Abstract
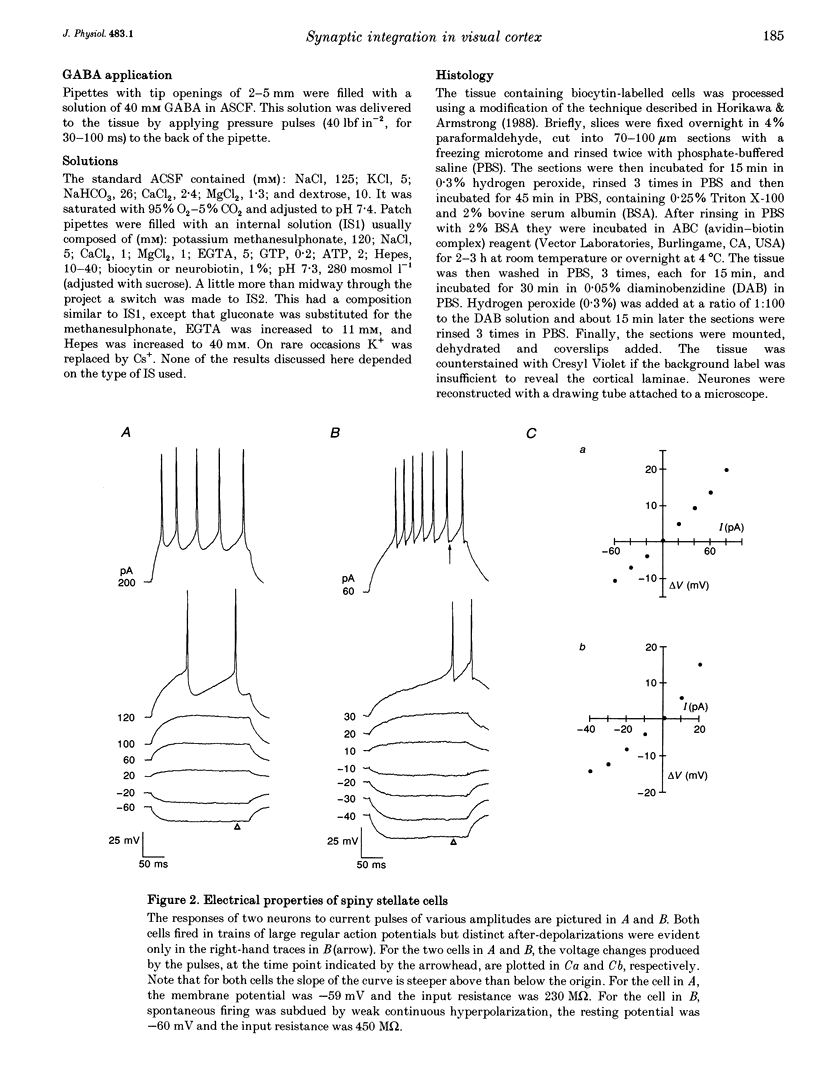
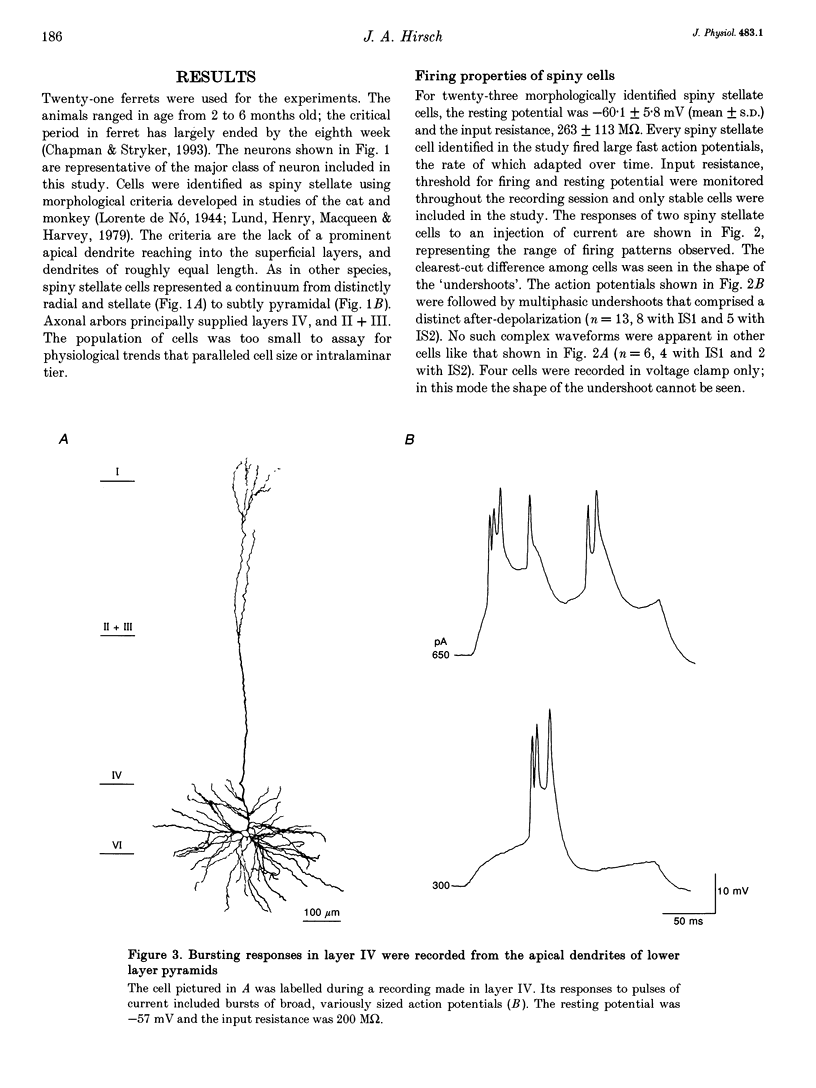
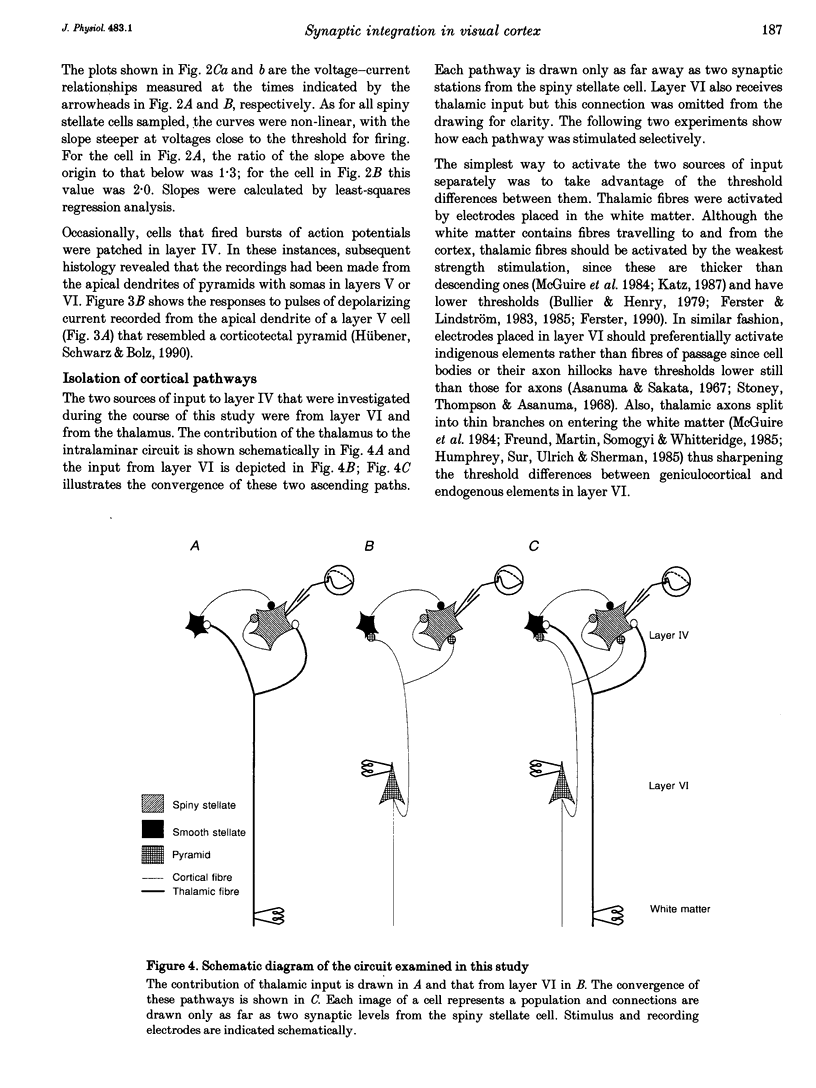
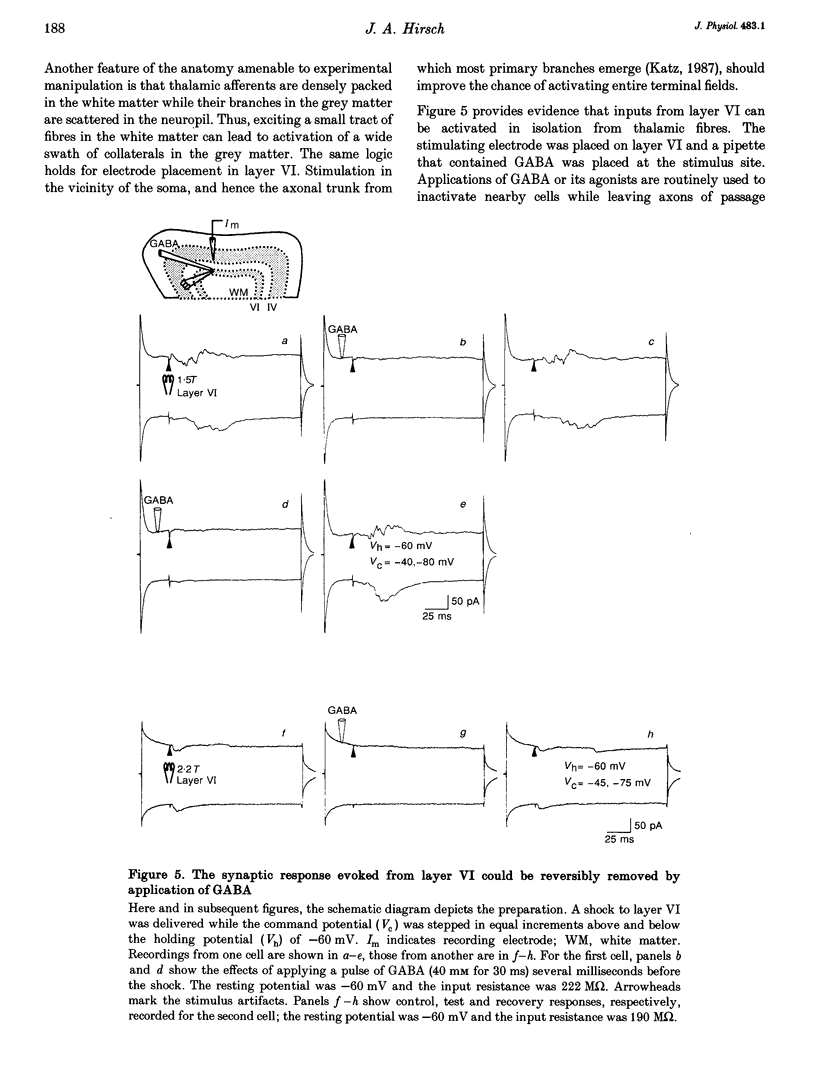
1. Whole-cell patch recording were made with dye-filled electrodes from layer IV in slices of the ferret striate cortex. Projections from the thalamus and layer VI provide most of the extralaminar input to layer IV. Interactions between these two pathways are thought to play a role in the generation of suppressive non-linearities such as end-inhibition. Thus, synaptic responses evoked by stimulating each pathway individually were compared with those produced by activating both projections together. 2. Spiny stellate cells are the majority population in layer IV and were the most frequently patched neurons (n = 23); all fired adapting trains of large, fast action potentials. About half of those tested (n = 13) were progressively inhibited by strengthening the stimulus to layer VI, while the rest became more excited. For the former, the response evoked by stimulating both pathways in coincidence was often more hyperpolarizing than would have been predicted by summing the responses to either projection alone (n = 4). Hence, the inputs from the thalamus and layer VI are integrated by circuits that can produce strong and non-linear inhibition. 3. Recordings from various basket cells, which are inhibitory, have provided a first view of the suppressive circuits in layer IV (n = 5). Two cells were excited by stimulation of both pathways. The remaining three cells were mainly excited by activation of thalamic afferents but were largely inhibited by stimulation of layer VI. Thus, inhibition seen at the level of the spiny stellate cells could result from two mechanisms operating via presynaptic smooth cells: convergent excitation provided by both ascending pathways on the one hand, and a push-pull relationship between pathways on the other.
Full text
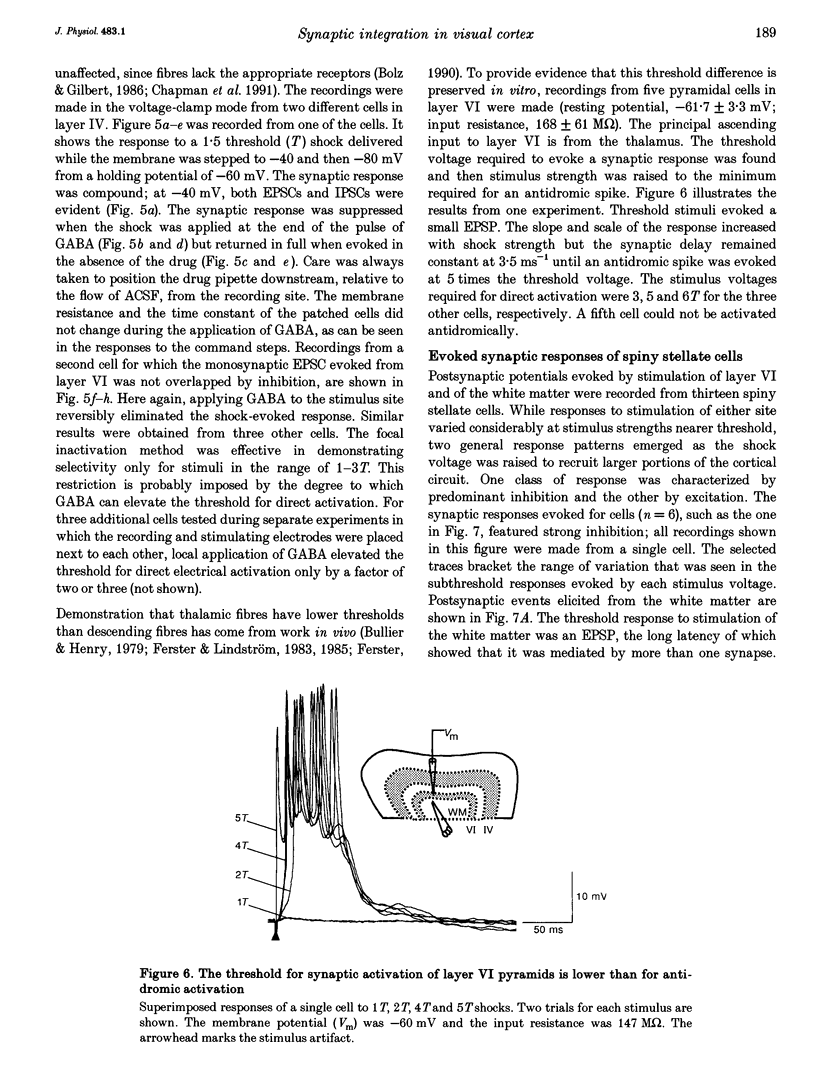
PDF
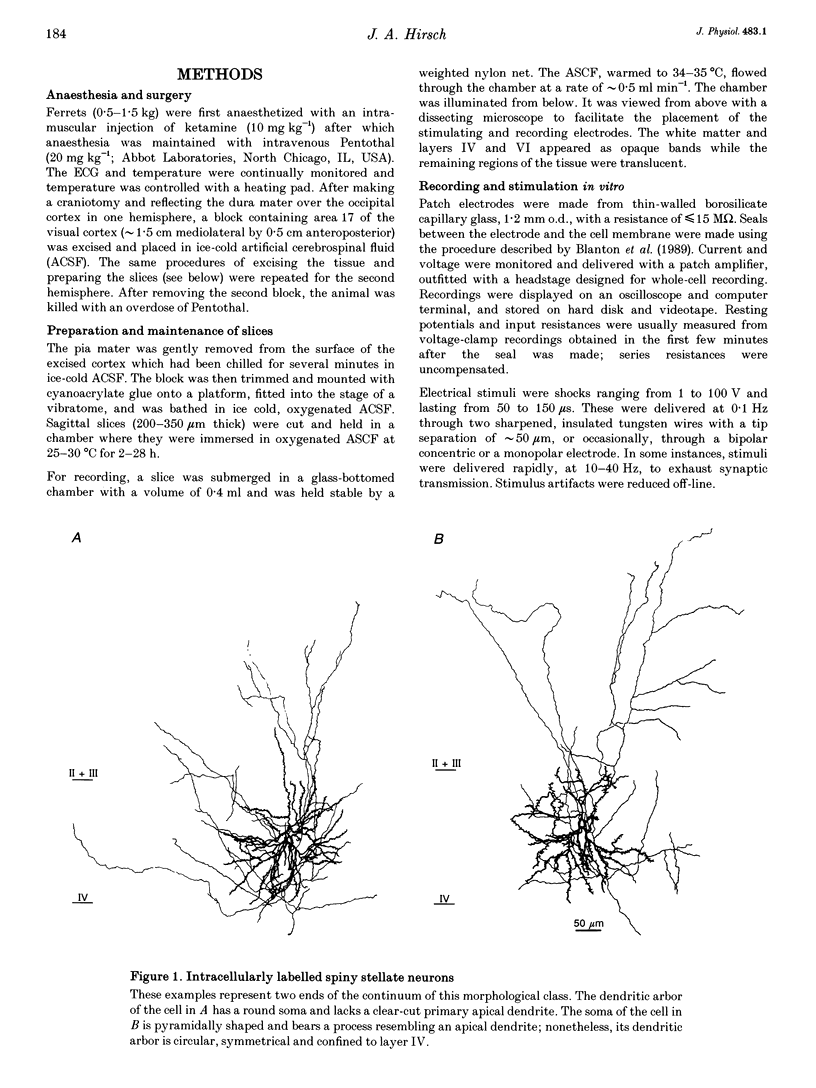




Images in this article
Selected References
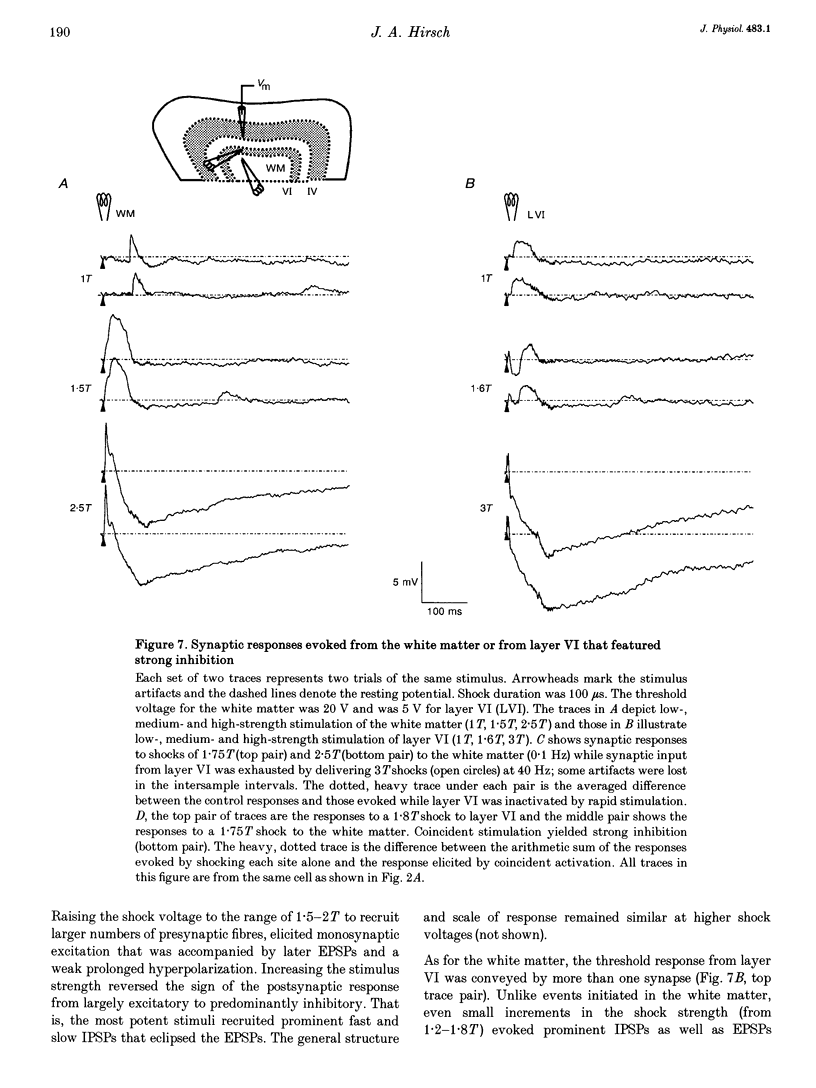
These references are in PubMed. This may not be the complete list of references from this article.
- Amitai Y., Friedman A., Connors B. W., Gutnick M. J. Regenerative activity in apical dendrites of pyramidal cells in neocortex. Cereb Cortex. 1993 Jan-Feb;3(1):26–38. doi: 10.1093/cercor/3.1.26. [DOI] [PubMed] [Google Scholar]
- Berman N. J., Douglas R. J., Martin K. A., Whitteridge D. Mechanisms of inhibition in cat visual cortex. J Physiol. 1991;440:697–722. doi: 10.1113/jphysiol.1991.sp018731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton M. G., Lo Turco J. J., Kriegstein A. R. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989 Dec;30(3):203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bolz J., Gilbert C. D. Generation of end-inhibition in the visual cortex via interlaminar connections. 1986 Mar 27-Apr 2Nature. 320(6060):362–365. doi: 10.1038/320362a0. [DOI] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Laminar distribution of first-order neurons and afferent terminals in cat striate cortex. J Neurophysiol. 1979 Sep;42(5):1271–1281. doi: 10.1152/jn.1979.42.5.1271. [DOI] [PubMed] [Google Scholar]
- Chagnac-Amitai Y., Connors B. W. Synchronized excitation and inhibition driven by intrinsically bursting neurons in neocortex. J Neurophysiol. 1989 Nov;62(5):1149–1162. doi: 10.1152/jn.1989.62.5.1149. [DOI] [PubMed] [Google Scholar]
- Chapman B., Stryker M. P. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci. 1993 Dec;13(12):5251–5262. doi: 10.1523/JNEUROSCI.13-12-05251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B., Zahs K. R., Stryker M. P. Relation of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. J Neurosci. 1991 May;11(5):1347–1358. doi: 10.1523/JNEUROSCI.11-05-01347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. J., Martin K. A., Whitteridge D. Selective responses of visual cortical cells do not depend on shunting inhibition. Nature. 1988 Apr 14;332(6165):642–644. doi: 10.1038/332642a0. [DOI] [PubMed] [Google Scholar]
- Dreher B. Hypercomplex cells in the cat's striate cortex. Invest Ophthalmol. 1972 May;11(5):355–356. [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
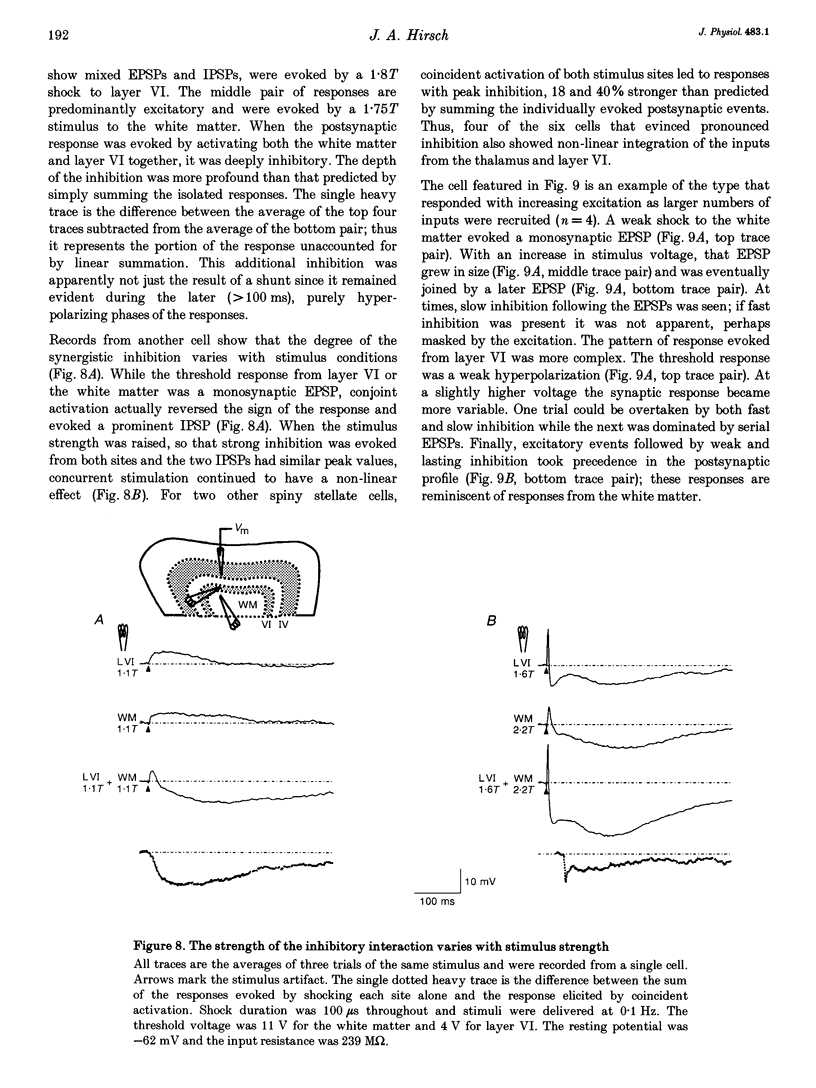
- Ferster D., Jagadeesh B. EPSP-IPSP interactions in cat visual cortex studied with in vivo whole-cell patch recording. J Neurosci. 1992 Apr;12(4):1262–1274. doi: 10.1523/JNEUROSCI.12-04-01262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D., Lindström S. An intracellular analysis of geniculo-cortical connectivity in area 17 of the cat. J Physiol. 1983 Sep;342:181–215. doi: 10.1113/jphysiol.1983.sp014846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D., Lindström S. Synaptic excitation of neurones in area 17 of the cat by intracortical axon collaterals of cortico-geniculate cells. J Physiol. 1985 Oct;367:233–252. doi: 10.1113/jphysiol.1985.sp015822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. X- and Y-mediated synaptic potentials in neurons of areas 17 and 18 of cat visual cortex. Vis Neurosci. 1990 Feb;4(2):115–133. doi: 10.1017/s0952523800002285. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Martin K. A., Whitteridge D. Innervation of cat visual areas 17 and 18 by physiologically identified X- and Y- type thalamic afferents. I. Arborization patterns and quantitative distribution of postsynaptic elements. J Comp Neurol. 1985 Dec 8;242(2):263–274. doi: 10.1002/cne.902420208. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve K. L., Sillito A. M. A re-appraisal of the role of layer VI of the visual cortex in the generation of cortical end inhibition. Exp Brain Res. 1991;87(3):521–529. doi: 10.1007/BF00227077. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirsch J. A., Gilbert C. D. Synaptic physiology of horizontal connections in the cat's visual cortex. J Neurosci. 1991 Jun;11(6):1800–1809. doi: 10.1523/JNEUROSCI.11-06-01800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K., Armstrong W. E. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods. 1988 Aug;25(1):1–11. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Humphrey A. L., Sur M., Uhlrich D. J., Sherman S. M. Projection patterns of individual X- and Y-cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol. 1985 Mar 8;233(2):159–189. doi: 10.1002/cne.902330203. [DOI] [PubMed] [Google Scholar]
- Hübener M., Schwarz C., Bolz J. Morphological types of projection neurons in layer 5 of cat visual cortex. J Comp Neurol. 1990 Nov 22;301(4):655–674. doi: 10.1002/cne.903010412. [DOI] [PubMed] [Google Scholar]
- Katz L. C. Local circuitry of identified projection neurons in cat visual cortex brain slices. J Neurosci. 1987 Apr;7(4):1223–1249. doi: 10.1523/JNEUROSCI.07-04-01223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol. 1993 Feb;69(2):416–431. doi: 10.1152/jn.1993.69.2.416. [DOI] [PubMed] [Google Scholar]
- Kisvárday Z. F., Martin K. A., Whitteridge D., Somogyi P. Synaptic connections of intracellularly filled clutch cells: a type of small basket cell in the visual cortex of the cat. J Comp Neurol. 1985 Nov 8;241(2):111–137. doi: 10.1002/cne.902410202. [DOI] [PubMed] [Google Scholar]
- LeVay S., McConnell S. K., Luskin M. B. Functional organization of primary visual cortex in the mink (Mustela vison), and a comparison with the cat. J Comp Neurol. 1987 Mar 15;257(3):422–441. doi: 10.1002/cne.902570310. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Somogyi P., Whitteridge D. Physiological and morphological properties of identified basket cells in the cat's visual cortex. Exp Brain Res. 1983;50(2-3):193–200. doi: 10.1007/BF00239183. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Connors B. W., Lighthall J. W., Prince D. A. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985 Oct;54(4):782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McGuire B. A., Hornung J. P., Gilbert C. D., Wiesel T. N. Patterns of synaptic input to layer 4 of cat striate cortex. J Neurosci. 1984 Dec;4(12):3021–3033. doi: 10.1523/JNEUROSCI.04-12-03021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
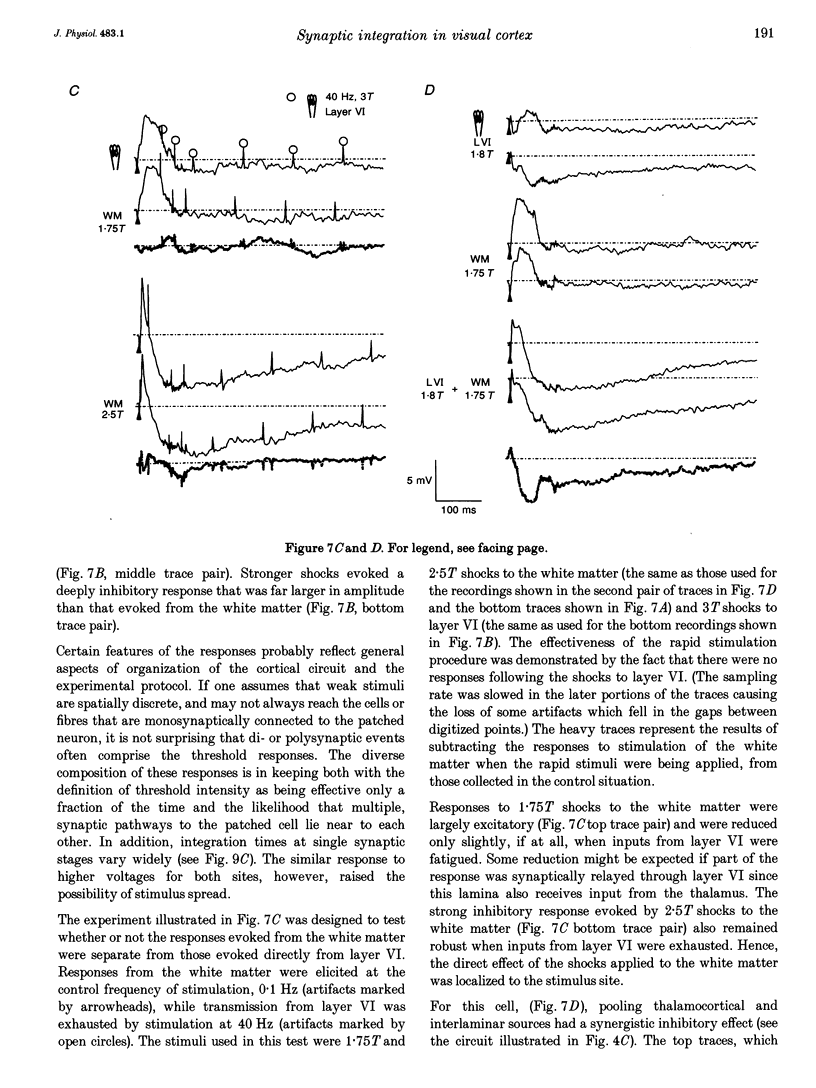
- Miles R., Wong R. K. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J Physiol. 1984 Nov;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Marie R. L., Peters A. The morphology and synaptic connections of spiny stellate neurons in monkey visual cortex (area 17): a Golgi-electron microscopic study. J Comp Neurol. 1985 Mar 8;233(2):213–235. doi: 10.1002/cne.902330205. [DOI] [PubMed] [Google Scholar]
- Sherk H., LeVay S. The visual claustrum of the cat. III. Receptive field properties. J Neurosci. 1981 Sep;1(9):993–1002. doi: 10.1523/JNEUROSCI.01-09-00993.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Stern P., Edwards F. A., Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol. 1992 Apr;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoney S. D., Jr, Thompson W. D., Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol. 1968 Sep;31(5):659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]