Abstract
Purpose
We retrospectively analyzed the effect of Bevacizumab (BEV) on recurrent high-grade glioma (rHGG) and examined the relationship between dose and efficacy.
Methods
A total of 182 patients with rHGG were included in this study. Patients were divided into a non-BEV group and a BEV group according to the treatment they received, and the BEV group was further divided into a low-dose group and a high-dose group based on the dose. Depending on the number of groups and the characteristics of numerical variables, t-test, ANOVA, or rank-sum test were selected. Categorical variables were compared using the chi-squared test.
Results
Progression-free survival (PFS) was lower in the non-BEV group compared to the BEV group, while overall survival (OS) was not different between the two groups. There was no difference in PFS and OS between low-dose group and high-dose group. Notably, we found that patients with longer PFS and OS were more likely to be from the BEV group. In addition, differences in Karnofsky Performance Score (KPS), steroid dose, and brain edema were observed in the non-BEV, low-dose, and high-dose groups from 3 to 12 months after treatment.
Conclusion
BEV can improve PFS in patients with rHGG, although its impact on OS is limited. There was no difference in the efficacy of different doses of BEV on rHGG. Interestingly, patients with longer PFS and OS were more likely to be from the BEV group. Based on these findings, long-term low-dose BEV appears to be an effective treatment option for rHGG.
Keywords: bevacizumab, temozolomide, recurrent high-grade glioma, progression free survival, overall survival
Introduction
The World Health Organization (WHO) classifies gliomas into grades I–IV, with grades III and IV designated as high-grade glioma (HGG).1 These HGG are among the most aggressive neoplasms of the central nervous system, typically treated with surgical resection, radiotherapy, and chemotherapy.2 Despite these treatments, tumor recurrence is common in recurrent high-grade glioma (rHGG). The treatment methods for rHGG include re-surgery, radiotherapy, and chemotherapy (alone or in combination). However, there is currently no established optimal treatment approach. Temozolomide (TMZ) is the most widely used chemotherapy drug for this condition.3–5 A standard 5-day TMZ regimen, consisting of 150–200 mg/m2 administered for 5 days out of every 28-day cycle, is typically recommended as first-line therapy for rHGG.6
Overexpression of vascular endothelial growth factor (VEGF), microvascular proliferation, and blood-brain barrier damage have been observed in rHGG. Bevacizumab (BEV), a humanized monoclonal antibody, exerts anti-tumor effects by inhibiting tumor neovascularization and has been applied to various tumors, including rHGG.7 In addition to its well-documented anti-angiogenic properties, recent studies have shown that BEV can also modulate the immune system. Specifically, increased expression of VEGF can lead to immune suppression by inhibiting the maturation of dendritic cells, reducing T-cell infiltration into tumors, and promoting the presence of inhibitory cell types in the tumor microenvironment. BEV has been demonstrated to potentially reverse this immune suppression by enhancing T-cell activity.8 Although BEV has been reported to carry a risk of side effects in treating rHGG, survival has been improved, but only in the study based on the systematic analysis of data from the published literature.9 Two randomized clinical trials have also evaluated the efficacy of BEV in newly diagnosed glioblastoma (GBM). Despite improvements in progression-free survival (PFS), these trials failed to demonstrate any significant improvement in overall survival (OS).10 Conflicting results from Phase II and Phase III clinical trials have demonstrated heterogeneity in the response to BEV in rHGG.11 Although BEV is commonly used for rHGG, its benefits in improving quality of life and neurocognitive function remain controversial.12 The relationship between BEV dose and adverse reactions is unclear. The purpose of this study was to investigate the effects of BEV on rHGG and explore the relationship between dose and efficacy.
Material and Methods
Patients
This retrospective study included patients based on the following inclusion criteria: (1) The first operation pathologically confirmed HGG; (2) Imaging findings or pathology from reoperation proved tumor recurrence; (3) The patient had received surgery and chemoradiotherapy, but not BEV; (4) Patients have normal coagulation, liver, and kidney function. (5) Patients have complete clinical data. The exclusion criteria are: (1) Patients with newly diagnosed HGG; (2) Patients with a history of other brain tumors; (3) The patient has a history of abnormal bleeding within the last six months. The determination of the type of resection was based on the postoperative imaging findings. A complete resection was considered when no enhanced tumor signal was observed on the imaging. If the residual enhancement signal did not exceed 5% of the preoperative signal, it was considered a subtotal resection. On the other hand, if the residual enhancement exceeded 5% of the preoperative volume, it was categorized as a partial resection.13 A total of 182 patients were included in this retrospective study, comprising 95 males and 87 females, with a median age of 54.50 ± 16.76 years. Thirty-nine patients refused reoperation for various reasons. Imaging evaluation was conducted by two experienced neurosurgeons to exclude pseudoprogression. Table 1 presents a summary of the patients’ demographic characteristics. This study adhered to the principles of the Declaration of Helsinki. Since it was a retrospective observational study that did not involve the disclosure of personal information and written informed consent had been obtained from all participants beforehand, the Ethics Review Committee of the Chinese Academy of Medical Sciences and Peking Union Medical College Hospital decided to waive the requirement for ethical approval for these reasons.
Table 1.
Baseline Characteristics of All Patients
| Parameter | Non-BEV (n=72) | Low-Dose BEV (n=57) | High-Dose BEV (n=53) | P-value |
|---|---|---|---|---|
| Age (median, years) | 58±17.64 | 54±16.71 | 52±15.80 | 0.719 |
| Sex (N) | 0.971 | |||
| Male | 38 | 29 | 28 | |
| Female | 34 | 28 | 25 | |
| Pathological grade (N) | 0.682 | |||
| WHO III | 23 | 16 | 19 | |
| WHO IV | 49 | 41 | 34 | |
| Extent of resection (N) | 0.826 | |||
| Complete resection | 38 | 32 | 33 | |
| Subtotal resection | 10 | 10 | 9 | |
| Partial resection | 6 | 3 | 2 | |
| No reoperation | 18 | 12 | 9 | |
| Tumor location (N) | 0.079 | |||
| Frontal lobe | 19 | 7 | 9 | |
| Temporal lobe | 9 | 15 | 7 | |
| Parietal lobe | 10 | 4 | 13 | |
| Frontotemporal lobe | 15 | 18 | 9 | |
| Parietooccipital lobe | 14 | 9 | 10 | |
| Brainstem | 5 | 4 | 5 | |
| IDH (N) | 0.413 | |||
| Mutation | 25 | 14 | 18 | |
| Wild type | 47 | 43 | 35 | |
| MGMT (N) | 0.998 | |||
| Methylated | 20 | 16 | 15 | |
| Unmethylated | 52 | 41 | 38 | |
| Reoperation to Bev(months) | / | 2±1.36 | 2±1.03 | <0.001* |
| KPS | 60±17.92 | 60±14.35 | 60±13.01 | 0.508 |
| Edema (ccm) | 24.50±12.95 | 31±12.40 | 27±11.56 | 0.130 |
| Steroids (mg) | 20±25.19 | 20±26.91 | 20±28.08 | 0.811 |
| Steroids (day) | 5±1.37 | 5±1.42 | 6±1.55 | 0.137 |
Notes: * indicates p-value < 0.05.
Abbreviations: BEV, bevacizumab; WHO, world health organization; KPS, karnofsky performance score.
Drug Administration
All patients received the standard 5-day TMZ regimen, consisting of 200 mg/m2 administered for 5 days every 28 days. The maximum course of TMZ treatment in this study was 26 cycles. Hematological examinations were conducted prior to each cycle of treatment. Long-term TMZ therapy has the potential to enhance the prognosis of patients with HGG; however, discontinuation of the drug should only be considered when severe toxic effects emerge.14 The recommended dose for BEV is 5–15 mg/kg, with an interval of 2–3 weeks.15 Patients were divided into a non-BEV group (TMZ alone, n=72) and a BEV group based on whether they received BEV treatment. The decision to use BEV was influenced by several factors, including patient concerns about potential adverse reactions and financial considerations, with cost being a significant factor in many cases. The BEV group was further categorized into a low-dose group (3mg/kg, interval: 2 weeks, n=57) and a high-dose group (10mg/kg, interval: 2 weeks, n=53) depending on the dose of BEV administered. Each 28-day period constituted one treatment cycle.
Efficacy Evaluation
The therapeutic response was evaluated according to the Response Assessment in Neuro-Oncology (RANO) criteria.16 Complete response (CR) was defined as the disappearance of the tumor signal. Partial response (PR) was defined as a ≥50% reduction in tumor area on contrast-enhanced scanning. Stable disease (SD) was defined as a decrease in tumor size of <50% or an increase in tumor size of <25%. Disease progression (PD) was defined as an increase in tumor size of ≥25%. The overall response rate (OR) included CR and PR. PFS and OS were calculated from the initiation of drug therapy. MRI was performed every 4–8 weeks. Tumor and edema volumes were obtained by accumulating the volumes on each axial image.17 Two experienced neurosurgeons separately evaluated the patients’ KPS and recorded steroid doses.
Statistical Analysis
The continuous variables are presented as the median ± standard deviation (median ± SD). All categorical variables are described as the number of patients or as a percentage (%). Depending on the number of groups and the characteristics of numerical variables, t-test, ANOVA, or rank-sum test were selected. Categorical variables were compared using the chi-squared test. Univariate and multivariate regression analyses were conducted to assess the impact of various factors on PFS and OS. The survival curves for PFS and OS were plotted using the Kaplan-Meier method. All data in this study were analyzed with SPSS (version 27.0, IBM). The multivariate mixed-effects model was developed using R software (version 4.4.1). A P-value of < 0.05 was considered statistically significant.
Results
Univariate and Multivariate Regression Analysis
In this study, 17 potential factors associated with PFS and OS were analyzed. A univariate Cox regression analysis was conducted on these factors, revealing that 8 significantly influenced PFS (P < 0.05), including BEV usage, BEV cycles, and reoperation, among others. These 8 factors were then incorporated into a multivariate mixed-effects regression model, which identified BEV usage, BEV cycles, TMZ cycles, reoperation, and pathological grade as independent risk factors for PFS (P < 0.05). For OS, 7 factors that showed statistical significance in the univariate analysis were selected for inclusion in the multivariate model. The analysis indicated that only TMZ cycles and reoperation were independent risk factors for OS (P < 0.001). The results of the univariate and multivariate regression analyses are summarized in Table 2.
Table 2.
Univariate and Multivariate Regression Analysis
| Parameter | UV HR (95% CI) | UV p | MV HR (95% CI) | MV p* |
|---|---|---|---|---|
| Progression-free survival | ||||
| BEV usage | 0.676 (0.496–0.921) | 0.013 | 0.789 (0.653–0.954) | 0.014* |
| BEV cycles | 0.934 (0.910–0.958) | <0.001 | 1.534 (1.045–2.253) | 0.029* |
| TMZ cycles | 0.816 (0.785–0.848) | <0.001 | 0.840 (0.803–0.879) | <0.001* |
| IDH | 1.414 (1.018–1.965) | 0.039 | ||
| MGMT | 1.928 (1.362–2.729) | <0.001 | ||
| Reoperation | 3.524 (2.359–5.263) | <0.001 | 2.123 (1.427–3.160) | <0.001* |
| Pathological grade | 1.662 (1.196–2.309) | 0.002 | 1.579 (1.103–2.259) | 0.013* |
| Reoperation to BEV | 0.872 (0.769–0.988) | 0.032 | ||
| Overall survival | ||||
| BEV cycles | 0.942 (0.920–0.965) | <0.001 | ||
| TMZ cycles | 0.722 (0.691–0.756) | <0.001 | 0.729 (0.693–0.767) | <0.001* |
| IDH | 1.582 (1.133–2.208) | 0.007 | ||
| MGMT | 1.891 (1.328–2.691) | <0.001 | ||
| Reoperation | 3.789 (2.536–5.661) | <0.001 | 2.325 (1.544–3.501) | <0.001* |
| Pathological grade | 1.665 (1.195–2.321) | 0.003 | ||
| KPS | 0.988 (0.978–0.998) | 0.023 |
Notes: * indicates p-value < 0.05.
Abbreviations: UV, univariable; MV, multivariable; CI, confidence interval; HR, hazard ratio; BEV, bevacizumab; TMZ, Temozolomide; KPS, karnofsky performance score.
Therapeutic Effect
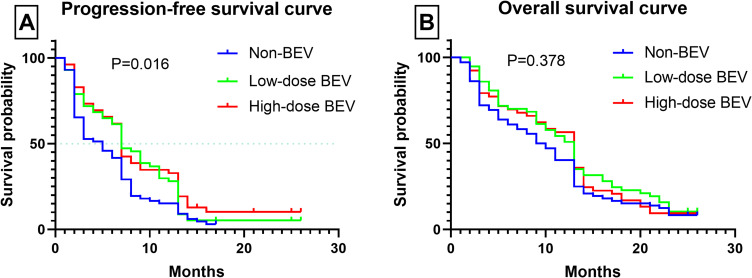
The median PFS was 5 months in the non-BEV group and 7 months in BEV groups (P < 0.05). OS was 9.5 months in the non-BEV group compared with 13 months in the BEV group (P > 0.05). There was no difference in PFS and OS between low-dose BEV group and high-dose BEV group (P > 0.05). The PFS and OS of 39 patients who did not undergo reoperation were 3 months and 5 months, respectively. For patients who underwent reoperation, PFS was 7 months and OS was 13 months (P < 0.05). The median PFS for all patients was 7 months. Of the 112 patients with PFS≤7 months, 61 (54.5%) received BEV and 51 (45.5%) did not (P > 0.05). Of the 70 patients with PFS > 7 months, 49 patients (70.0%) received BEV and 21 (30.0%) did not (P < 0.05). The median OS of enrolled patients was 11 months. Of the 92 patients with OS≤11 months, 49 (53.3%) received BEV and 43 (46.7%) did not (P > 0.05). Of the 90 patients with OS > 11 months, 61 (67.8%) received BEV and 29 (32.2%) did not (P < 0.05). Regarding overall response rates, the response rates at 3, 6, 12, and 24 months were 75.3%, 59.9%, 34.1%, and 8.2%, respectively. Figure 1 shows the survival curves of PFS and OS for the three groups.
Figure 1.
The survival curves of progression-free survival (PFS) and overall survival (OS) were plotted by Kaplan-Meier method. (Panel A): Survival curves of PFS in the three groups. (Panel B): Survival curves of OS in the three groups.
Clinical Manifestation
There were no significant differences in baseline levels of brain edema, steroid dose, and KPS among the three groups. Table 3 presents a summary of the changes in brain edema, KPS, and steroid dose during treatment for all patients. After 3 months of treatment, KPS was lower in the non-BEV group than in the BEV group, with a median difference of 20 points (P<0.05). At 6 and 12 months, the difference in KPS between the non-BEV and BEV groups was smaller, with a median difference of 10 points (P<0.05). Steroid doses were higher in the non-BEV group than in the BEV group from 3 to 12 months (P<0.05). Meanwhile, a significant difference in brain edema was observed between the non-BEV and BEV groups (P<0.05). At 24 months after treatment, there were no differences in KPS, steroid dose, and brain edema among the non-BEV, low-dose BEV, and high-dose BEV groups (P>0.05). Throughout the course of treatment, there was no difference in KPS, steroid dose, and brain edema between the low-dose and high-dose BEV groups (P>0.05).
Table 3.
Changes in KPS, Steroids, and Brain Edema in the Three Groups During Treatment
| Parameter | Non-BEV (n=72) | Low-Dose BEV (n=57) | High-Dose BEV (n=53) | P-value |
|---|---|---|---|---|
| KPS | ||||
| 3 months | 50±12.84 | 70±10.25 | 70±14.97 | <0.001* |
| 6 months | 50±12.72 | 60±11.50 | 60±11.27 | <0.001* |
| 12 months | 50±12.24 | 60±11.23 | 60±9.80 | 0.042* |
| 24 months | 45±10.49 | 55±5.48 | 60±11.40 | 0.122 |
| Steroid (mg) | ||||
| 3 months | 40±19.72 | 20±13.25 | 20±20.02 | <0.001* |
| 6 months | 40±17.16 | 20±16.32 | 20±21.05 | <0.001* |
| 12 months | 40±18.34 | 20±21.99 | 20±20.30 | 0.007* |
| 24 months | 40±24.50 | 20±24.50 | 20±26.83 | 0.108 |
| Brain edema (ccm) | ||||
| 3 months | 21±11.02 | 15±7.13 | 14.50±7.25 | 0.017* |
| 6 months | 24±7.60 | 16±5.74 | 12±6.51 | <0.001* |
| 12 months | 30±5.96 | 26±5.93 | 24±7.38 | 0.003* |
| 24 months | 29±14.42 | 32.50±17.07 | 27±5.54 | 0.934 |
Notes: * indicates p-value < 0.05.
Abbreviations: BEV, bevacizumab; KPS, karnofsky performance score.
Adverse Drug Events
BEV-associated adverse effects included various types of bleeding, headaches, hypertension, blood toxicity, thrombosis, proteinuria, gastrointestinal perforation, delayed wound healing, congestive heart failure, sepsis, and nephrotic syndrome.18–20 The incidence of BEV-related adverse events may be dose-dependent.21 Thirty-seven patients in the three groups had nausea, 23 had thrombocytopenia, which may be related to TMZ. Among the patients treated with BEV in the two groups, 47 were considered to have new-onset hypertension, and 32 were considered to be fatigued. Both hypertension and fatigue were considered to be related to BEV. There was no difference in adverse reactions between different dose groups. No serious adverse reactions, such as gastrointestinal perforation, cerebral hemorrhage, or pulmonary embolism, were observed in this study, which may be related to the low dose of BEV.
Discussion
VEGF serves as a potent mediator of angiogenesis, vascular permeability, and glioma growth in brain tumors.22 Increasing evidence indicates that VEGF not only promotes angiogenesis but also plays a crucial role in immune suppression within the tumor microenvironment.23 It has been demonstrated to inhibit dendritic cell maturation, reduce T cell tumor infiltration, and promote the accumulation of suppressive cell types, contributing to the formation of an immunosuppressive environment associated with poorer outcomes in cancer patients.8 Targeting VEGF with BEV may not only exert anti-angiogenic effects but also potentially reverse VEGF-mediated immune suppression. By modulating the VEGF signaling pathway, BEV could activate immune cell activity, enhance immune responses against tumors, and provide a dual mechanism of inhibiting tumor neovascularization while promoting immune surveillance within brain tumors.24
While large-scale studies have failed to demonstrate improved OS with BEV in GBM patients, its use in treating rHGG has gained widespread acceptance. However, it is crucial to acknowledge the limited penetration of antibodies, including BEV, through the blood-brain barrier (BBB), significantly limiting their effectiveness in brain tissue. Furthermore, BEV tightens the BBB, potentially offsetting its beneficial effects on immune cells and potential angiogenesis. Despite reports of VEGF concentrations in the cyst fluid of GBM patients being 200 to 300 times higher than in serum, VEGF remains undetectable in peripheral blood using enzyme-linked immunosorbent assay (ELISA). In contrast, other cancer types may exhibit lower VEGF levels in tumors but detectable concentrations in peripheral blood.25 This observation highlights the BBB’s ability to prevent peripheral VEGF leakage and neutralization, leading to significantly elevated VEGF concentrations within brain cancer tissue. Such high levels of VEGF markedly suppress immune cell activity.22 Under these conditions, traditional intravenous administration of BEV may be inefficient due to limited BBB penetration.
In addition to traditional intravenous administration, several advanced methods for drug delivery to the brain have been developed, including stereotactic convection-enhanced delivery,26 slow-release polymers,27 polymeric micelles,28 focused ultrasound,29 and intraarterial administration.30 Recent studies have shown that intra-arterial administration of BEV significantly improves OS in newly diagnosed GBM patients following surgical resection compared to historical data.31–33 Using radiolabeling techniques in animal models, permeability of the BBB through osmotic BBB opening (OBBBO) dramatically increases BEV penetration into brain tissue, enabling its action within brain cancer tissue instead of acting merely peripherally.34 This suggests that combining intra-arterial administration with OBBBO may offer a new therapeutic approach by facilitating direct drug delivery to brain cancer tissue.35 This enhanced delivery not only strengthens BEV’s anti-angiogenic effects within brain cancer tissue but also allows it to counteract VEGF-mediated immune suppression, potentially improving patient outcomes. Thus, the pivotal therapeutic action of BEV may lie in its enhancement of the immune system rather than its anti-angiogenic activity. Future research and clinical trials should further explore methods such as OBBBO to enhance BEV delivery to brain cancer tissue, optimizing its therapeutic efficacy and improving patient prognosis.
Additionally, BEV exhibits steroid-sparing effects and enhances the quality of life for patients with rHGG.36 BEV has been utilized in the treatment of HGG for over 10 years, yet the determination of an optimal dosage remains controversial.37 High-dose BEV can rapidly alleviate brain edema, but simultaneously promotes tumor hypoxia and accelerates tumor resistance, posing challenges in balancing its therapeutic benefits and potential drawbacks.38 Chronic exposure to high-dose BEV can lead to tumor resistance and promote a more aggressive tumor phenotype.39,40 When treating rHGG with standard doses of BEV (10mg/kg), it leads to reduced vascular permeability, thereby limiting the effectiveness of chemotherapy.41 In contrast, low-dose BEV has demonstrated efficacy in alleviating cerebral edema and treating radiation necrosis.42,43 Therefore, the exploration of low-dose BEV strategies to improve survival outcomes appears feasible.
In this study, PFS in the non-BEV group was shorter than in the BEV groups, indicating that BEV can prolong PFS in rHGG patients. While the OS was longer in the BEV group compared to the non-BEV group, the observed difference did not reach statistical significance, indicating a limited impact of BEV on OS. The results of both univariate and multivariate regression analyses further confirmed this point. Interestingly, our analysis revealed that approximately 70% of patients with both longer PFS and OS belonged to the BEV group. These findings suggest a potential positive impact of BEV on the OS of rHGG patients. However, the limited impact of BEV on OS may also be influenced by the effects of prior radiotherapy. Evidence from a primate study demonstrated that 82% of healthy rhesus monkeys developed de novo GBM within 2.5 to 8 years following fractionated whole-brain radiation therapy (WBRT), suggesting a high rate of radiation-induced gliomagenesis.44 This phenomenon implies that radiotherapy may contribute to the emergence of new brain tumors or exacerbate tumor progression, potentially offsetting the benefits of subsequent therapies such as BEV over the long term. This aspect could help explain why BEV’s effect on extending OS has been less pronounced, as long-term post-radiation changes in the tumor microenvironment may present additional therapeutic challenges.
Our study found no significant differences in PFS and OS between the low-dose BEV group and the high-dose BEV group. However, it is important to note that these findings may be under-powered due to the small sample size in each group, which could limit our ability to detect equivalence between the two dosages. These findings suggest the feasibility of utilizing low-dose BEV in the management of rHGG patients, pending further studies with larger cohorts to confirm these results. Previous studies have demonstrated a slight superiority in PFS when low-dose BEV is administered, further supporting its potential as a treatment strategy for rHGG patients.45,46
The PFS and OS of the reoperation group were longer than those of the non-reoperation group, suggesting that surgery is an effective means to improve the prognosis of rHGG. This is consistent with previous reports, and the results of the multivariate mixed model in this study also support this conclusion.47 The KPS in the non-BEV group was consistently lower than that in the BEV group, indicating that BEV can improve the clinical symptoms of HGG patients. At the same time, the lower dose of steroids in the BEV group compared to the non-BEV group indicates that BEV can reduce steroid use. From 3 months to 12 months, brain edema was more severe in the non-BEV group compared to the BEV group, suggesting that BEV can effectively alleviate brain edema. However, at 24 months after treatment, there was no significant difference in brain edema among the three treatment groups (BEV low-dose, BEV high-dose, and non-BEV). These findings suggest that BEV’s efficacy in treating brain edema caused by tumor recurrence is limited.
The relationship between BEV dose and the incidence of adverse events is unknown.21 A lower dose may reduce the incidence of adverse events.48 In this study, there was no significant difference in the incidence of adverse events between the low-dose and high-dose groups. This may be attributed to the fact that the drug dose used in the study was lower than the standard level. Additionally, a separate study involving 49 patients demonstrated that by lowering the BEV dose, it was estimated to achieve cost savings of $1,439,726.08. This suggests that using a lower dose not only maintains efficacy but also has the potential to significantly reduce treatment costs.49 In this study, the median treatment duration was 5 months in the high-dose group and 7 months in the low-dose group. The total amount of drug used in the high-dose group was 3.26 times that in the low-dose group. While lowering the dose may prolong the treatment period, it will reduce the financial burden on patients.
However, several limitations of this study should be acknowledged. Firstly, as a single-center study, the results may not be fully representative and could benefit from replication in multicenter studies. Secondly, due to its retrospective nature, our study was limited in terms of the evidence it could gather. Therefore, there is a need for more prospective studies to be conducted to comprehensively evaluate the efficacy of BEV in patients with rHGG.
Conclusion
This study aimed to investigate the impact of the absence of BEV and varying dosages of BEV on PFS and OS among patients with rHGG. The findings indicate that BEV administration can prolong PFS in rHGG patients and potentially confer benefits for long-term OS. Notably, the efficacy of low-dose BEV was comparable to that of high-dose BEV. In conclusion, long-term low-dose BEV administration may serve as a viable therapeutic alternative for the treatment of rHGG.
Acknowledgments
The authors are grateful to all patients included in this study for their support.
Funding Statement
This work was supported by the National High Level Hospital Clinical Research Funding(2022-PUMCH-C-012).
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Consent to Participate
Written informed consent was obtained from all individual participants included in the study.
Consent to Publish
We confirm that the manuscript submitted for publication does not contain any personal data or sensitive information that could identify individual patients or research participants. All authors have provided their consent for the publication of this manuscript.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
- 1.Komori T. The 2021 WHO classification of tumors, 5th edition, central nervous system tumors: the 10 basic principles. Brain Tumor Pathol. 2022;39(2):47–50. doi: 10.1007/s10014-022-00428-3 [DOI] [PubMed] [Google Scholar]
- 2.Detti B, Scoccianti S, Teriaca MA, et al. Bevacizumab in recurrent high-grade glioma: a single institution retrospective analysis on 92 patients. Radiol Med. 2021;126(9):1249–1254. doi: 10.1007/s11547-021-01381-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled Phase 2 trial. Lancet Oncol. 2014;15(9):943–953. doi: 10.1016/s1470-2045(14)70314-6 [DOI] [PubMed] [Google Scholar]
- 4.Badruddoja MA, Pazzi M, Sanan A, et al. Phase II study of bi-weekly temozolomide plus bevacizumab for adult patients with recurrent glioblastoma. Cancer Chemother Pharmacol. 2017;80(4):715–721. doi: 10.1007/s00280-017-3405-7 [DOI] [PubMed] [Google Scholar]
- 5.Wick W, Gorlia T, Bendszus M, et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med. 2017;377(20):1954–1963. doi: 10.1056/NEJMoa1707358 [DOI] [PubMed] [Google Scholar]
- 6.Wei W, Chen X, Ma X, Wang D, Guo Z. The efficacy and safety of various dose-dense regimens of temozolomide for recurrent high-grade glioma: a systematic review with meta-analysis. J Neurooncol. 2015;125(2):339–349. doi: 10.1007/s11060-015-1920-0 [DOI] [PubMed] [Google Scholar]
- 7.Robles Irizarry L, Hambardzumyan D, Nakano I, Gladson CL, Ahluwalia MS. Therapeutic targeting of VEGF in the treatment of glioblastoma. Expert Opin Ther Targets. 2012;16(10):973–984. doi: 10.1517/14728222.2012.711817 [DOI] [PubMed] [Google Scholar]
- 8.Ribatti D. Immunosuppressive effects of vascular endothelial growth factor. Oncol Lett. 2022;24(4):369. doi: 10.3892/ol.2022.13489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz RJ, Ali S, Qadir MG, De La Fuente MI, Ivan ME, Komotar RJ. The role of bevacizumab in the treatment of glioblastoma. J Neurooncol. 2017;133(3):455–467. doi: 10.1007/s11060-017-2477-x [DOI] [PubMed] [Google Scholar]
- 10.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Field KM, Simes J, Nowak AK, et al. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015;17(11):1504–1513. doi: 10.1093/neuonc/nov104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MM, Umemura Y, Leung D. Bevacizumab and Glioblastoma: past, Present, and Future Directions. Cancer J. 2018;24(4):180–186. doi: 10.1097/ppo.0000000000000326 [DOI] [PubMed] [Google Scholar]
- 13.Hervey-Jumper SL, Berger MS. Reoperation for recurrent high-grade glioma: a current perspective of the literature. Neurosurgery. 2014;75(5):491–499. doi: 10.1227/neu.0000000000000486 [DOI] [PubMed] [Google Scholar]
- 14.Yuan J, Liu J, Fan R, Liu Z. Effect of Temozolomide Combined with Intensity Modulated Radiation Therapy on Serum Factor, Immune Function and Clinical Efficacy in Postoperative Glioma Patients. Radiat Res. 2023;200(3):289–295. doi: 10.1667/rade-22-00198 [DOI] [PubMed] [Google Scholar]
- 15.Pillay Smiley N, Alden T, Hartsell W, Fangusaro J. Severe Radiation Necrosis Successfully Treated With Bevacizumab in an Infant with Low-Grade Glioma and Tumor-Associated Intractable Trigeminal Neuralgia. Pediatr Blood Cancer. 2016;63(9):1671–1673. doi: 10.1002/pbc.26055 [DOI] [PubMed] [Google Scholar]
- 16.Imber BS, Lin AL, Zhang Z, et al. Comparison of Radiographic Approaches to Assess Treatment Response in Pituitary Adenomas: is RECIST or RANO Good Enough? J Endocr Soc. 2019;3(9):1693–1706. doi: 10.1210/js.2019-00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baris MM, Celik AO, Gezer NS, Ada E. Role of mass effect, tumor volume and peritumoral edema volume in the differential diagnosis of primary brain tumor and metastasis. Clin Neurol Neurosurg. 2016;148:67–71. doi: 10.1016/j.clineuro.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Lu Y, Zheng Y. Incidence and risk of hypertension with bevacizumab in non-small-cell lung cancer patients: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2015;9:4751–4760. doi: 10.2147/dddt.S87258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matikas A, Kentepozidis Ν, Ardavanis A, et al. Efficacy and tolerance of frontline bevacizumab-based chemotherapy for advanced non-small cell lung cancer patients: a multicenter, Phase IV study of the Hellenic Oncology Research Group (HORG). Cancer Chemother Pharmacol. 2016;78(2):369–376. doi: 10.1007/s00280-016-3094-7 [DOI] [PubMed] [Google Scholar]
- 20.Zhong J, Ali AN, Voloschin AD, et al. Bevacizumab-induced hypertension is a predictive marker for improved outcomes in patients with recurrent glioblastoma treated with bevacizumab. Cancer. 2015;121(9):1456–1462. doi: 10.1002/cncr.29234 [DOI] [PubMed] [Google Scholar]
- 21.Blumenthal DT, Mendel L, Bokstein F. The optimal regimen of bevacizumab for recurrent glioblastoma: does dose matter? J Neurooncol. 2016;127(3):493–502. doi: 10.1007/s11060-015-2025-5 [DOI] [PubMed] [Google Scholar]
- 22.Takano S, Yoshii Y, Kondo S, et al. Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Res. 1996;56(9):2185–2190. [PubMed] [Google Scholar]
- 23.Soto-Ortiz L, Finley SD. A cancer treatment based on synergy between anti-angiogenic and immune cell therapies. J Theor Biol. 2016;394:197–211. doi: 10.1016/j.jtbi.2016.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacal PM, Graziani G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res. 2018;136:97–107. doi: 10.1016/j.phrs.2018.08.023 [DOI] [PubMed] [Google Scholar]
- 25.Kraft A, Weindel K, Ochs A, et al. Vascular endothelial growth factor in the sera and effusions of patients with malignant and nonmalignant disease. Cancer. 1999;85(1):178–187. doi: [DOI] [PubMed] [Google Scholar]
- 26.Jahangiri A, Chin AT, Flanigan PM, Chen R, Bankiewicz K, Aghi MK. Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies. J Neurosurg. 2017;126(1):191–200. doi: 10.3171/2016.1.Jns151591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossman R, Burger P, Soudry E, et al. MGMT inactivation and clinical response in newly diagnosed GBM patients treated with Gliadel. J Clin Neurosci. 2015;22(12):1938–1942. doi: 10.1016/j.jocn.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 28.Morshed RA, Cheng Y, Auffinger B, Wegscheid ML, Lesniak MS. The potential of polymeric micelles in the context of glioblastoma therapy. Front Pharmacol. 2013;4:157. doi: 10.3389/fphar.2013.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hersh DS, Kim AJ, Winkles JA, Eisenberg HM, Woodworth GF, Frenkel V. Emerging Applications of Therapeutic Ultrasound in Neuro-oncology: moving Beyond Tumor Ablation. Neurosurgery. 2016;79(5):643–654. doi: 10.1227/neu.0000000000001399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burkhardt JK, Riina HA, Shin BJ, Moliterno JA, Hofstetter CP, Boockvar JA. Intra-arterial chemotherapy for malignant gliomas: a critical analysis. Interv Neuroradiol. 2011;17(3):286–295. doi: 10.1177/159101991101700302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lesniak WG, Chu C, Jablonska A, et al. A Distinct Advantage to Intraarterial Delivery of (89)Zr-Bevacizumab in PET Imaging of Mice With and Without Osmotic Opening of the Blood-Brain Barrier. J Nucl Med. 2019;60(5):617–622. doi: 10.2967/jnumed.118.218792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkhardt JK, Santillan A, Hofstetter CP, et al. Intra-arterial bevacizumab with blood brain barrier disruption in a glioblastoma xenograft model. J Exp Ther Oncol. 2012;10(1):31–37. [PubMed] [Google Scholar]
- 33.Kaprin AD, Zaitsev AM, Rerberg AG, Fedenko AA, Datsenko PV, Kirsanova ON. Superselective intra-arterial administration of bevacizumab with blood-brain barrier disruption in patients with recurrent malignant gliomas: case report and literature review. Zh Vopr Neirokhir Im N N Burdenko. 2021;85(5):64–70. doi: 10.17116/neiro20218505164 [DOI] [PubMed] [Google Scholar]
- 34.Chu C, Jablonska A, Lesniak WG, et al. Optimization of osmotic blood-brain barrier opening to enable intravital microscopy studies on drug delivery in mouse cortex. J Control Release. 2020;317:312–321. doi: 10.1016/j.jconrel.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 35.D’Amico RS, Khatri D, Reichman N, et al. Super selective intra-arterial cerebral infusion of modern chemotherapeutics after blood-brain barrier disruption: where are we now, and where we are going. J Neurooncol. 2020;147(2):261–278. doi: 10.1007/s11060-020-03435-6 [DOI] [PubMed] [Google Scholar]
- 36.Funakoshi Y, Takigawa K, Hata N, et al. Changes in the Relapse Pattern and Prognosis of Glioblastoma After Approval of First-Line Bevacizumab: a Single-Center Retrospective Study. World Neurosurg. 2022;159:e479–e87. doi: 10.1016/j.wneu.2021.12.075 [DOI] [PubMed] [Google Scholar]
- 37.Chen Y, Guo L, Li X, Liu R, Ren C, Du S. Reduced-dose bevacizumab vs. standard-dose bevacizumab in recurrent high-grade glioma: which one is better? A meta-analysis. Clin Neurol Neurosurg. 2020;198:106239. doi: 10.1016/j.clineuro.2020.106239 [DOI] [PubMed] [Google Scholar]
- 38.de Groot JF. High-dose antiangiogenic therapy for glioblastoma: less may be more? Clin Cancer Res. 2011;17(19):6109–6111. doi: 10.1158/1078-0432.Ccr-11-1853 [DOI] [PubMed] [Google Scholar]
- 39.Piao Y, Liang J, Holmes L, Henry V, Sulman E, de Groot JF. Acquired resistance to anti-VEGF therapy in glioblastoma is associated with a mesenchymal transition. Clin Cancer Res. 2013;19(16):4392–4403. doi: 10.1158/1078-0432.Ccr-12-1557 [DOI] [PubMed] [Google Scholar]
- 40.Onishi M, Kurozumi K, Ichikawa T, Date I. Mechanisms of tumor development and anti-angiogenic therapy in glioblastoma multiforme. Neurol Med Chir. 2013;53(11):755–763. doi: 10.2176/nmc.ra2013-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerstner ER, Emblem KE, Chang K, et al. Bevacizumab Reduces Permeability and Concurrent Temozolomide Delivery in a Subset of Patients with Recurrent Glioblastoma. Clin Cancer Res. 2020;26(1):206–212. doi: 10.1158/1078-0432.Ccr-19-1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiangying M, Rugang Z, Lijuan D, et al. Low-dose bevacizumab as an effective pre-treatment for peri-tumoral brain edema prior to CyberKnife radiosurgery: a case report. Cancer Biol Ther. 2018;19(6):461–464. doi: 10.1080/15384047.2018.1433499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuang H, Zhuang H, Shi S, Wang Y. Ultra-Low-Dose Bevacizumab For Cerebral Radiation Necrosis: a Prospective Phase II Clinical Study. Onco Targets Ther. 2019;12:8447–8453. doi: 10.2147/ott.S223258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lonser RR, Walbridge S, Vortmeyer AO, et al. Induction of glioblastoma multiforme in nonhuman primates after therapeutic doses of fractionated whole-brain radiation therapy. J Neurosurg. 2002;97(6):1378–1389. doi: 10.3171/jns.2002.97.6.1378 [DOI] [PubMed] [Google Scholar]
- 45.Levin VA, Mendelssohn ND, Chan J, et al. Impact of bevacizumab administered dose on overall survival of patients with progressive glioblastoma. J Neurooncol. 2015;122(1):145–150. doi: 10.1007/s11060-014-1693-x [DOI] [PubMed] [Google Scholar]
- 46.Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw. 2011;9(4):403–407. doi: 10.6004/jnccn.2011.0037 [DOI] [PubMed] [Google Scholar]
- 47.Champeaux Depond C, Bauchet L, Elhairech D, et al. Survival After Newly-Diagnosed High-Grade Glioma Surgery: what Can We Learn From the French National Healthcare Database? Brain Tumor Res Treat. 2024;12(3):162–171. doi: 10.14791/btrt.2024.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirven-Villaros L, Bourg V, Suissa L, et al. Bevacizumab: is the lower the better for glioblastoma patients in progression? Bull Cancer. 2018;105(12):1135–1146. doi: 10.1016/j.bulcan.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 49.Gleeson JP, Keane F, Keegan NM, et al. Similar overall survival with reduced vs. standard dose bevacizumab monotherapy in progressive glioblastoma. Cancer Med. 2020;9(2):469–475. doi: 10.1002/cam4.2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.