Abstract
1. The cellular mechanisms underlying temporal synaptic signalling of tectothalamic pathways were investigated in rat medial geniculate body (MGB) maintained in vitro. Stimulation of the brachium of the inferior colliculus elicited either a short latency, single- (or dual-) spike or a long latency (10-80 ms) burst in MGB neurones. The delayed burst response was found in most non-lemniscal or caudodorsal MGB (MGd) neurones, whereas single-spike units were mainly seen in the lemniscal ventral MGB (MGv). Population latency analysis revealed that the overall relay time of tectothalamic transmission is approximately 50 ms, with at least two excitation peaks occurring around 8 and 15 ms, respectively. 2. Intracellular recordings showed that the delayed burst responses in MGd neurones were mediated by an EPSP-triggered low threshold spike (LTS). Small variations in either the membrane voltage or in EPSP amplitude induced significant shifts of LTS latency. 3. Compared with MGv cells, MGd neurones exhibited a more negative resting membrane potential and a prolonged EPSP; they lacked an apparent hyperpolarization-activated inward rectifier (Ih). These factors seem to lead collectively to a dominant occurrence of long latency burst response in the MGd. In the majority of single-spiking MGv cells that expressed a clear Ih, application of Cs+ consistently hyperpolarized the cell, which transformed a single-spike synaptic response into an EPSP-LTS burst or a subthreshold EPSP. 4. Taken together, these data suggest that the monosynaptic tectothalamic pathways are capable of introducing a ventrodorsal gradient in auditory response time. This synaptic activity pattern is probably dominantly regulated by a set of membrane conductances expressed endogenously in thalamocortical neurones.
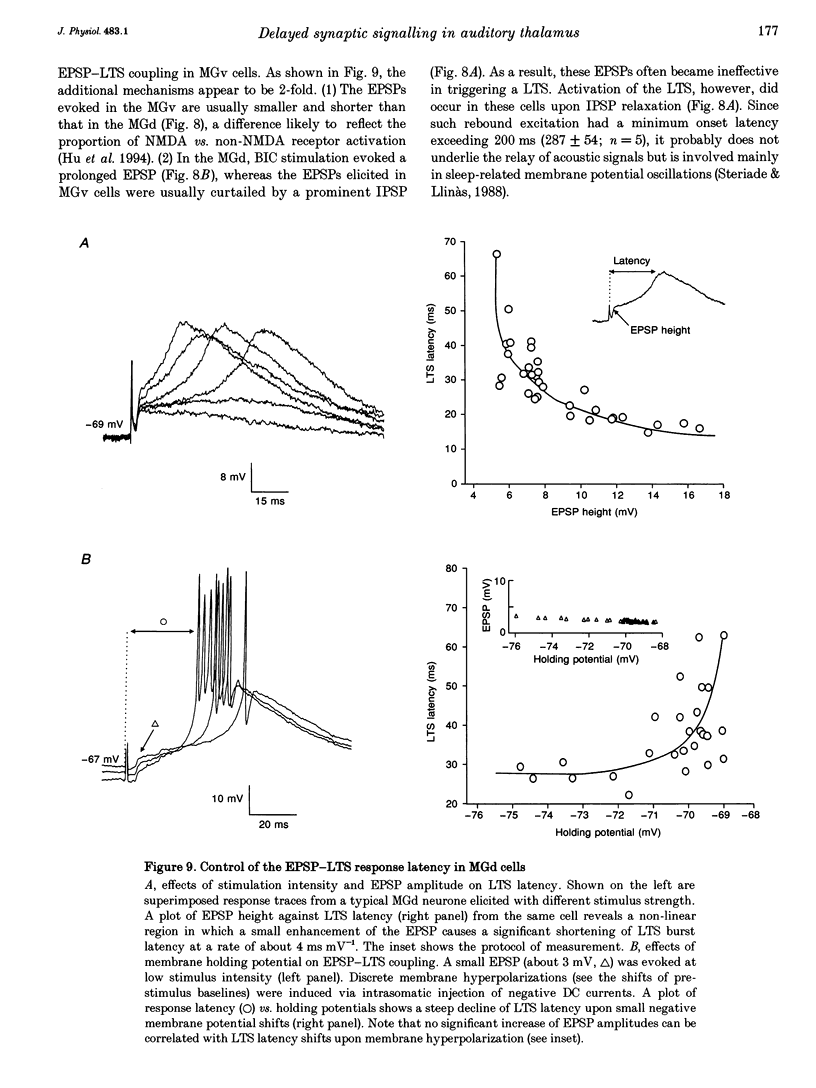
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitkin L. M., Prain S. M. Medial geniculate body: unit responses in the awake cat. J Neurophysiol. 1974 May;37(3):512–521. doi: 10.1152/jn.1974.37.3.512. [DOI] [PubMed] [Google Scholar]
- Calford M. B., Aitkin L. M. Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through thalamus. J Neurosci. 1983 Nov;3(11):2365–2380. doi: 10.1523/JNEUROSCI.03-11-02365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford M. B. The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurosci. 1983 Nov;3(11):2350–2364. doi: 10.1523/JNEUROSCI.03-11-02350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford M. B., Webster W. R. Auditory representation within principal division of cat medial geniculate body: an electrophysiology study. J Neurophysiol. 1981 Jun;45(6):1013–1028. doi: 10.1152/jn.1981.45.6.1013. [DOI] [PubMed] [Google Scholar]
- Carr C. E. Processing of temporal information in the brain. Annu Rev Neurosci. 1993;16:223–243. doi: 10.1146/annurev.ne.16.030193.001255. [DOI] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J Physiol. 1989 Jul;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Lightowler S., Pollard C. E. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol. 1989 Jun;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschêenes Martin, Hu Bin. Electrophysiology and Pharmacology of the Corticothalamic Input to Lateral Thalamic Nuclei: an Intracellular Study in the Cat. Eur J Neurosci. 1990 Feb;2(2):140–152. doi: 10.1111/j.1460-9568.1990.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Di S., Barth D. S. The functional anatomy of middle-latency auditory evoked potentials: thalamocortical connections. J Neurophysiol. 1992 Aug;68(2):425–431. doi: 10.1152/jn.1992.68.2.425. [DOI] [PubMed] [Google Scholar]
- Engel A. K., König P., Kreiter A. K., Schillen T. B., Singer W. Temporal coding in the visual cortex: new vistas on integration in the nervous system. Trends Neurosci. 1992 Jun;15(6):218–226. doi: 10.1016/0166-2236(92)90039-b. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M. Some fiber pathways related to the posterior thalamic region in the cat. Brain Behav Evol. 1972;6(1):363–393. doi: 10.1159/000123723. [DOI] [PubMed] [Google Scholar]
- Hu B., Senatorov V., Mooney D. Lemniscal and non-lemniscal synaptic transmission in rat auditory thalamus. J Physiol. 1994 Sep 1;479(Pt 2):217–231. doi: 10.1113/jphysiol.1994.sp020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. On the resting potential of isolated frog sympathetic neurons. Neuron. 1989 Aug;3(2):153–161. doi: 10.1016/0896-6273(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Lennartz R. C., Weinberger N. M. Frequency selectivity is related to temporal processing in parallel thalamocortical auditory pathways. Brain Res. 1992 Jun 26;583(1-2):81–92. doi: 10.1016/s0006-8993(10)80011-3. [DOI] [PubMed] [Google Scholar]
- Leresche N., Lightowler S., Soltesz I., Jassik-Gerschenfeld D., Crunelli V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. J Physiol. 1991 Sep;441:155–174. doi: 10.1113/jphysiol.1991.sp018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Lu S. M., Guido W., Sherman S. M. Effects of membrane voltage on receptive field properties of lateral geniculate neurons in the cat: contributions of the low-threshold Ca2+ conductance. J Neurophysiol. 1992 Dec;68(6):2185–2198. doi: 10.1152/jn.1992.68.6.2185. [DOI] [PubMed] [Google Scholar]
- McClurkin J. W., Optican L. M., Richmond B. J., Gawne T. J. Concurrent processing and complexity of temporally encoded neuronal messages in visual perception. Science. 1991 Aug 9;253(5020):675–677. doi: 10.1126/science.1908118. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Feeser H. R. Functional implications of burst firing and single spike activity in lateral geniculate relay neurons. Neuroscience. 1990;39(1):103–113. doi: 10.1016/0306-4522(90)90225-s. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee T., Kraus N., Littman T., Nicol T. Contributions of medial geniculate body subdivisions to the middle latency response. Hear Res. 1992 Aug;61(1-2):147–154. doi: 10.1016/0378-5955(92)90045-o. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Schreiner C., Jenkins W., Wang X. Neural mechanisms underlying temporal integration, segmentation, and input sequence representation: some implications for the origin of learning disabilities. Ann N Y Acad Sci. 1993 Jun 14;682:1–22. doi: 10.1111/j.1749-6632.1993.tb22955.x. [DOI] [PubMed] [Google Scholar]
- Middlebrooks J. C., Clock A. E., Xu L., Green D. M. A panoramic code for sound location by cortical neurons. Science. 1994 May 6;264(5160):842–844. doi: 10.1126/science.8171339. [DOI] [PubMed] [Google Scholar]
- Nicolelis M. A., Lin R. C., Woodward D. J., Chapin J. K. Dynamic and distributed properties of many-neuron ensembles in the ventral posterior medial thalamus of awake rats. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2212–2216. doi: 10.1073/pnas.90.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H. C., McCormick D. A. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature. 1989 Aug 31;340(6236):715–718. doi: 10.1038/340715a0. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Lu S. M., Guido W., Adams P. R., Sherman S. M. N-methyl-D-aspartate receptors contribute to excitatory postsynaptic potentials of cat lateral geniculate neurons recorded in thalamic slices. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4548–4552. doi: 10.1073/pnas.87.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J. S., Doyle J. F., Burkhalter A., Nerbonne J. M. Differential expression of hyperpolarization-activated currents reveals distinct classes of visual cortical projection neurons. J Neurosci. 1993 Dec;13(12):5082–5091. doi: 10.1523/JNEUROSCI.13-12-05082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I., Lightowler S., Leresche N., Jassik-Gerschenfeld D., Pollard C. E., Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol. 1991 Sep;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K. J., Otis T. S., Mody I. Membrane properties of dentate gyrus granule cells: comparison of sharp microelectrode and whole-cell recordings. J Neurophysiol. 1992 May;67(5):1346–1358. doi: 10.1152/jn.1992.67.5.1346. [DOI] [PubMed] [Google Scholar]
- Steriade M., Llinás R. R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988 Jul;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Suga N., Olsen J. F., Butman J. A. Specialized subsystems for processing biologically important complex sounds: cross-correlation analysis for ranging in the bat's brain. Cold Spring Harb Symp Quant Biol. 1990;55:585–597. doi: 10.1101/sqb.1990.055.01.056. [DOI] [PubMed] [Google Scholar]
- Winer J. A., Larue D. T. Anatomy of glutamic acid decarboxylase immunoreactive neurons and axons in the rat medial geniculate body. J Comp Neurol. 1988 Dec 1;278(1):47–68. doi: 10.1002/cne.902780104. [DOI] [PubMed] [Google Scholar]
- Winer J. A., Larue D. T. Patterns of reciprocity in auditory thalamocortical and corticothalamic connections: study with horseradish peroxidase and autoradiographic methods in the rat medial geniculate body. J Comp Neurol. 1987 Mar 8;257(2):282–315. doi: 10.1002/cne.902570212. [DOI] [PubMed] [Google Scholar]