Abstract
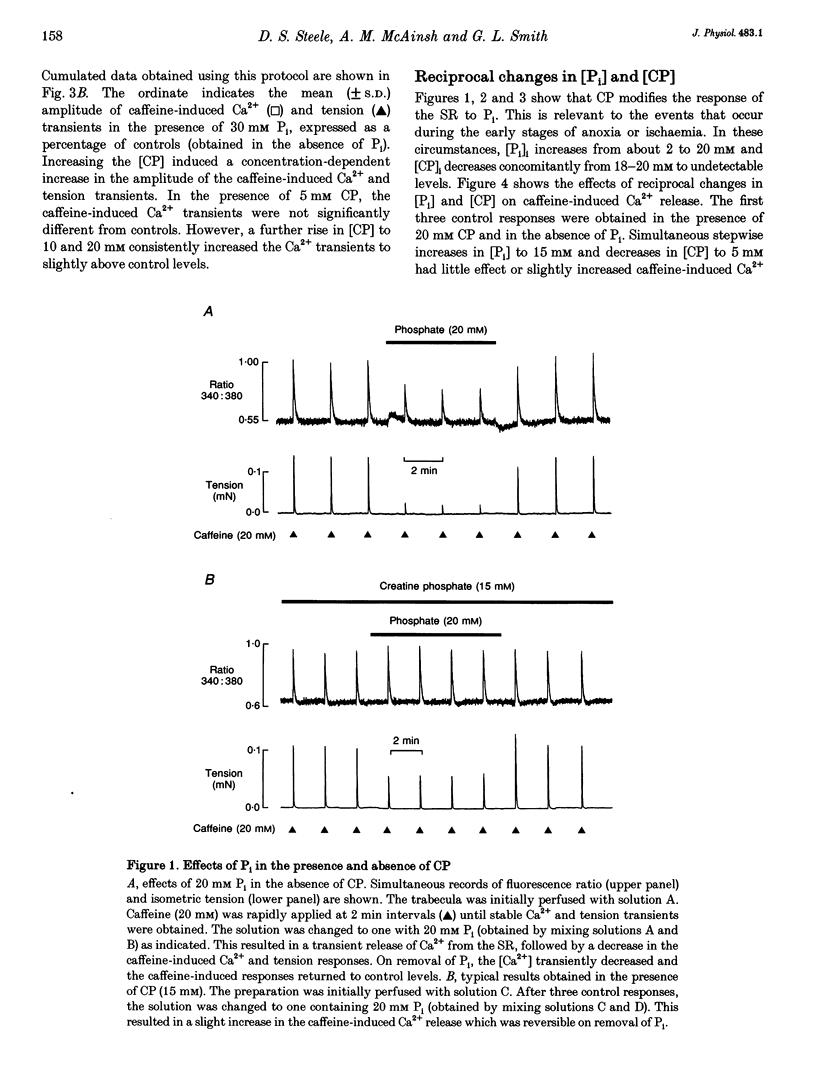
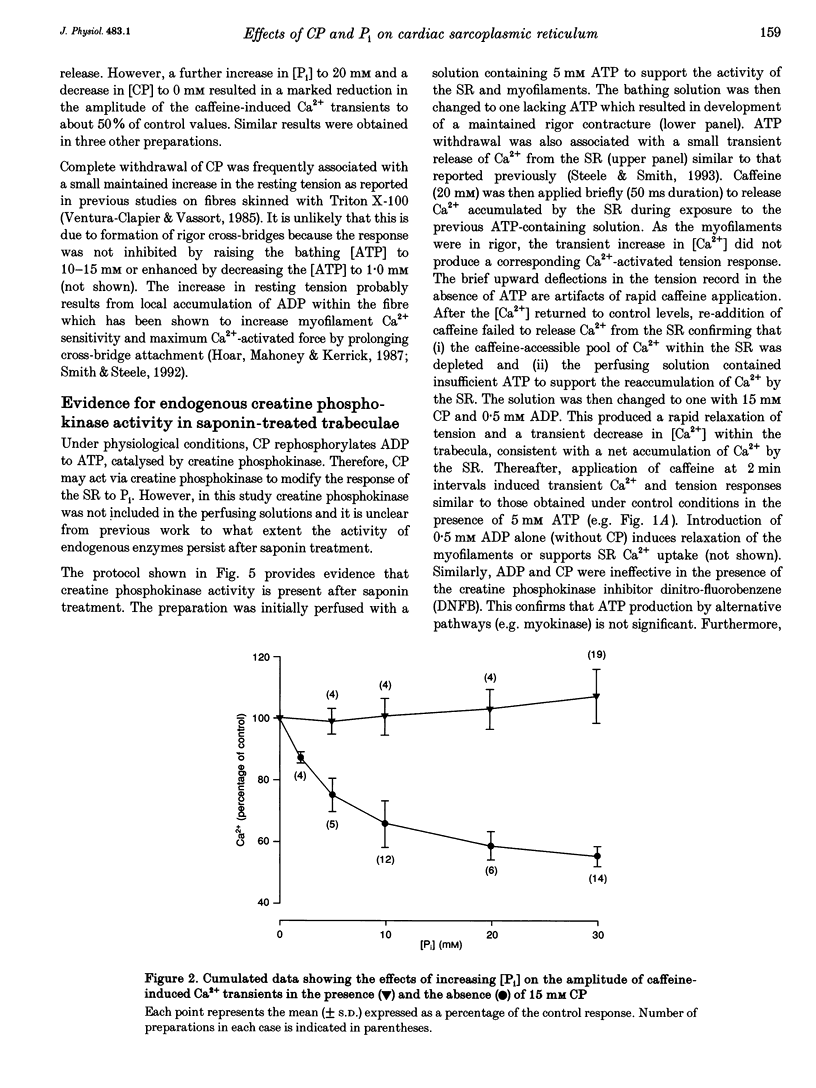
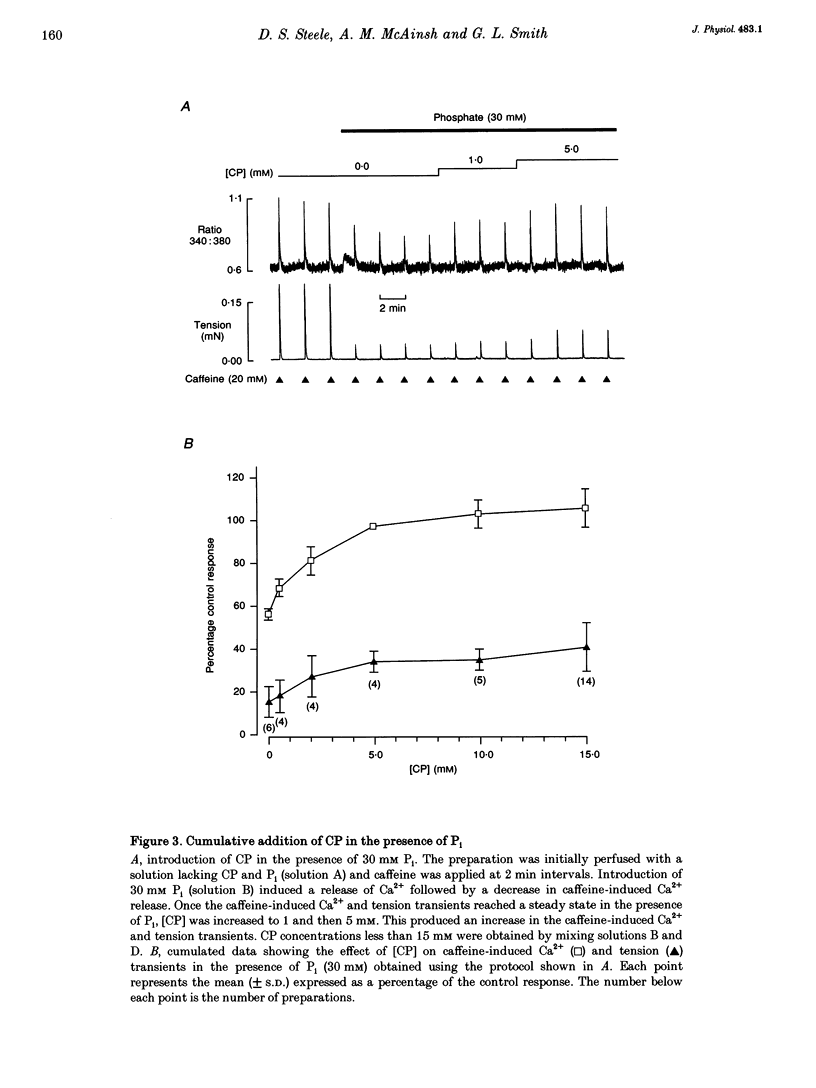
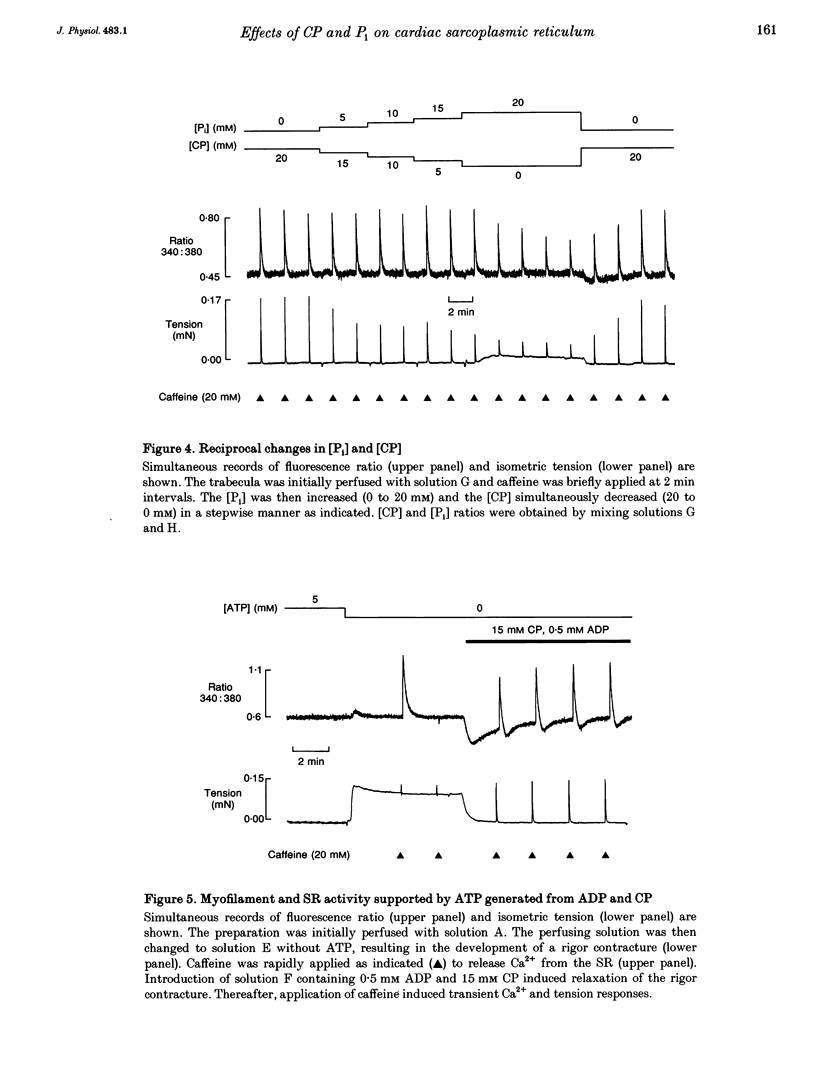
1. Ventricular trabeculae from rat heart were permeabilized by treatment with saponin. In the presence of 150 nM Ca2+, application of 20 mM caffeine released Ca2+ from the sarcoplasmic reticulum (SR), resulting in a transient contracture. Ca2+ released from the SR was detected using fura-2 fluorescence. The amplitudes of the caffeine-induced Ca2+ transients were used to assess SR Ca2+ content. 2. In the absence of creatine phosphate (CP), introduction of 5-30 mM inorganic phosphate (Pi) caused a net release of Ca2+ from the SR. Subsequent caffeine-induced Ca2+ and tension transients were smaller in the presence of Pi. Under these conditions, 30 mM Pi decreased the caffeine-induced Ca2+ transients by 45 +/- 3.1% (mean +/- S.D., n = 14). On removal of Pi, the [Ca2+] transiently decreased and the caffeine-induced Ca2+ transients returned to control levels over 4-6 min. 3. In the presence of CP (5-15 mM), the Ca2+ transients were unaffected by the introduction of Pi (5-30 mM) or slightly increased in amplitude. Pi (30 mM) significantly increased the caffeine-induced Ca2+ transients by 7 +/- 8.8% (mean +/- S.D., n = 19, P < 0.05) in the presence of 15 mM CP. The release of Ca2+ on addition of Pi and decrease in [Ca2+] on Pi withdrawal was less pronounced or absent completely in the presence of CP. The inhibitory effects of Pi on caffeine-induced Ca2+ release became apparent as the [CP] was decreased from 5 to 0 mM. 4. In the presence of the creatine phosphokinase inhibitor dinitro-fluorobenzene (DNFB) the effects of Pi (in the presence of CP) were qualitatively similar to the results obtained in the absence of CP, although the decrease in caffeine-induced Ca2+ release was less pronounced. 5. These results suggest that the rise in [Pi]i during ischaemia or anoxia will have little effect on the regulation of Ca2+ by the SR while the [CP]i remains above 5 mM. However, as the [CP] decreases below 5 mM, the accumulation of Pi within the cytosol will progressively reduce the SR Ca2+ content. CP may act in conjunction with endogenous creatine phosphokinase to modify the response of the SR to Pi, and possible mechanisms are considered.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Lee J. A., Smith G. L. The consequences of simulated ischaemia on intracellular Ca2+ and tension in isolated ferret ventricular muscle. J Physiol. 1989 Mar;410:297–323. doi: 10.1113/jphysiol.1989.sp017534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Intracellular calcium concentration during hypoxia and metabolic inhibition in mammalian ventricular muscle. J Physiol. 1983 Jun;339:107–122. doi: 10.1113/jphysiol.1983.sp014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987 Feb;60(2):153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Hasselbach W., Makinose M. Activation of calcium efflux by ADP and inorganic phosphate. FEBS Lett. 1971 Jan 30;12(5):267–268. doi: 10.1016/0014-5793(71)80194-1. [DOI] [PubMed] [Google Scholar]
- Deamer D. W., Baskin R. J. ATP synthesis in sarcoplasmic reticulum. Arch Biochem Biophys. 1972 Nov;153(1):47–54. doi: 10.1016/0003-9861(72)90418-3. [DOI] [PubMed] [Google Scholar]
- Elliott A. C., Smith G. L., Allen D. G. The metabolic consequences of an increase in the frequency of stimulation in isolated ferret hearts. J Physiol. 1994 Jan 1;474(1):147–159. doi: 10.1113/jphysiol.1994.sp020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A. C., Smith G. L., Eisner D. A., Allen D. G. Metabolic changes during ischaemia and their role in contractile failure in isolated ferret hearts. J Physiol. 1992 Aug;454:467–490. doi: 10.1113/jphysiol.1992.sp019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J. J., Briggs F. N. Determinants of calcium loading at steady state in sarcoplasmic reticulum. Biochim Biophys Acta. 1983 Jan 19;727(2):389–402. doi: 10.1016/0005-2736(83)90424-8. [DOI] [PubMed] [Google Scholar]
- Fruen B. R., Mickelson J. R., Shomer N. H., Roghair T. J., Louis C. F. Regulation of the sarcoplasmic reticulum ryanodine receptor by inorganic phosphate. J Biol Chem. 1994 Jan 7;269(1):192–198. [PubMed] [Google Scholar]
- Goldhaber J. I., Parker J. M., Weiss J. N. Mechanisms of excitation-contraction coupling failure during metabolic inhibition in guinea-pig ventricular myocytes. J Physiol. 1991 Nov;443:371–386. doi: 10.1113/jphysiol.1991.sp018838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineman F. W., Balaban R. S. Control of mitochondrial respiration in the heart in vivo. Annu Rev Physiol. 1990;52:523–542. doi: 10.1146/annurev.ph.52.030190.002515. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Mahoney C. W., Kerrick W. G. MgADP- increases maximum tension and Ca2+ sensitivity in skinned rabbit soleus fibers. Pflugers Arch. 1987 Sep;410(1-2):30–36. doi: 10.1007/BF00581892. [DOI] [PubMed] [Google Scholar]
- Hoerter J. A., Miceli M. V., Renlund D. G., Jacobus W. E., Gerstenblith G., Lakatta E. G. A phosphorus-31 nuclear magnetic resonance study of the metabolic, contractile, and ionic consequences of induced calcium alterations in the isovolumic rat heart. Circ Res. 1986 Apr;58(4):539–551. doi: 10.1161/01.res.58.4.539. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Swain J. A., Portman M. A., Balaban R. S. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol. 1989 Jan;256(1 Pt 2):H265–H274. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y., Grossman W., Morgan J. P. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res. 1989 Oct;65(4):1029–1044. doi: 10.1161/01.res.65.4.1029. [DOI] [PubMed] [Google Scholar]
- Koretsune Y., Marban E. Relative roles of Ca2(+)-dependent and Ca2(+)-independent mechanisms in hypoxic contractile dysfunction. Circulation. 1990 Aug;82(2):528–535. doi: 10.1161/01.cir.82.2.528. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. The effects of repeated exposure to anoxia on intracellular calcium, glycogen and lactate in isolated ferret heart muscle. Pflugers Arch. 1988 Nov;413(1):83–89. doi: 10.1007/BF00581232. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Gwathmey J. K., Morgan J. P. Differential effects of reoxygenation on intracellular calcium and isometric tension. Pflugers Arch. 1987 Aug;409(4-5):448–453. doi: 10.1007/BF00583800. [DOI] [PubMed] [Google Scholar]
- Makinose M., Hasselbach W. ATP synthesis by the reverse of the sarcoplasmic calcium pump. FEBS Lett. 1971 Jan 30;12(5):271–272. doi: 10.1016/0014-5793(71)80196-5. [DOI] [PubMed] [Google Scholar]
- Miller D. J., Smith G. L. EGTA purity and the buffering of calcium ions in physiological solutions. Am J Physiol. 1984 Jan;246(1 Pt 1):C160–C166. doi: 10.1152/ajpcell.1984.246.1.C160. [DOI] [PubMed] [Google Scholar]
- Mohabir R., Lee H. C., Kurz R. W., Clusin W. T. Effects of ischemia and hypercarbic acidosis on myocyte calcium transients, contraction, and pHi in perfused rabbit hearts. Circ Res. 1991 Dec;69(6):1525–1537. doi: 10.1161/01.res.69.6.1525. [DOI] [PubMed] [Google Scholar]
- Northover B. J. Continuous fluorimetric assessment of the changes in cytoplasmic calcium concentration during exposure of rat isolated myocardium to conditions of simulated ischaemia. Br J Pharmacol. 1990 Jul;100(3):477–482. doi: 10.1111/j.1476-5381.1990.tb15832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H., Kentish J. C. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990 Jun;258(6 Pt 1):C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Chernousova G. B., Vetter R., Smirnov V. N., Chazov E. I. Kinetic properties and the functional role of particulate MM-isoenzyme of creatine phosphokinase bound to heart muscle myofibrils. FEBS Lett. 1976 Mar 1;62(3):293–296. doi: 10.1016/0014-5793(76)80078-6. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Allen D. G. Effects of metabolic blockade on intracellular calcium concentration in isolated ferret ventricular muscle. Circ Res. 1988 Jun;62(6):1223–1236. doi: 10.1161/01.res.62.6.1223. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Donoso P., Bauer C. J., Eisner D. A. Relationship between intracellular pH and metabolite concentrations during metabolic inhibition in isolated ferret heart. J Physiol. 1993 Dec;472:11–22. doi: 10.1113/jphysiol.1993.sp019932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Steele D. S. Inorganic phosphate decreases the Ca2+ content of the sarcoplasmic reticulum in saponin-treated rat cardiac trabeculae. J Physiol. 1992 Dec;458:457–473. doi: 10.1113/jphysiol.1992.sp019427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler F., Teruel J. A., Fernandez-Belda F., Gomez-Fernandez J. C. Characterization of the steady-state calcium fluxes in skeletal sarcoplasmic reticulum vesicles. Role of the Ca2+ pump. Eur J Biochem. 1990 Sep 11;192(2):347–354. doi: 10.1111/j.1432-1033.1990.tb19233.x. [DOI] [PubMed] [Google Scholar]
- Steele D. S., Smith G. L. Effects of 2,3-butanedione monoxime on sarcoplasmic reticulum of saponin-treated rat cardiac muscle. Am J Physiol. 1993 Nov;265(5 Pt 2):H1493–H1500. doi: 10.1152/ajpheart.1993.265.5.H1493. [DOI] [PubMed] [Google Scholar]
- Stern M. D., Silverman H. S., Houser S. R., Josephson R. A., Capogrossi M. C., Nichols C. G., Lederer W. J., Lakatta E. G. Anoxic contractile failure in rat heart myocytes is caused by failure of intracellular calcium release due to alteration of the action potential. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6954–6958. doi: 10.1073/pnas.85.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R., Vassort G. Role of myofibrillar creatine kinase in the relaxation of rigor tension in skinned cardiac muscle. Pflugers Arch. 1985 May;404(2):157–161. doi: 10.1007/BF00585412. [DOI] [PubMed] [Google Scholar]
- Waas W., Hasselbach W. Interference of nucleoside diphosphates and inorganic phosphate with nucleoside-triphosphate-dependent calcium fluxes and calcium-dependent nucleoside-triphosphate hydrolysis in membranes of sarcoplasmic-reticulum vesicles. Eur J Biochem. 1981 Jun 1;116(3):601–608. doi: 10.1111/j.1432-1033.1981.tb05378.x. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Nosek T. M. Intracellular milieu changes associated with hypoxia impair sarcoplasmic reticulum Ca2+ transport in cardiac muscle. Am J Physiol. 1991 Sep;261(3 Pt 2):H620–H626. doi: 10.1152/ajpheart.1991.261.3.H620. [DOI] [PubMed] [Google Scholar]


