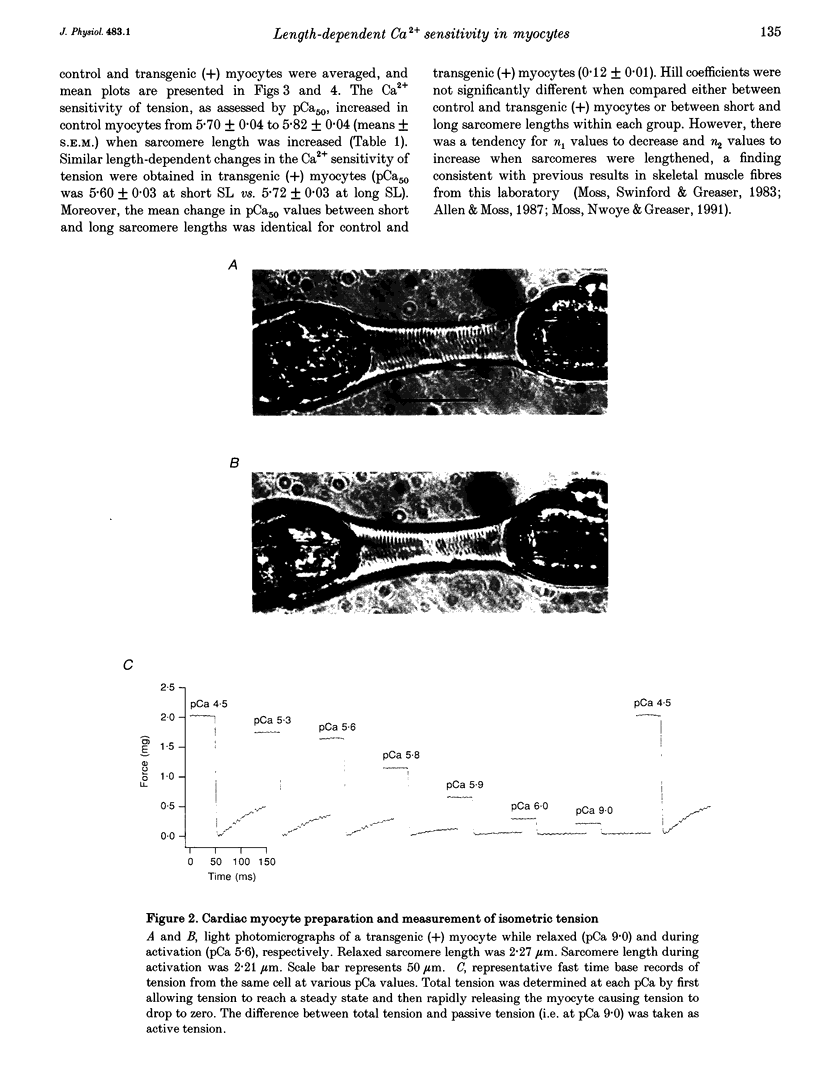
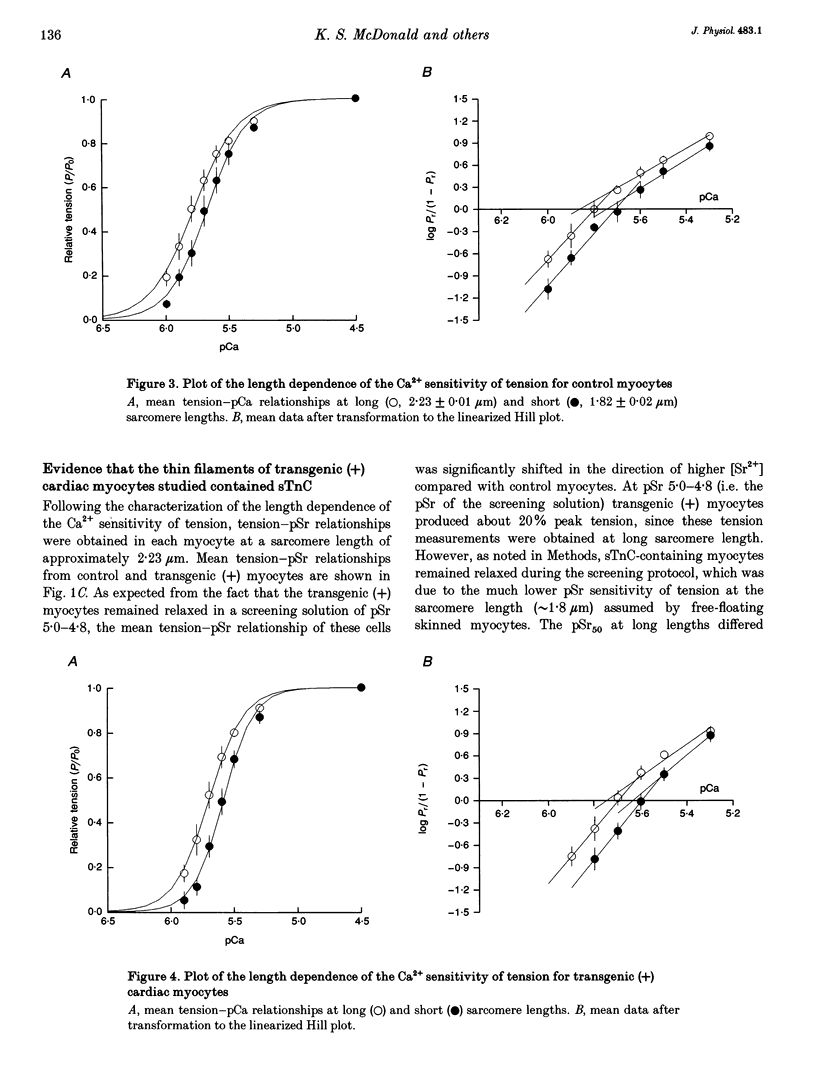
1. Beat-to-beat performance of myocardium is highly dependent on sarcomere length. The physiological basis for this effect is not well understood but presumably includes alterations in the extent of overlap between thick and thin filaments. Sarcomere length dependence of activation also appears to be involved since length-tension relationships in cardiac muscle are usually steeper than those in skeletal muscle. 2. An explanation recently proposed to account for the difference between length-tension relationships is that the cardiac isoform of troponin C (cTnC) has intrinsic properties that confer greater length-dependent changes in the Ca2+ sensitivity of tension than does skeletal troponin C (sTnC), presumably due to greater length-dependent changes in the Ca(2+)-binding affinity of cTnC. To test this hypothesis, transgenic mice were developed in which fast sTnC was expressed ectopically in the heart. This allowed a comparison of the length dependence of the Ca2+ sensitivity of tension between myocytes having thin filaments that contained either endogenous cTnC or primarily sTnC. 3. In myocytes from both transgenic and normal mice, the Ca2+ sensitivity of tension increased similarly when mean sarcomere length was increased from approximately 1.83 to 2.23 microns. In both cases, the mid-point (pCa50) of the tension-pCa (i.e. -log[Ca2+]) relationship shifted 0.12 +/- 0.01 pCa units (mean +/- S.E.M.) in the direction of lower Ca2+. 4. We conclude that the Ca2+ sensitivity of tension in myocytes changes as a function of sarcomere length but is independent of the isoform of troponin C present in the thin filaments.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Kentish J. C. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Nichols C. G., Smith G. L. The effects of changes in muscle length during diastole on the calcium transient in ferret ventricular muscle. J Physiol. 1988 Dec;406:359–370. doi: 10.1113/jphysiol.1988.sp017385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. D., Moss R. L. Factors influencing the ascending limb of the sarcomere length-tension relationship in rabbit skinned muscle fibres. J Physiol. 1987 Sep;390:119–136. doi: 10.1113/jphysiol.1987.sp016689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu A., Sonnenblick E., Gulati J. Molecular basis for the influence of muscle length on myocardial performance. Science. 1988 Apr 1;240(4848):74–76. doi: 10.1126/science.3353709. [DOI] [PubMed] [Google Scholar]
- Close R. I. The relations between sarcomere length and characteristics of isometric twitch contractions of frog sartorius muscle. J Physiol. 1972 Feb;220(3):745–762. doi: 10.1113/jphysiol.1972.sp009733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature. 1975 Jul 3;256(5512):54–56. doi: 10.1038/256054a0. [DOI] [PubMed] [Google Scholar]
- Field L. J. Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice. Science. 1988 Feb 26;239(4843):1029–1033. doi: 10.1126/science.2964082. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Scordilis S., Babu A. Effect of troponin C on the cooperativity in Ca2+ activation of cardiac muscle. FEBS Lett. 1988 Aug 29;236(2):441–444. doi: 10.1016/0014-5793(88)80073-5. [DOI] [PubMed] [Google Scholar]
- Gulati J., Sonnenblick E., Babu A. The role of troponin C in the length dependence of Ca(2+)-sensitive force of mammalian skeletal and cardiac muscles. J Physiol. 1991 Sep;441:305–324. doi: 10.1113/jphysiol.1991.sp018753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. M., Lamont C., Miller D. J. Hysteresis and the length dependence of calcium sensitivity in chemically skinned rat cardiac muscle. J Physiol. 1988 Jul;401:115–143. doi: 10.1113/jphysiol.1988.sp017154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibberd M. G., Jewell B. R. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol. 1982 Aug;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoar P. E., Potter J. D., Kerrick W. G. Skinned ventricular fibres: troponin C extraction is species-dependent and its replacement with skeletal troponin C changes Sr2+ activation properties. J Muscle Res Cell Motil. 1988 Apr;9(2):165–173. doi: 10.1007/BF01773738. [DOI] [PubMed] [Google Scholar]
- Kentish J. C., ter Keurs H. E., Ricciardi L., Bucx J. J., Noble M. I. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986 Jun;58(6):755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Malencik D. A., Hoar P. E., Potter J. D., Coby R. L., Pocinwong S., Fischer E. H. Ca2+ and Sr2+ activation: comparison of cardiac and skeletal muscle contraction models. Pflugers Arch. 1980 Aug;386(3):207–213. doi: 10.1007/BF00587470. [DOI] [PubMed] [Google Scholar]
- Martyn D. A., Gordon A. M. Length and myofilament spacing-dependent changes in calcium sensitivity of skeletal fibres: effects of pH and ionic strength. J Muscle Res Cell Motil. 1988 Oct;9(5):428–445. doi: 10.1007/BF01774069. [DOI] [PubMed] [Google Scholar]
- Maughan D. W., Godt R. E. Inhibition of force production in compressed skinned muscle fibers of the frog. Pflugers Arch. 1981 May;390(2):161–163. doi: 10.1007/BF00590200. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Parmacek M. S., Barr E., Pasyk K., Lin W. I., Cochrane K. L., Field L. J., Leiden J. M. Skeletal troponin C reduces contractile sensitivity to acidosis in cardiac myocytes from transgenic mice. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9036–9040. doi: 10.1073/pnas.90.19.9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. The effects of partial extraction of TnC upon the tension-pCa relationship in rabbit skinned skeletal muscle fibers. J Gen Physiol. 1985 Oct;86(4):585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Lauer M. R., Giulian G. G., Greaser M. L. Altered Ca2+ dependence of tension development in skinned skeletal muscle fibers following modification of troponin by partial substitution with cardiac troponin C. J Biol Chem. 1986 May 5;261(13):6096–6099. [PubMed] [Google Scholar]
- Moss R. L., Nwoye L. O., Greaser M. L. Substitution of cardiac troponin C into rabbit muscle does not alter the length dependence of Ca2+ sensitivity of tension. J Physiol. 1991;440:273–289. doi: 10.1113/jphysiol.1991.sp018708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L., Swinford A. E., Greaser M. L. Alterations in the Ca2+ sensitivity of tension development by single skeletal muscle fibers at stretched lengths. Biophys J. 1983 Jul;43(1):115–119. doi: 10.1016/S0006-3495(83)84329-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmacek M. S., Bengur A. R., Vora A. J., Leiden J. M. The structure and regulation of expression of the murine fast skeletal troponin C gene. Identification of a developmentally regulated, muscle-specific transcriptional enhancer. J Biol Chem. 1990 Sep 15;265(26):15970–15976. [PubMed] [Google Scholar]
- Parmacek M. S., Leiden J. M. Structure and expression of the murine slow/cardiac troponin C gene. J Biol Chem. 1989 Aug 5;264(22):13217–13225. [PubMed] [Google Scholar]
- Rome E. X-ray diffraction studies of the filament lattice of striated muscle in various bathing media. J Mol Biol. 1968 Oct 28;37(2):331–344. doi: 10.1016/0022-2836(68)90272-6. [DOI] [PubMed] [Google Scholar]
- Shiner J. S., Solaro R. J. The hill coefficient for the Ca2+-activation of striated muscle contraction. Biophys J. 1984 Oct;46(4):541–543. doi: 10.1016/S0006-3495(84)84051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Wendt I. R. Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibres. J Muscle Res Cell Motil. 1984 Jun;5(3):243–272. doi: 10.1007/BF00713107. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol. 1982 Dec;333:637–653. doi: 10.1113/jphysiol.1982.sp014473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer N. K., Moss R. L. The effect of altered temperature on Ca2(+)-sensitive force in permeabilized myocardium and skeletal muscle. Evidence for force dependence of thin filament activation. J Gen Physiol. 1990 Dec;96(6):1221–1245. doi: 10.1085/jgp.96.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Keurs H. E., Rijnsburger W. H., van Heuningen R., Nagelsmit M. J. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res. 1980 May;46(5):703–714. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]