Abstract
In the past decade, there have been substantial changes in diagnostic nomenclature. This study investigated sex differences in attention-deficit/hyperactivity disorder (ADHD) symptom severity based on Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV, DSM-IV(TR), and DSM-5 criteria, separating rating scale and clinical interview data in children and adults with ADHD. PubMed, PsycINFO, and Scopus were searched for published studies (1996–2021) reporting severity of attention, and hyperactivity/impulsivity in males and females. We compared data: (1) across the entire lifespan aggregating rating scale and clinical interview data (51 studies), (2) drawing solely on rating scale data (18 studies), and (3) drawing solely on clinical interview data (33 studies). Fifty-two studies met inclusion criteria comparing data for females (n = 8423) and males (n = 9985) with ADHD across childhood and/or adulthood. In total, 15 meta-analyses were conducted. Pooled data across the lifespan aggregating both rating scale and clinical diagnostic interview data, showed males had significantly more severe hyperactivity/impulsivity symptoms than females. Rating scale data were similar; boys had significantly more severe hyperactivity/impulsivity than girls. In adulthood, men were rated to have significantly more severe inattention than women with no difference in the hyperactivity/impulsivity dimension. All significant differences were of small effect size. No significant sex differences in the severity of symptoms emerged for clinical interview data for children or adults, in contrast. Possible reasons for the discrepancy in findings between rating scales and clinical diagnostic interviews are discussed.
Keywords: attention-deficit/hyperactivity disorder (ADHD), boys, females, girls, males, men, meta-analysis, sex differences, systematic review, women
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is characterized by inattention and/or hyperactivity/impulsivity (HI) (APA, 2022; WHO, 2019). Three primary presentations are described as predominantly inattentive, hyperactive/impulsive, and combined symptom presentations. To meet the diagnostic thresholds, symptoms must persist over time, be pervasive across situations, and cause significant impairment (Asherson, 2016).
The overall prevalence of ADHD is estimated to be 7.1% in children (Thomas, Sanders, Doust, Beller, & Glasziou, 2015) and 2.5–5% in adults (Simon, Czobor, Bálint, Mészáros, & Bitter, 2009; Willcutt, 2012). A preponderance of males with ADHD is widely recognized both in clinical samples where male/female ratios range from 3:1 to 16:1 (Nøvik et al., 2006) and in community samples where the ratio of 3:1 is reported (Willcutt, 2012).
Empirical research reports heterogeneity over time in the symptom presentation for both sexes. Epidemiological samples identify that the hyperactive/impulsive subtype predominates in young children, whereas the inattentive subtype is the more common presentation for adolescents and adults (Simon et al., 2009; Willcutt, 2012). In contrast, studies of clinical samples identify a greater prevalence of combined-type ADHD, perhaps reflecting that individuals of greater severity in their presentation are more likely to be referred for diagnosis (Du Rietz et al., 2016; Larsson, Dilshad, Lichtenstein, & Barker, 2011; Michielsen et al., 2012).
To date, there have been two meta-analyses of sex effects reporting the severity of ADHD symptoms. The first, published in 1997, yielded 18 studies (including one unpublished dissertation) comparing sex differences in children with ADHD (the search criteria were for children aged 13 years and younger) (Gaub & Carlson, 1997). Although published in 1997, all the included studies were conducted prior to 1992 and therefore were prior to the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) nomenclature. Only five studies reported outcomes for symptoms of inattention, nine for hyperactivity, and three for impulsivity. Girls were reported to have significantly lower symptoms of hyperactivity and inattention; there were no significant differences in symptoms of impulsivity. Furthermore, girls had greater intellectual impairments and lower ratings of externalizing and internalizing problems.
The second meta-analysis (Gershon, 2002) imposed no age limit, included 38 studies (including unpublished studies) and two research reports. Few studies included adult samples or data reporting ADHD subtypes. Females with ADHD were found to have less severe symptoms of inattention, hyperactivity, and impulsivity than males with ADHD (low effect sizes). Females had significantly fewer externalizing problems, more internalizing problems, and a lower IQ than males. No significant sex differences emerged on measures of academic achievement or social functioning.
Gershon's (2002) study also separated effect sizes from clinical and community samples. Using community samples only, females were rated as significantly less severe than males on all the dependent measures, particularly regarding hyperactivity (large effect size). The author recommended that future studies include community-based samples, as well as clinical samples.
These early reviews suggest that girls have substantially less severe ADHD symptoms than boys; however, they are subject to methodological limitations (including limited power, a lack of adult studies, and the use of unpublished non-peer-reviewed data). Nevertheless, the reported sexual dimorphism in the prevalence and presentation of ADHD has fueled speculation over possible contributory factors (including genetic, endocrine, and psychosocial) (Greven, Richards, & Buitelaar, 2018; Hinshaw, Owens, Sami, & Fargeon, 2006).
ADHD is now recognized to be a condition affecting many individuals over their lifespan. In parallel, there has been an exponential increase in international research reporting the prevalence of ADHD, associated impairments, and long-term outcomes (Arnold, Hodgkins, Caci, Kahle, & Young, 2015; Hinshaw et al., 2006; Hodgkins et al., 2012; Shaw et al., 2012). This research has resulted in substantial revisions to the diagnostic criteria since the early DSM-II, DSM-III, and DSM-IIIR studies included by Gaub and Carlson (1997) and extended to include some DSM-IV studies by Gershon (2002).
In 2012, Willcutt (2012) conducted a meta-analytic review of the literature published between 1994 and 2010 investigating the prevalence rates of ADHD subtypes in children and adults diagnosed using the DSM-IV criteria. Unlike the previous studies, the primary aim was to investigate prevalence rates and they were generally similar across the rating sources for children (parent, teacher, diagnostic procedure: 5.9–7.1%) and self-reporting in young adults (5%). The diagnostic outcomes, however, differed between the sexes. A significantly greater proportion of females than males met the diagnostic criteria for the inattentive subtype. In contrast, males were more likely than females to meet the diagnostic criteria for the combined type.
It has become clear that we need to develop better insights into the presentation and difficulties of ADHD in girls and women (Willcutt, 2012). Our understanding has been hampered, however, because the existing evidence predominantly draws on male samples (Gershon, 2016; Willcutt, 2012). It is unknown to what extent ADHD is missed or misdiagnosed in females. A key question is, does the presentation of ADHD symptoms differ between boys and girls in childhood and/or in their adulthood? A prevalence study does not directly answer this question, whereas a study investigating sex differences in the severity of symptoms is more informative because it provides a dimensional perspective rather than focusing on the outcomes of a categorical threshold (Kraemer, Noda, & O'Hara, 2004).
In the past decade, there have been substantial changes in the diagnostic nomenclature and this study conducts an updated and contemporary systematic review of the data reporting severity of ADHD symptoms which is not present in the current literature. The unique features of the present study are the reliance on more refined symptom criteria (using the DSM-IV [APA, 1994], DSM-IV(TR) [APA, 2000], and DSM-5 [APA, 2022]), the separation of clinical diagnostic assessment data from rating scale data, and the inclusion of a broader age range of adults.
Given the exploratory nature of the study, a priori hypotheses were not formulated. Rather, we examined the following research question: Does the severity of ADHD symptoms differ between boys and girls in childhood (aged <18) and/or in adulthood?
Materials and methods
The main objective of the analysis was to compare the severity of ADHD between females and males. The secondary objective was to evaluate possible gender differences in children and in adults. The severity of ADHD was stated as the core ADHD symptoms score (inattention, hyperactivity, impulsivity) based on established rating scales or diagnostic criteria scores.
We followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Page et al., 2021). The protocol of the present study was registered on PROSPERO (registration number: CRD42020103830). The protocol on PROSPERO also includes a review question on the difference in ADHD outcomes between males and females which will be published in another paper. We also followed the MOOSE guidelines for reporting meta-analysis of observational studies (Stroup et al., 2000).
We searched the PubMed, PsycINFO, and Scopus literature databases to include articles published through 28 May 2021. The specific search terms related to (1) attention-deficit and hyperactivity disorder and (2) either gender or sex (the articles included studies that compared males/females + only male + only female) were applied, including ‘Attention Deficit Hyperactivity Disorder’, ‘ADHD’, ‘attention deficit disorder’, ‘disturbance of activity and attention’, ‘TDAH’, ‘hyperkine’, ‘Hyperkinetic disorder’, and ‘Hyperkinetic syndrome’. Comparator terms included ‘female’, ‘girl’, ‘woman’, ‘women’, ‘mother’, ‘male’, ‘boy’, ‘men’, ‘man’, ‘father’, ‘gender’, and ‘sex’. Term indexing using free Boolean operators was employed. No age restrictions were applied. The entire search strings are provided in online Supplementary File S1.
Study selection
The inclusion criteria were peer-reviewed articles written in the English language; documentation of empirical, primary research regarding ADHD symptoms assessed using the DSM-IV, DSM-IV-TR, or DSM-5 criteria; and results reported separately for males and females. As the diagnostic criteria for ADHD were substantially different before DSM-IV, we excluded the studies where patients were diagnosed using earlier versions of DSM (i.e. DSM-III and earlier versions).
The search results were imported from the databases to COVIDENCE. Covidence.org was used to store the search results, identify duplicates, and track screening decisions. After removing duplicate articles, a first round of screening titles and abstracts was used to eliminate the articles that did not meet the inclusion criteria. A second round of screening was carried out by reading the complete text of the articles. Articles that reported data from the same dataset, that focused on conditions other than ADHD, or that presented aggregated data for females and males were excluded.
For both rounds of screening, any two of the following reviewers, including two psychiatrists, four clinical psychologists, a neuroscientist, and a medical student (O.K., J.K., A.S.-K., J.H., B.G., N.S., K.C., and/or U.E.Y.) independently evaluated the potential articles for inclusion. Disagreements were resolved by discussion with a lead author (O.K.). When articles reported data from the same dataset, data from the most recent and biggest dataset were considered.
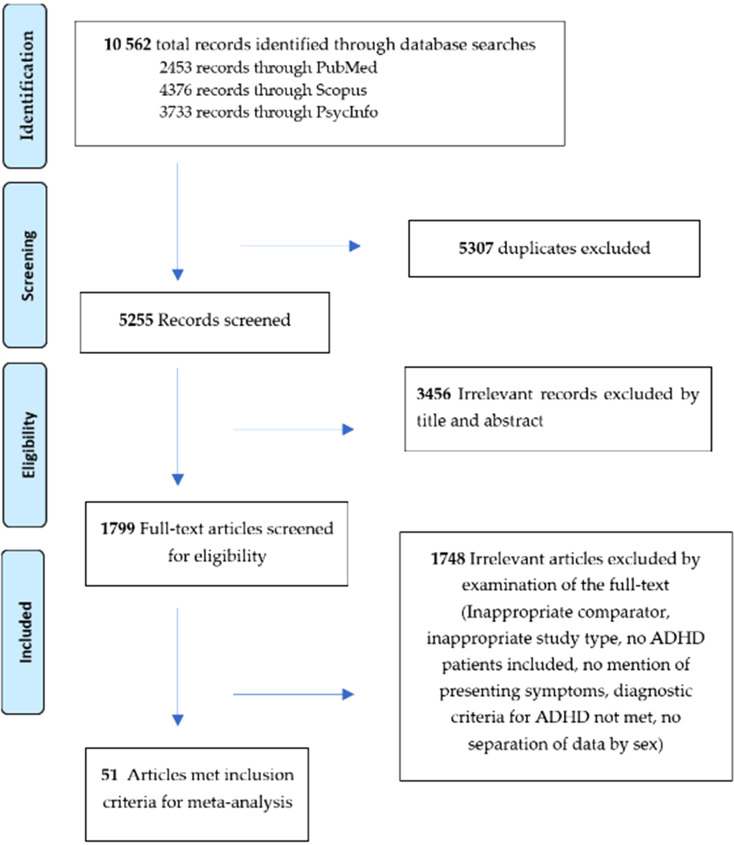
Figure 1 shows the PRISMA flow chart of the articles yielded by the initial search and the article screening process.
Figure 1.
PRISMA flow chart of article disposition.
Data extraction
The following data were extracted into an Excel spreadsheet by any two of the following reviewers independently (O.K., A.S.-K., B.G., N.S., K.C., J.H., and/or U.E.Y.): first author, year of publication (we used this information instead of the year of data collection because the latter was missing in 43.1% of the studies); geographic location; sample size; population (adults v. youths); study setting (clinic v. community); participant characteristics (age, sex); rater source (parent-report, self-report); assessment tools (e.g. rating scales, clinical diagnostic assessment tools such as interviews based on DSM-IV, DSM-IV-TR; K-SADS; DISC), and diagnostic criteria (DSM-IV, DSM-IV-TR, or DSM-5) and the scores and standard deviations from the assessment tool as the severity of ADHD measure. All rating scales to measure severity of ADHD in the included articles were established and valid measures in the field. For children, we included parent-rated data only for consistency across the studies as this is the most common source of reporting for children with ADHD. Child and adult studies were distinguished by the mean age of the sample reported in the study, the cut-off being <17 for children (as applied by DSM-5). If neither the mean nor median scores of the data were published, a mid-range value was calculated as ((high age − low age)/2) + low age = mid-range age (Cochran, 1954; Viechtbauer, 2010).
Statistical analysis
We used Jamovi statistical software (version 1.8) for analysis (The Jamovi Project, 2022). The main planned meta-analysis combining all 52 selected studies was performed to determine if females differ from males in the severity of ADHD throughout their lifespan. Subgroup analyses were performed to determine (1) sex differences in the severity of ADHD during childhood and adulthood and (2) differences when severity was determined using clinical interviews (diagnostic) v. rating scales (not diagnostic). The standardized mean difference was used as the outcome variable in the analysis and is used in the present study as an effect size. The data were fitted with a random effects model, chosen based on the variability of individual studies regarding the diagnostic criteria and other sample characteristics, as recommended in these cases (e.g. Borenstein, Hedges, Higgins, & Rothstein, 2021) and consistent with previous meta-analyses in the field (e.g. Cortese et al., 2018). Forest plots were generated to visualize the outcome variables: differences in severity of inattention and hyperactivity/impulsivity. The restricted maximum-likelihood estimator was used to estimate the degree of heterogeneity (i.e. tau2) (Arcia & Conners, 2007). Along with the tau2 estimate, the Q-test for heterogeneity (Ebejer et al., 2013) and the I2 statistic indicating the percentage of heterogeneity due to true heterogeneity were calculated. Heterogeneity represents to what extent the results of the studies are consistent and the variation in study outcomes between the studies. An I2 value higher than 75% represented high heterogeneity whereas an I2 value lower than 25% represented small heterogeneity. We used I2 and Q to explore the heterogeneity of the studies.
A publication bias analysis was conducted, and the Funnel plots were inspected for inattention, hyperactivity/impulsivity, and combined presentations of ADHD. Egger's test was conducted (Egger, Davey Smith, Schneider, & Minder, 1997). Egger's regression with significance was given in each meta-analytic result. Fifteen separate meta-analyses were conducted.
Sensitivity analysis of the main analysis in total sample data was performed excluding those articles reporting ‘poor-quality’ studies, as determined in the risk of bias assessment.
Risk of bias assessment
The risk of bias and methodological quality of the included observational studies in the systematic review and meta-analysis were assessed using the Newcastle–Ottawa Scale for observational studies and the modified version of the Newcastle–Ottawa Scale for cross-sectional studies assessing representativeness, sample size, respondents and non-respondents, ascertainment of the presentation of ADHD, comparability of subjects, assessment of outcome, and quality of the statistics reported (seven items with three subscales and with a total maximum score of 9) (Wells et al., 1997). Any two of the authors independently assessed the quality of the studies (O.K., A.S.-K., B.G., N.S., K.C., J.H., and/or U.E.Y.). The Newcastle–Ottawa Scale for case–control or cohort studies consists of eight items with three subscales, with a total maximum score of 9. A standard criterion for a high-quality study has not yet been universally established. In the present study, we considered a score ⩾7 to indicate a high-quality study, 5–6 a fair-quality study, and ⩽4 a poor-quality study. National Institute of Health assessment tool for controlled intervention studies was used for the one controlled study. The results of this classification and individual scoring can be found in online Supplementary File S2.
Results
From a total of 10 562 potentially eligible references, 51 manuscripts were retained consisting of 52 studies (two independent studies were drawn from the study by DuPaul et al., 2001) and comprised of a total of 18 408 participants (8423 females and 9985 males). The studies were separated into groups of articles that present data for inattention, hyperactivity/impulsivity, and combined domains. For clarity and succinctness, Table 1 provides a summary of the findings presented in Figs 2–16 representing 15 separate meta-analyses. See online Supplementary Files S3 and S4 for a summary of the included child (Bianchini et al., 2013; Bröring, Rommelse, Sergeant, & Scherder, 2008; Castellanos et al., 2002; Chen et al., 2008; DuPaul et al., 1998, 2016; El Hamrawy, El Sayed, Soltan, & Abd El-Gwad, 2017; Fliers et al., 2013; Gabel, Schmitz, & Fulker, 1996; Gadow, Sprafkin, & Nolan, 2001; Ghanizadeh, Mohammadi, & Moini, 2008; Graetz, Sawyer, Baghurst, & Ettridge, 2006; Gudjonsson, Sigurdsson, Adalsteinsson, & Young, 2013; Hartung et al., 2002; Hellström, Wagner, Nilsson, Leppert, & Åslund, 2017; Hogue, Dauber, Lichvar, & Spiewak, 2014; Kean et al., 2017; Kim et al., 2018; Lahey et al., 2007; Lefler, Hartung, Bartgis, & Thomas, 2015; Major, Martinussen, & Wiener, 2013; Nøvik et al., 2006; Øie, Hovik, Andersen, Czajkowski, & Skogli, 2018; Paavonen et al., 2009; Riddle et al., 2013; Rosch, Dirlikov, & Mostofsky, 2015; Serra-Pinheiro, Mattos, & Angélica Regalla, 2008; Seymour, Mostofsky, & Rosch, 2016; Sihvola et al., 2011; Skogli, Teicher, Andersen, Hovik, & Øie, 2013; Thorell & Rydell, 2008; Tseng et al., 2012; Waschbusch & King, 2006; Willcutt & Pennington, 2000; Yoo et al., 2004) and adult (Amador-Campos, Gómez-Benito, & Ramos-Quiroga, 2014; DuPaul et al., 2001a; DuPaul et al.,, 2001b; Ebejer et al., 2013; Edebol, Helldin, & Norlander, 2013; Fedele, Hartung, Canu, & Wilkowski, 2010; Fredriksen et al., 2014; Gomez, 2016; Jaconis et al., 2016; Levitan, Jain, & Katzman, 1999; Millenet et al., 2018; Mosalanejad, Mosalanejad, & Lashkarpour, 2013; Murphy & Barkley, 1996; Onnink et al., 2014; Park & Park, 2016; Retz-Junginger, Rösler, Jacob, Alm, & Retz, 2010; Robison et al., 2008) studies, respectively.
Table 1.
Summary of findings for sex differences
| Core symptoms | Figure | Sample | Number of studies | Total n | Measure | z-Value | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Inattention | 2 | Total | 48 | 14 601 | All + | −1.37 | −0.2 | 0.04 |
| Hyperactivity/impulsivity | 3 | Total | 43 | 12 874 | All + | −2.50* | −0.27 | −0.03 |
| Combined symptoms | 4 | Total | 19 | 7272 | All + | −0.21 | −0.17 | 0.14 |
| Inattention | 5 | Child | 12 | 3978 | Rating scale | −1.88 | −0.052 | 0.01 |
| Hyperactivity/impulsivity | 7 | Child | 12 | 3917 | Rating scale | −4.62*** | −0.51 | −0.21 |
| Combined symptoms | 9 | Child | 5 | 1639 | Rating scale | −1.20 | −0.43 | 0.1 |
| Inattention | 6 | Adult | 4 | 1587 | Rating scale | −3.25** | −0.28 | −0.07 |
| Hyperactivity/impulsivity | 8 | Adult | 3 | 1042 | Rating scale | 0.85 | −0.3 | 0.76 |
| Combined symptoms | 10 | Adult | 3 | 1042 | Rating scale | 0.64 | −0.39 | 0.76 |
| Inattention | 11 | Child | 23 | 4907 | Clinical interview | −0.10 | −0.18 | 0.16 |
| Hyperactivity/impulsivity | 13 | Child | 22 | 4626 | Clinical interview | −0.82 | −0.32 | 0.13 |
| Combined symptoms | 15 | Child | 6 | 1272 | Clinical interview | 0.47 | −0.11 | 0.19 |
| Inattention | 12 | Adult | 9 | 2371 | Clinical interview | 0.18 | −0.27 | 0.23 |
| Hyperactivity/impulsivity | 14 | Adult | 8 | 2175 | Clinical interview | −1.20 | −0.14 | 0.03 |
| Combined symptoms | 16 | Adult | 4 | 1468 | Clinical interview | 0.8 | −0.21 | 0.51 |
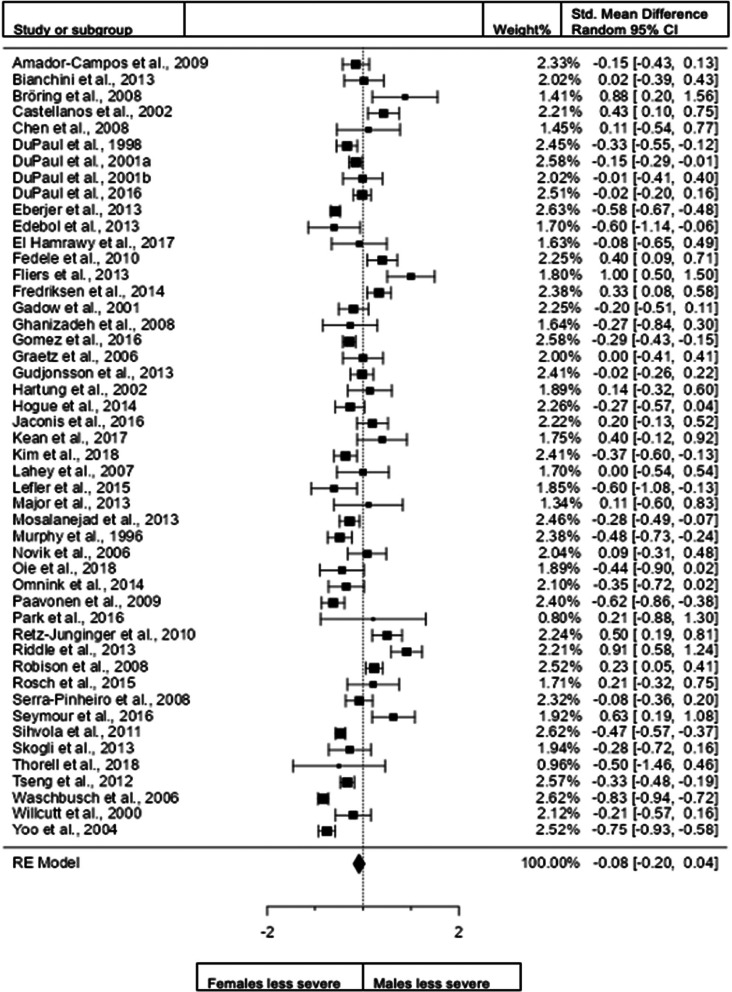
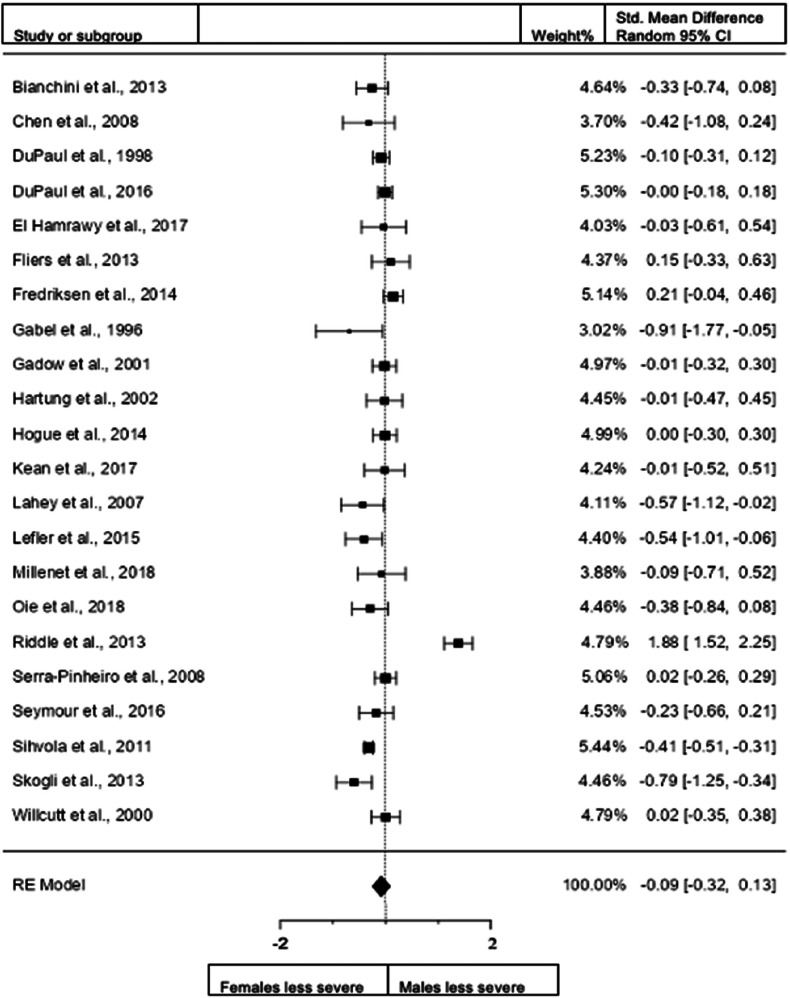
Figure 2.
Forest plot showing differences in inattention severity in males and females in the total sample (n = 48 – DuPaul et al. (2001)a and DuPaul(2001)b are different data from the same study).
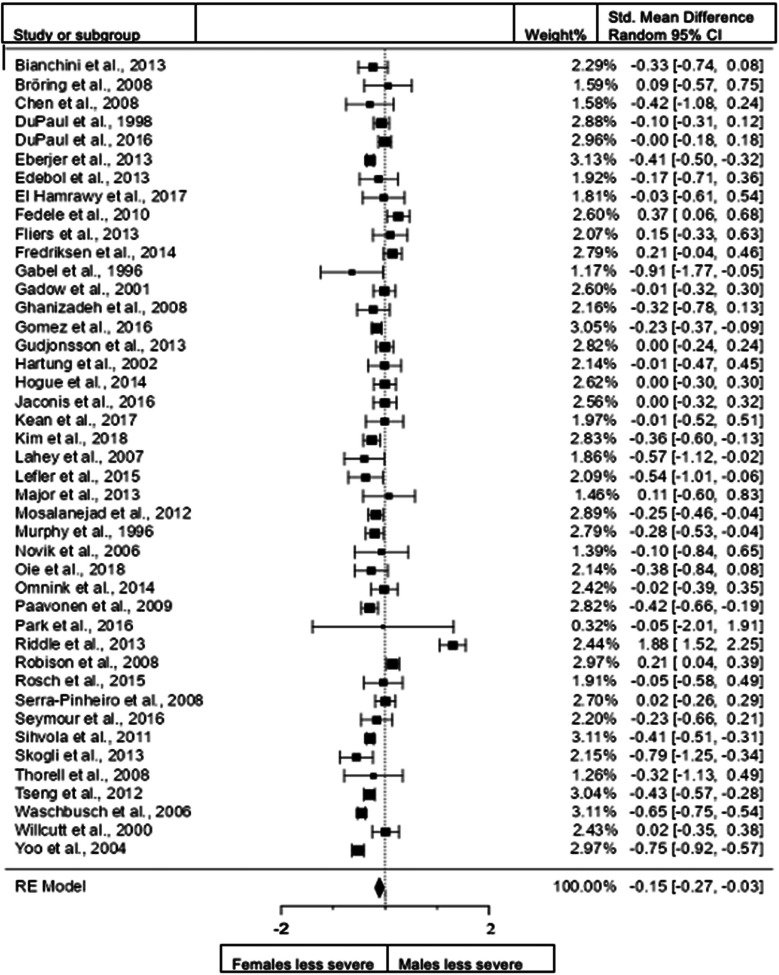
Figure 3.
Forest plot of hyperactivity/impulsivity presentation in the total sample.
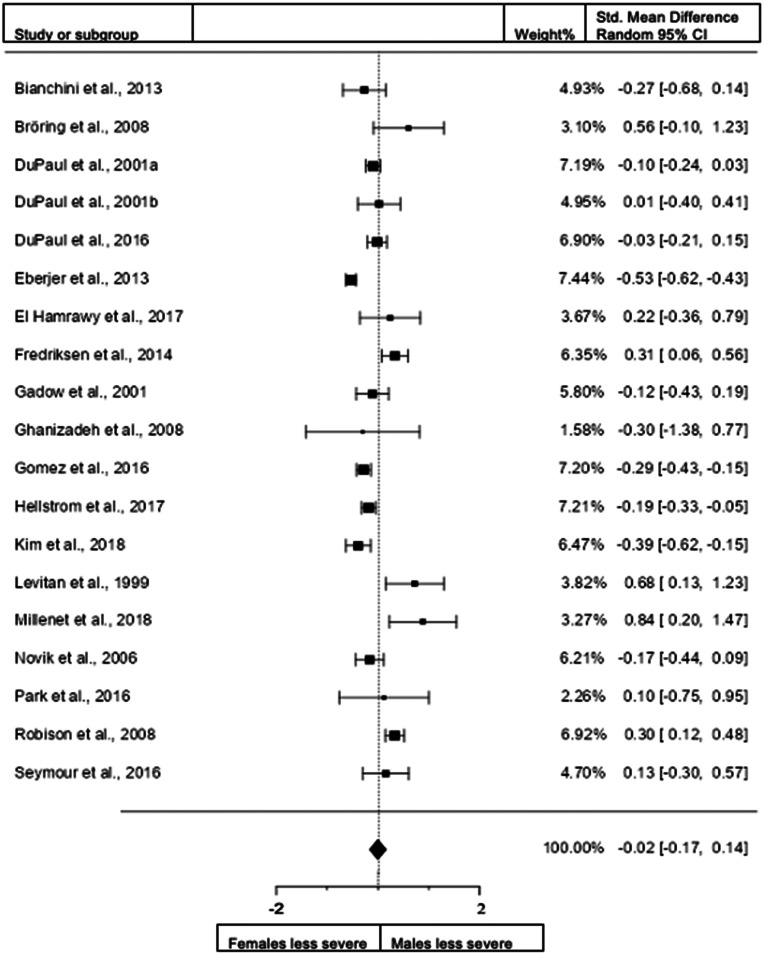
Figure 4.
Forest plot of combined hyperactivity/impulsivity and inattention presentation in the total sample. DuPaul et al. (2001)a = data from the USA; DuPaul et al.( 2001)b = data from Italy.
Figure 5.
Forest plot of inattention presentation in children from the rating scales sample.
Figure 6.
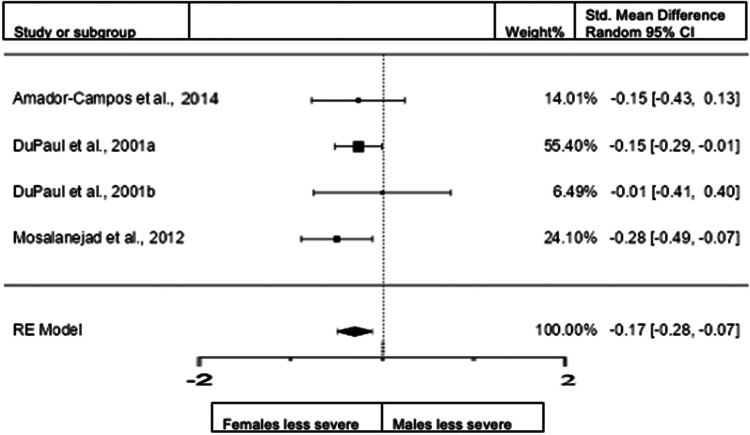
Forest plot of inattention presentation in adults from the rating scales sample.
Figure 7.
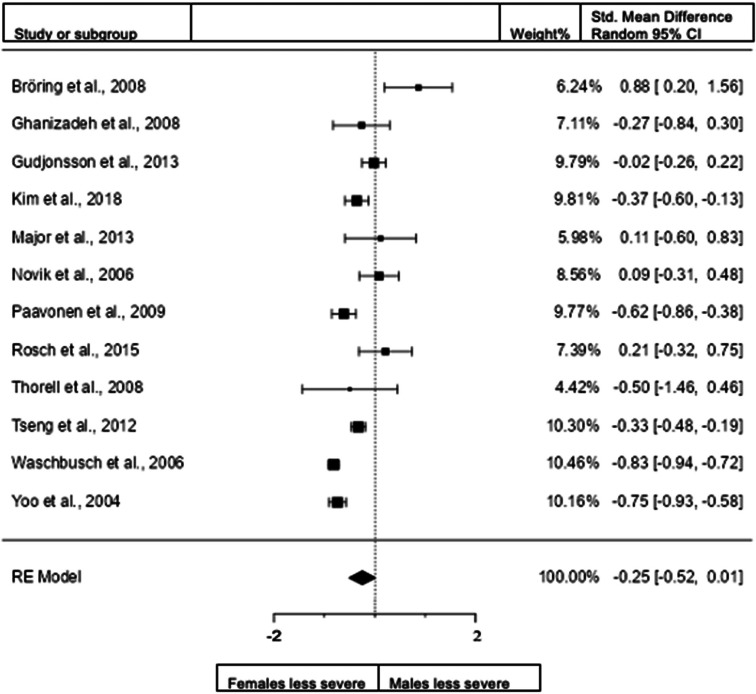
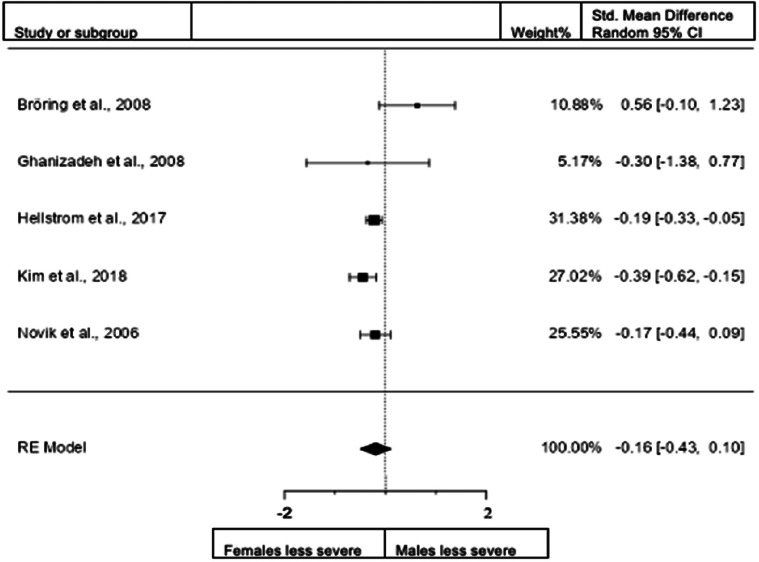
Forest plot of hyperactivity/impulsivity presentation in children from the rating scales sample.
Figure 8.
Forest plot of hyperactivity/impulsivity presentation in adults from the rating scales sample.
Figure 9.
Forest plot of combined presentation in children from the rating scales sample.
Figure 10.
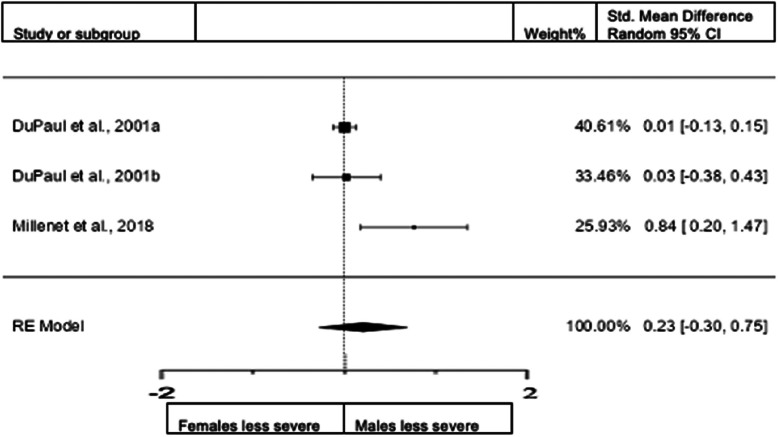
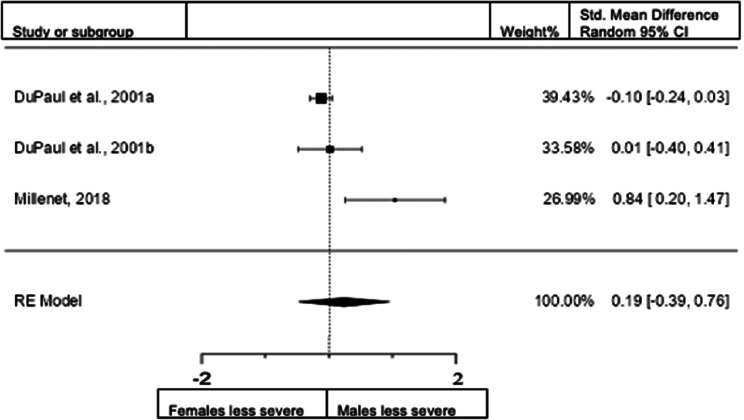
Forest plot of combined presentation in adults from the rating scales sample.
Figure 11.
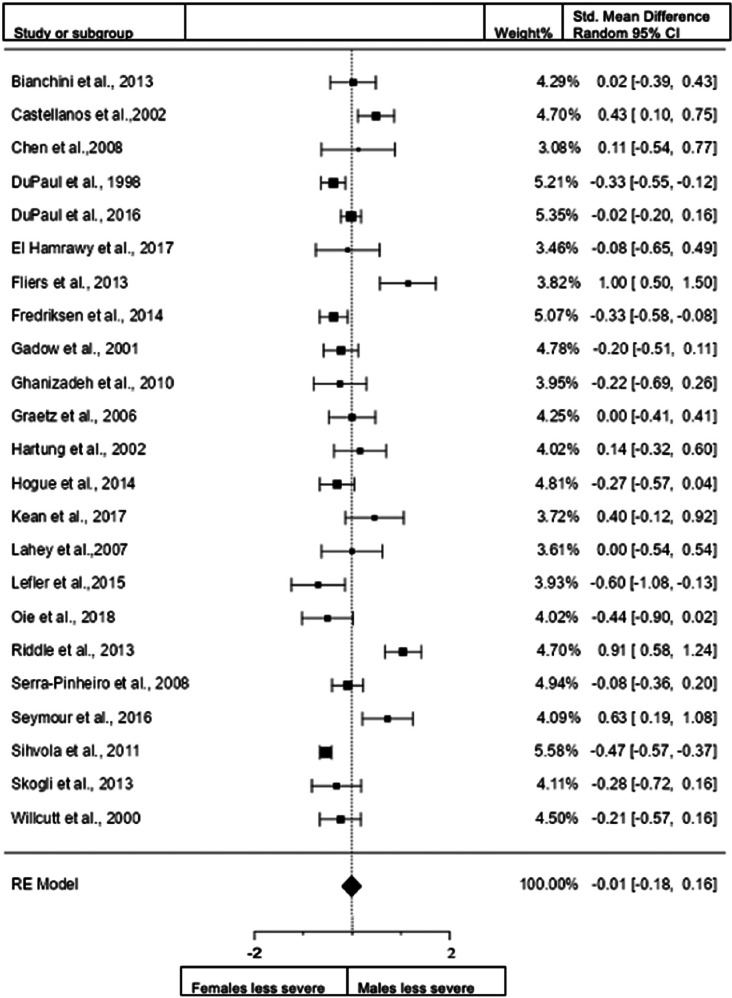
Forest plot of inattention presentation in children from the clinical diagnostic interview sample.
Figure 12.
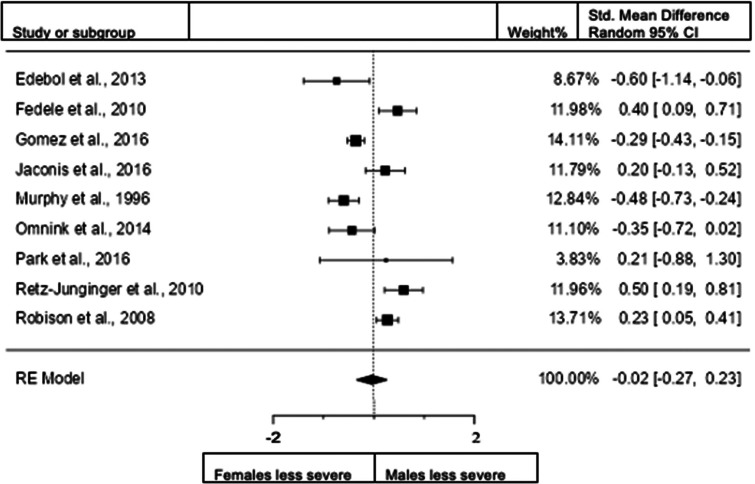
Forest plot of inattention presentation in adults from the clinical diagnostic interview sample.
Figure 13.
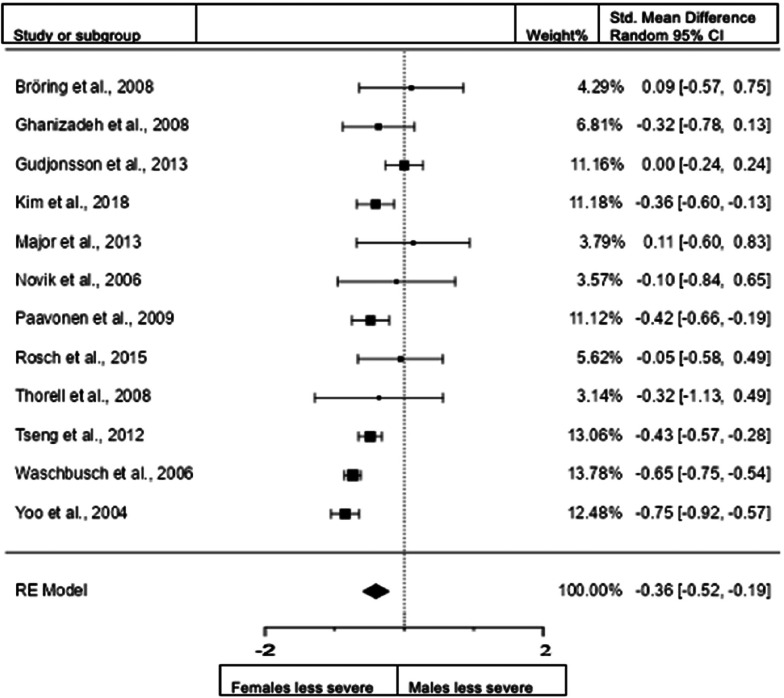
Forest plot of hyperactivity/impulsivity presentation in children from the clinical diagnostic interview sample.
Figure 14.
Forest plot of hyperactivity/impulsivity presentation in adults from the clinical diagnostic interview sample.
Figure 15.
Forest plot of combined presentation in children from the clinical diagnostic interview sample.
Figure 16.
Forest plot of combined presentation in adults from the clinical diagnostic interview sample.
Using these data, we conducted meta-analyses in three broad areas: (1) total sample data (three analyses), (2) rating scale data (six analyses), and (3) clinical interview diagnostic data (six analyses). Fifteen meta-analyses were conducted in total. The results report sex differences in the severity of symptoms of ADHD: (1) in the ‘total sample’ across the entire lifespan when aggregating rating scale and clinical diagnostic assessment data (52 studies); (2) in a ‘rating scales sample’ that we obtained by extracting the rating scale data sample (18 studies; 83.3% community-based studies), and (3) in a ‘clinical diagnostic interview sample’ which exclusively focused on studies employing clinical diagnostic assessment data in samples of child and adult study participants (33 studies; 57.6% in clinical settings).
As shown in Table 1, out of 15 meta-analyses, only three showed significant sex differences in Figs 3, 6, and 7. The most significant sex difference was found in the rating scale data for hyperactivity/impulsivity where male children exhibited significantly higher symptoms (z value = −4.62).
Meta-analyses of the total sample
For the initial analysis, no study was excluded based on any age, mode of assessment, or setting representing a sample that aggregated all data across the lifespan (i.e. including both rating scale and clinical diagnostic interview data). This was comprised of a total of 52 studies: n = 8451 male and n = 7304 female participants. The most common adult rating scales used in the studies were the Adult ADHD Rating Scale and the Wender Utah rating scale for adults. For children, the most common were the Conners’ scales, Child Behavior Checklist, Barkley Current Symptoms Scale, and the Strength and Difficulties Questionnaire. The most common clinical interviews used in the studies were reported to be based on DSM-IV and DSM-IV-TR criteria for adults and, for children, the Kiddie Schedule for Affective Disorders and Schizophrenia and the Diagnostic Interview Schedule for Children.
Sex differences in symptoms of inattention: total sample
When we included all studies both in children and adults and studies that use both diagnostic interviews and rating scales for assessment (48 studies; n = female 6890; male 7711), the overall observed standardized mean sex difference of −0.0819 (95% confidence interval [CI] −0.1988 to 0.0350) was not significant (test of overall effect: z = −1.3726, p = 0.1699). The heterogeneity data resulted as I2 = 89.9%, tau2 = 0.13, Q(47) = 471.3260, and df = 47, p < 0.001. Egger's regression value = 2.483, p = 0.013. The data are presented in Fig. 2.
Sex differences in symptoms of hyperactivity/impulsivity: total sample
When we included all studies both in children and adults and studies that use diagnostic interviews and rating scales for assessment (43 studies; n = female 6014; male 6860), the overall observed standardized mean sex differences for hyperactivity/impulsivity of −0.1489 (95% CI −0.2658 to −0.0321) was significant (z = −2.4982, p = 0.0125) indicating that females scored significantly lower than males in the domain of hyperactivity/impulsivity. The heterogeneity data resulted as I2 = 88%, tau2 = 0.11, Q(42) = 347.8804, and df = 42, p < 0.001. Egger's regression value = 0.297, p = 0.767. The data are presented in Fig. 3.
Sex differences in combined presentation: total sample
When we included all studies both in children and adults and studies that use diagnostic interviews and rating scales for assessment (19 studies; n = female 3422; male 3850), the overall observed standardized mean difference was not significant (z = −0.21, p = 0.835) with a standardized mean difference of −0.0161 (95% CI −0.1682 to 0.1360). The heterogeneity data resulted as I2 = 86%, tau2 = 0.08, Q(18) = 134.1339, and df = 18, p < 0.001. Egger's regression value = 2.231, p = 0.026. Data are presented in Fig. 4. The data of one study (DuPaul et al., 2001) conducted in New Zealand were removed because the study was an extreme outlier (this study also reported data for the USA and Italy). The data are presented in Fig. 4.
Meta-analyses of rating scale data (n = 17)
A secondary analysis of the ‘total sample’ data was performed by extracting the rating scale data across the lifespan, representing a ‘rating scales sample’. The sample consisted of a total of 17 studies (n = female 3890; male 4451).
Sex differences in inattention for children: rating scales
A total of 12 studies (n = female 1865; male 2113) were included in this analysis. The overall observed standardized mean difference was not significant (z = −1.88, p = 0.060) with −0.2531 (95% CI −0.5170 to 0.0108). The heterogeneity data resulted as I2 = 91.9%, tau2 = 0.17, Q(11) = 101.2418, and p < 0.0001. Egger's regression value = 2.075, p = 0.038. The data are presented in Fig. 5.
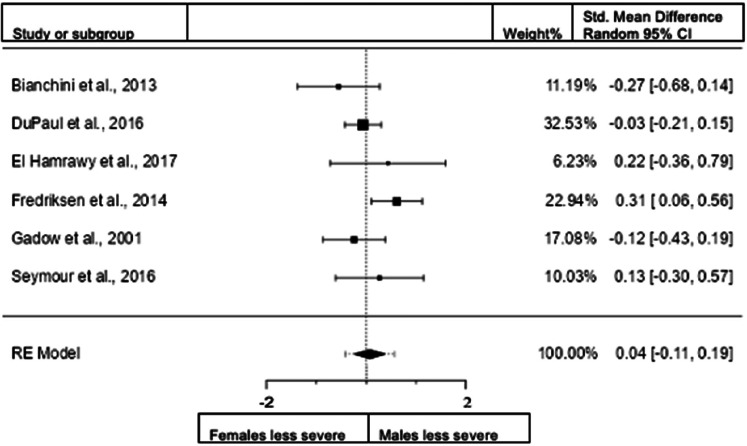
Sex differences in inattention for adults: rating scales
A total of four studies (n = 890 female; 697 male) were included in this analysis. The overall observed standardized mean difference was significant (z = −3.25, p = 0.001) with a standardized mean difference of −0.1716 (95% CI −0.2751 to −0.0682) indicating that women scored significantly lower than men in the domain of inattention. The heterogeneity data resulted as I2 = 0%, tau2 = 0, Q(3) = 1.7343, and p = 0.6293. Egger's regression value = 0.353, p = 0.724. The data are presented in Fig. 6.
Sex differences in hyperactivity/impulsivity for children: rating scales
A total of 12 studies (n = female 1867; male 2050) were included in the analysis. The overall observed standardized mean sex difference was significant (z = −4.6, p < 0.001) indicating that girls scored significantly lower than boys in the domain of hyperactivity/impulsivity. Standardized mean difference = −0.3587 (95% CI −0.5109 to −0.2065). Test of overall effect: z = −4.3170, p < 0.0001. The heterogeneity data resulted as I2 = 75.4%, tau2 = 0.05, Q(11) = 45 961, and p < 0.001. Egger's regression value = 2.574, p = 0.01. The data are presented in Fig. 7.
Sex differences in hyperactivity/impulsivity for adults: rating scales
A total of three studies (n = female 576; male 466) were included in the analysis. The overall observed standardized mean difference was not significant (z = 0.85, p = 0.3941). Standardized mean difference = 0.2287 (95% CI −0.2972 to 0.7546). Heterogeneity: I2 = 83.4%, tau2 = 0.17, Q(2) = 6.23477, and p = 0.0443. Egger's regression value = 1.433, p = 0.152. The data are presented in Fig. 8.
Sex differences in combined presentation for children: rating scales
A total of five studies (n = female 688; male 951) were included in the analysis. The overall observed standardized mean slightly favored girls exhibiting a less severe combined symptom presentation compared with boys, but this difference (−0.1629 [95% CI −0.4287 to 0.1029]) was not significant (z = −1.20, p = 0.2297). Heterogeneity: I2 = 72.4%, tau2 = 0.05, Q(4) = 7.6175, and p = 0.1066. Egger's regression value = 0.648, p = 0.517. The data are presented in Fig. 9.
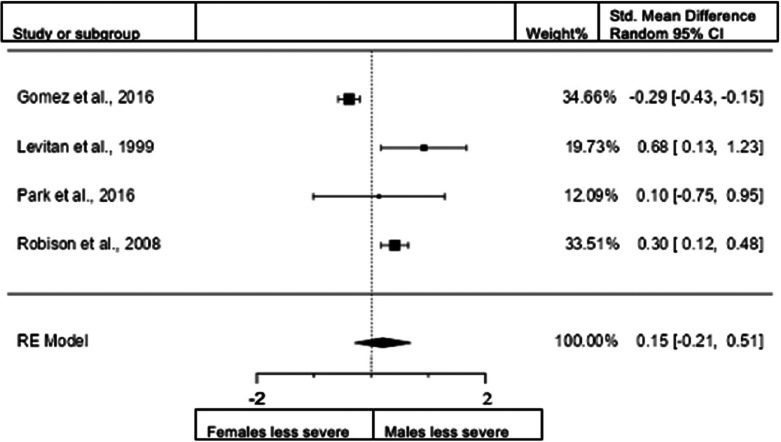
Sex differences in combined presentation for adults: rating scales
A total of three studies (n = female 576; male 466) were included in the analysis. The overall observed standardized mean difference was not significant (z = 0.64, p = 0.524). Standardized mean difference = 0.1866 (95% CI −0.3877 to 0.7609). Heterogeneity: I2 = 86%, tau2 = 0.21, Q(2) = 8.1081, and p = 0.0174. Egger's regression value = 1.791, p = 0.073. The data are presented in Fig. 10.
Meta-analyses of clinical interview diagnostic sample
This analysis exclusively analyzed data from child and adult samples obtained from clinical diagnostic assessment tools. This ‘clinical interview diagnostic sample’ consisted of 34 studies. The child studies had 2121 female and 3110 male participants with ADHD and the adult studies had 2266 female and 2290 male participants with ADHD. The most common clinical interviews used in the studies were DSM-IV and DSM-IV-TR for adults and the K-SADS and DISC for children.
Sex differences in inattention for children: clinical interview
When we included the studies in children that use clinical interview data for assessment (23 studies; n = female 2073; male 2834), the overall observed standardized mean difference was not significant. Standardized mean difference = −0.0089 (95% CI −0.1788 to 0.1610). Test of overall effect: z = −0.10, p = 0.918. Heterogeneity: I2 = 84.7%, tau2 = 0.13, Q(22) = 142.7029, df = 22, and p < 0.0001. Egger's regression value = 1.353, p = 0.176. The data are presented in Fig. 11.
Sex differences in inattention for adults: clinical interview
When we included the studies in adults that use interview data for assessment (9 studies; n = female 1148; male 1223), the observed standardized mean difference was not significant. Standardized mean difference = −0.0231 (95% CI −0.2721 to 0.2258). Test of overall effect: z = 0.18, p = 0.855. Heterogeneity: I2 = 85.4%, tau2 = 0.11, Q(8) = 61.6453, df = 9, and p < 0.0001. Egger's regression value = −0.015, p = 0.988. The data are presented in Fig. 12.
Sex differences in hyperactivity/impulsivity for children: clinical interview
When we included the studies in children assessing hyperactivity/impulsivity with interview data (22 studies; n = female 1979; male 2647), the overall observed standardized mean difference was not significant. Standardized mean difference = −0.0928 (95% CI −0.3152 to 0.1296). Test of overall effect: z = −0.82, p = 0.413. Heterogeneity: I2 = 90.5%, tau2 = 0.23, Q(21) = 181.5585, df = 21, and p < 0.0001. Egger's regression value = −1.276, p = 0.202. The data are presented in Fig. 13.
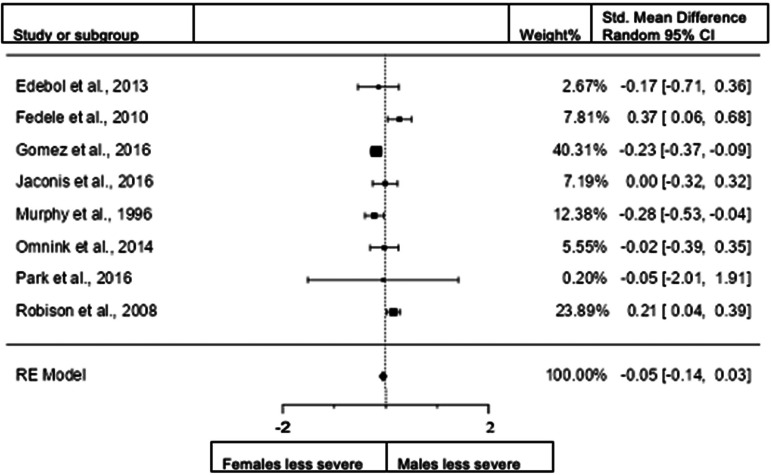
Sex differences in hyperactivity/impulsivity for adults: clinical interview
When we included the studies in adults assessing hyperactivity/impulsivity with interview data (8 studies; n = female 1085; male 1090), the overall observed standardized mean difference was not significant. Standardized mean difference = −0.0535 (95% CI −0.1405 to 0.0334). Test of overall effect: z = −1.2, p = 0.227. Heterogeneity: I2 = 0%, tau2 = 0, Q(7) = 25.8101, df = 7 and p = 0.0005. Egger's regression value = 0.153, p = 0.878. The data are presented in Fig. 14.
Sex differences in combined presentation for children: clinical interview
When we included the studies in children assessing a combined presentation with interview data (6 studies; n = female 501; male 771), the overall observed standardized mean difference was not significant. Standardized mean difference = 0.0366 (95% CI −0.1144 to 0.1876). Test of overall effect: z = 0.47, p = 0.634. Heterogeneity: I2 = 28%, tau2 = 0.01, Q(5) = 8.7297, df = 5, and p = 0.1203. Egger's regression value = 0.006, p = 0.995. The data are presented in Fig. 15.
Sex differences in combined presentation for adults: clinical interview
When we included the studies in adults assessing a combined presentation with interview data (4 studies; n = female 720; male 748). The overall observed standardized mean was not significant. Standardized mean difference = 0.1473 (95% CI −0.2138 to 0.5085). Test of overall effect: z = 0.7996, p = 0.4239. Heterogeneity: I2 = 84.7%, tau2 = 0.09, (Q(3) = 33.3378, df = 3 and p < 0.0001. Egger's regression value = 0.665, p = 0.506. The data are presented in Fig. 16.
Sensitivity analyses excluding ‘poor-quality’ studies
Sensitivity analyses removing ‘poor-quality’ studies from the meta-analyses in the total sample that used diagnostic interviews and rating scales for assessment demonstrated no differences in the results in the comparison of hyperactivity/impulsivity and combined symptoms. One comparison that was not significantly different in the total sample that used diagnostic interviews and rating scales for assessment exhibited significance in the sensitivity analysis (females exhibited less severe inattention than males). The effect size in this comparison was small in both analyses (−0.08 and −0.16). The results of the sensitivity analyses are presented in online Supplementary File 5.
Discussion
Our review of the literature identified 51 manuscripts (52 studies) that included male (n = 9985) and female (n = 8423) participants with ADHD across childhood and/or adulthood. The included studies that only used diagnoses based on DSM-IV or DSM-5, reflecting an updated understanding of ADHD. Drawing on this substantially greater and updated sample than those applied over 20 years previously, our findings shed a different perspective on the symptom presentation of females with ADHD compared with males with ADHD.
First, we examined all 52 studies by aggregating all the available data across the lifespan, all settings, and modes of assessment. Next, we examined rating scale data only, which was based predominantly on community samples in children and adults. Then, we exclusively focused on clinical diagnostic interview data, analyzing the data independently for child and adult study participants. Most of the studies in this group were conducted in clinical settings.
As can be seen from Table 1 there were only significant differences between groups in the comparison of rating scale data. When solely clinical interview data were analyzed there were no significant differences between sexes (for both child and adult data).
There were three main findings. First, the average standardized mean difference, based on a random effects model, in the pooled sample data of all 52 studies, aggregating both rating scale and clinical diagnostic interview data, showed that males had significantly more severe hyperactivity and impulsivity symptoms than females (small effect size). Second, using the same statistical method, a further analysis that investigated solely rating scale studies of symptoms showed that among children, boys had significantly more severe symptoms of hyperactivity/impulsivity (small effect size). In contrast, among the adult sample, the sex difference was significantly greater for inattention among men (small effect size) with no difference in the hyperactivity/impulsivity dimension. Third, the average standardized mean difference showed no significant difference between females and males in clinical diagnostic interview sample studies, either for children or adults. The essence of the three significant findings was that the effect sizes estimating the population parameters using the standardized mean difference were all small (i.e. below 0.50).
The sex difference in the pattern of rating scale findings among the children and adult samples in ADHD symptoms raises important questions about the role of sex in the remittance of symptoms over time. Children are evaluated for ADHD mainly using scales completed by parents or teachers of children whereby the symptom of hyperactivity/impulsivity is more obvious to refer for clinical evaluation. In contrast, inattention, being a more subtle symptom, may be less noticable. The rating scales that are used to screen, evaluate, and monitor ADHD symptoms but are not diagnostic may not be ideal for capturing the complex clinical picture of inattention in adults. As shown in a study by Young et al. (2009), rating scales are associated with both false-positive and false-negative symptoms; however, when diagnostic interviews were conducted, it may become clearer that the severity of inattention is similar in males and females both in children and adults.
The findings from our rating scale data, drawing on childhood samples, are consistent with previous meta-analyses reporting significant differences in symptom severity between males and females with ADHD (Gershon, 2002; Thomas et al., 2015). Importantly, the largest sex difference among the childhood rating scale data was the lower rates of hyperactivity/impulsivity in girls. This subset of symptoms is particularly elevated in childhood and remits more quickly with age than inattention (Willcutt, 2012).
The rating scale samples drew predominantly on community samples (82%) and the larger sex differences identified may reflect participants presenting with a broader range of ADHD symptoms with the peak being at the lower end of the symptom dimension. Clinical samples are a more selective population and typically more severe in presentation. Indeed, only a small proportion of participants in community-based studies meet the diagnostic screening criteria for ADHD (Willcutt, 2012). In contrast, the distribution of scores among clinical diagnostic samples is likely to be the reverse of those found in community-based studies (Amador-Campos et al., 2014). This important point needs further research.
A key finding from the present study, however, was that the severity of ADHD symptoms among females and males did not differ significantly when only clinical diagnostic interviews were included in the analysis. This was evident for both children and adults. This diverges from clinical prevalence data; Willcutt (2012) investigated clinical prevalence (as opposed to the severity of symptoms) in a clinical population (in the literature published between 1994 and 2010) and reported that the clinical and community diagnostic patterns were consistent, i.e. that diagnostic outcomes differed between sexes with females being more likely to be diagnosed with the inattentive domain and males with the combined domains.
The present results are largely consistent with those reported by the Child and Adolescent Twin Study (CATSS), a large Swedish study that investigated sex differences in the severity and presentation of ADHD symptoms, conduct problems, and learning problems in boys and girls with and without clinically diagnosed ADHD. The participants were parents of 19 804 twins (50.64% male) who were assessed at 9 years of age (Anckarsäter et al., 2011). Children were linked to Patient Register data on clinical ADHD diagnosis and medication prescriptions. At a population level, boys had higher scores for all symptom domains, but a similar severity was observed in clinically diagnosed boys and girls. For girls, prediction analyses found that hyperactivity/impulsivity and conduct problems were stronger predictors of clinical diagnosis and the prescription of medication. The authors concluded that ‘females with ADHD may be more easily missed in the ADHD diagnostic process and less likely to be prescribed medication unless they have prominent externalizing problems’.
If the severity of symptom presentation is similar between boys and girls with ADHD, how can one understand the substantial difference in male/female ratios? (Willcutt, 2012). One possibility is that there may be a sex bias in the process of receiving a clinical diagnosis of ADHD but we cannot confirm this from the current data. The CATSS study reported that externalizing behaviors drive the referrals for ADHD, hence females with ADHD who are without prominent externalizing problems may be missed or misdiagnosed (Anckarsäter et al., 2011).
One explanation may be the ‘female protective effect’ theory which posits that girls and women may need to reach a higher threshold of genetic and environmental exposures for ADHD to be expressed (Taylor et al., 2016). This may also contribute to the lower prevalence of ADHD and less severe externalizing hyperactive-impulsive problems in females (Rhee & Waldman, 2016).
It is possible that the ‘zeitgeist’ of early findings, albeit important at the time, contributed to perceived differences in symptom profiles that then led to expectations of how females with ADHD present. Females may present with different forms of behavioral problems, yet the severity of symptom ratings remains similar. It may be the underlying expression, in terms of functional behavior, that differs; males may present with greater disruptive, aggressive, and conduct-related problems whereas the focus for females may be more social, relational, and emotional in nature, including deliberate self-harming behaviors (Young et al., 2020). Moreover, referrals, which are usually initiated by parents or teachers, may prioritize children who are difficult to manage.
Indeed, the different expectations in how females present with ADHD can be an influential factor. When teachers are presented with ADHD-like vignettes that vary solely on the use of male or female names and pronouns, boys’ names were more likely to be referred for additional support (Sciutto, Nolfi, & Bluhm, 2004) and considered more suitable for treatment (Pisecco, Huzinec, & Curtis, 2001). Parents may also overrate the severity of hyperactive/impulsive symptoms and impairments in boys and underestimate these same symptoms in girls (Mowlem, Agnew-Blais, Taylor, & Asherson, 2019a; 2019b).
Once referred for assessment, the expectation of sexual dimorphism may also influence the outcome for girls (Young et al., 2020). ADHD symptoms may not be detected because females may be more likely to camouflage and/or engage in compensatory behaviors (Mowlem et al., 2019a; 2019b; Quinn & Madhoo, 2014); this may delay the time to referral as well as misdirect the assessment process. For females, the diagnosis may be missed or misdiagnosed due to comorbid problems. Females with ADHD have been reported to have a higher presence of mental health problems (such as anxiety, depression, eating disorders, and self-harming behaviors), leading to admission for inpatient care (Cortese, Faraone, Bernardi, Wang, & Blanco, 2016; Dalsgaard et al., 2014; Dalsgaard, Mortensen, Frydenberg, & Thomsen, 2002).
In the last decade, it has become clear that by adulthood the gap in prevalence rates by sex is substantially reduced. Young adult studies (both epidemiological and clinical) show a more balanced distribution of prevalence of ADHD total symptoms and subtypes (combined, hyperactivity/impulsivity, and inattention) in men and women (Willcutt, 2012). Social relational difficulties (infrequently mentioned in academic reports) are unlikely to be seen as symptomatic, and as girls become young women, this may be explained by an increasing interface with health services and/or associated self-referral.
There are several reasons why our findings differ from those previous meta-analyses reported. Our study applied a more refined methodology by drawing on a much greater sample size than earlier meta-analyses conducted over 20 years ago (Gaub & Carlson, 1997; Gershon, 2002). This may reflect that substantial revisions have been made to the diagnostic nosology in the intervening years, including the recognition for some individuals that ADHD persists well into adulthood.
Furthermore, previous reports of studies that have found sex differences in presenting symptoms have discussed ideas that may explain this; for example, suggesting differences in hormones (Waddell & McCarthy, 2012) or chromosomes (Greven, Rijsdijk, & Plomin, 2011). The results of our present analysis, however, indicate that there are not large differences in presenting symptoms between the sexes, but rather suggest a systemic bias in the referral and assessment of people for ADHD. In other words, the previously described sex differences in presenting symptoms may not exist so much within the sexual biology of the people with ADHD but in the gender biases of their surrounding caretakers. It is reasonable to us also that documented differences in biology may well affect other aspects of ADHD and the resulting behavior; but it appears from our study that presenting symptoms are not largely affected by sex differences.
Much of the community data appears to be driven by information drawn from rating scales. Rating scales are helpful indicators that may be usefully applied to assess treatment outcomes; however, they are not diagnostic (Kooij et al., 2010; Young et al., 2020). There is a variation in how well they map onto clinical diagnostic criteria; indeed, many are not compliant with current diagnostic nosology (e.g. DSM-5 criteria). They may overclassify ADHD symptoms as a ‘current’ presenting problem; the etiology of which may be another distinct and predominant clinical presentation (e.g. anxiety, bipolar, and/or personality disorders). For example, an empirical study of ADHD in male prisoners identified that rating scales are associated with both false-positive and false-negative symptoms (Young et al., 2009), emphasizing the need for practitioners to move to a clinical diagnostic interview when borderline scores are obtained.
Importantly, a scoping search in PubMed limited to the papers published from May 28, 2021 to January 6, 2024 identified 30 studies. None of those consisted of relevant gender-disaggregated data on ADHD symptom severity. This aligns with our expectations as not examining characteristics of males and females separately is the gap in the literature that our meta-analysis has addressed. We therefore encourage future studies to prioritize gender-specific analyses in this area.
Another research direction that we suggest is acknowledging and exploring the effect of ethnicity. We expect that as more primary studies including reports of outcomes broken out by ethnicity are published, future systematic reviews and meta-analyses will be able to approach this research question, with the foundation laid by the present study of gender.
Strengths and limitations
The main advantages of the current meta-analysis study over the previous studies were the reliance on more refined symptom criteria (using the DSM-IV, DSM-IV-TR, and DSM-5), the separation of clinical diagnostic assessment and rating scale samples data, and the inclusion of both child and adult populations (the latter including a broader age range of adults). To provide a comprehensive picture of the issues we wished to investigate, we included a wide age range in our search which generated a substantial amount of data. The number of studies across the 15 meta-analyses shown in Table 1 ranged from 3 to 52 and the proportion of females v. males varied across the analysis. Some studies did not compare all three ADHD presentations (inattention, hyperactivity/impulsivity, and combined).
We only included studies in the English language as we did not have funding for translation. We did not include unpublished study data, because we wanted to maintain the level of data quality provided by the peer-review process. Nevertheless, 9 of the 51 manuscripts were rated as poor quality. When the analysis was rerun after removing the poor-quality studies in the total sample, the only change was inattention exhibiting significance in the sensitivity analysis (females exhibited less severe inattention than males) still with a small effect size. Significant heterogeneity was found in all but four meta-analyses (see Fig 6, 9, 14); the latter indicating diversity across the studies in terms of possible outliers, populations, methodology, comorbid factors, and/or publication bias. Publication bias was present for the data of inattention and combined presentations in studies that aggregated both children and adults and both rating scale and interview data (p < 0.05). For children, according to the rating scale data, publication bias was present for inattention and HI (p < 0.05). Once more publications are available, it would be helpful for future research to conduct similar analyses drawing solely on strong/good quality studies.
Another limitation is the small number of studies included in some of the sub-analyses using subsets of the full number of studies included in the systematic review. In some of the sub-analyses, the low number of studies included due to a lack of adult data that met the inclusion criteria for the community rating scale population, we were unable to analyze the contribution of these data for adults. Furthermore, there was insufficient data for a meta-regression which would be informative in establishing which variables influence the outcomes of inattention, hyperactivity/impulsivity, and combined classifications. Future research should focus on this to increase our understanding of the different types of ADHD compared with a general population sample.
Conclusions
The present study extended early systematic reviews and meta-analyses of data comparing the symptom presentation of males and females with ADHD. Our review of the literature identified 51 manuscripts (52 studies) that in total included male (n = 8451) and female (n = 7304) participants with ADHD across childhood and/or adulthood. Drawing on this substantially greater sample than those applied over 20 years previously, our findings yield a different perspective and an important insight on the symptom presentation of females with ADHD.
Both males and females appear to equally endorse the severity of ADHD symptoms when assessed using clinical diagnostic interview data (the great majority of which were from clinical settings). By contrast, rating scale data (predominantly drawn from community samples) showed males present with more severe symptoms than females, but the type of symptoms was different for the child and adult studies, suggesting an important sex differential in the remittance of symptoms from childhood into adulthood. This is a novel finding and needs further investigation. Taking the findings as a whole, there may be a sex bias in the initiation of the process of receiving a clinical diagnosis of ADHD possibly due to the perception that ADHD is a behavioral conduct-related problem. The bias may influence the ratings of clinicians and/or of individuals completing them. Perhaps it is the underlying expression and functional behaviors associated with ADHD symptoms that differ between sexes leading to the under-recognition and underestimation of the prevalence of ADHD in females. This means that many girls and women with ADHD are likely to be unidentified and untreated, which in turn may have implications for social, educational, and mental health outcomes.
Supporting information
Young et al. supplementary material
Young et al. supplementary material
Young et al. supplementary material
Young et al. supplementary material
Young et al. supplementary material
Acknowledgments
We thank Victoria Williams, Patrycja Garasimczyk, Emma Bush, Sonya McCrea, Sanushiya Ananthakumar, and Tamsin Crook for their support in the initial screening of abstracts.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724001600.
Data availability statement
The template data collection forms, data extracted from included studies, and data used for all analyses are not publicly available. Requests for accessing the datasets should be directed to O.K., drozgekilic@gmail.com.
Author contributions
S. Y. led the project in collaboration with O. K., J. K., and J. H. in the planning and scientific input of the study. O. K., J. K., A. S.-K., J. H., B. G., N. S., K. C., and U. E. Y. substantially assisted with the data screening and extraction process under the supervision of O. K. O. K. and O. U. conducted the statistical analysis. S. Y. wrote the manuscript with input from O. K., J. K., S. C., G. H. G., and B. S. All authors have read and agreed to the published version of the manuscript.
Competing interests
In the last 5 years, S. Y. has received honoraria for consultancy and educational talks from HB Pharma, Medice, and Takeda. She is the author of the ADHD Child Evaluation (ACE) and ACE+ for adults. J. K. is the owner of BPS International and IHS International, which have received consultancy fees in the last 5 years from Sage Therapeutics, Cognition Therapeutics, Jazz Pharmaceuticals, and Greenwich Biosciences; no projects were related to ADHD. S. C. declares honoraria and reimbursement for travel and accommodation expenses for lectures from the following non-profit associations: Association for Child and Adolescent Central Health (ACAMH), Canadian ADHD Alliance Resource (CADDRA), British Association of Pharmacology (BAP), and from Healthcare Convention for educational activity on ADHD. O. K., O. U., G. H. G., J. H., A. S.-K., B. G., K. C., N. S., U. E. Y., and B. S. declare no competing interests.
References
- Nøvik, T. S., Hervas, A., Ralston, S. J., Dalsgaard, S., Rodrigues Pereira, R., & Lorenzo, M. J., & ADORE Study Group. (2006). Influence of gender on attention-deficit/hyperactivity disorder in Europe – ADORE. European Child & Adolescent Psychiatry, 15(Suppl. 1), 1–24. doi: 10.1007/s00787-006-1003-z [DOI] [PubMed] [Google Scholar]
- Amador-Campos, J. A., Gómez-Benito, J., & Ramos-Quiroga, J. A. (2014). The Conners' Adult ADHD Rating Scales – Short self-report and observer forms: Psychometric properties of the Catalan version. Journal of Attention Disorders, 18, 671–679. doi: 10.1177/1087054712446831 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC, USA: American Psychiatric Association. Retrieved from https://psycnet.apa.org/record/1994-97698-000 [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC, USA: American Psychiatric Association. doi: 10.1176/appi.books.9780890420249.dsm-iv-tr [DOI] [Google Scholar]
- American Psychiatric Association. (2022). Diagnostic and statistical manual of mental disorders (5th ed., text rev.). Washington, DC, USA: American Psychiatric Association. doi: 10.1176/appi.books.9780890425787 [DOI] [Google Scholar]
- Anckarsäter, H., Lundström, S., Kollberg, L., Kerekes, N., Palm, C., Carlström, E., … Lichtenstein, P. (2011). The child and adolescent twin study in Sweden (CATSS). Twin Research and Human Genetics, 14, 495–508. doi: 10.1375/twin.14.6.495 [DOI] [PubMed] [Google Scholar]
- Arcia, E., & Conners, C.K. (2007). Gender differences in ADHD? Journal of Developmental & Behavioral Pediatrics, 19(2), 68, 77–83. doi: 10.1097/00004703-199804000-00003 [DOI] [PubMed] [Google Scholar]
- Arnold, L. E., Hodgkins, P., Caci, H., Kahle, J., & Young, S. (2015). Effect of treatment on long-term outcomes in ADHD: A systematic review. PLoS ONE, 10, e0116407. doi: 10.1371/journal.pone.0116407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asherson, P. (2016). ADHD across the lifespan. Medicine, 40, 623–627. doi: 10.1016/j.mpmed.2016.08.012 [DOI] [Google Scholar]
- Bianchini, R., Postorino, V., Grasso, R., Santoro, B., Migliore, S., Burlo, C., … Mazzone, L. (2013). Prevalence of ADHD in a sample of Italian students: A population-based study. Research in Developmental Disabilities, 34, 2543–2550. doi: 10.1016/j.ridd.2013.05.027 [DOI] [PubMed] [Google Scholar]
- Borenstein, M., Hedges, L.V., Higgins, J.P., & Rothstein, H.R. (2021). Introduction to meta-analysis. Hoboken, NJ, USA: John Wiley & Sons. doi: 10.1002/9780470743386 [DOI] [Google Scholar]
- Bröring, T., Rommelse, N., Sergeant, J., & Scherder, E. (2008). Sex differences in tactile defensiveness in children with ADHD and their siblings. Developmental Medicine and Child Neurology, 50(2), 129–133. doi: 10.1111/j.1469-8749.2007.02024.x [DOI] [PubMed] [Google Scholar]
- Castellanos, F. X., Lee, P. P., Sharp, W., Jeffries, N. O., Greenstein, D. K., Clasen, L. S., … Rapoport, J. L. (2002). Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA, 288, 1740–1748. doi: 10.1001/jama.288.14.1740 [DOI] [PubMed] [Google Scholar]
- Chen, W., Zhou, K., Sham, P., Franke, B., Kuntsi, J., Campbell, D., … Asherson, P. (2008). DSM-IV combined type ADHD shows familial association with sibling trait scores: A sampling strategy for QTL linkage. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 147, 1450–1460. doi: 10.1002/ajmg.b.30672 [DOI] [PubMed] [Google Scholar]
- Cochran, W. G. (1954). The combination of estimates from different experiments. Biometrics, 10, 101–129. doi: 10.2307/3001666 [DOI] [Google Scholar]
- Cortese, S., Faraone, S. V., Bernardi, S., Wang, S., & Blanco, C. (2016). Gender differences in adult attention-deficit/hyperactivity disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). The Journal of Clinical Psychiatry, 77, 762–766. doi: 10.4088/jcp.14m09630 [DOI] [PubMed] [Google Scholar]
- Cortese, S., Adamo, N., Del Giovane, C., Mohr-Jensen, C., Hayes, A. J., Carucci, S., … Cipriani, A. (2018). Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. The Lancet Psychiatry, 5, 727–738. doi: 10.1016/S2215-0366(18)30269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard, S., Mortensen, P. B., Frydenberg, M., & Thomsen, P. H. (2002). Conduct problems, gender, and adult psychiatric outcome of children with attention-deficit hyperactivity disorder. British Journal of Psychiatry, 181, 416–421. doi: 10.1192/bjp.181.5.416 [DOI] [PubMed] [Google Scholar]
- Dalsgaard, S., Mortensen, P. B., Frydenberg, M., Maibing, C. M., Nordentoft, M., & Thomsen, P. H. (2014). Association between attention-deficit hyperactivity disorder in childhood and schizophrenia later in adulthood. European Psychiatry, 29, 259–263. doi: 10.1016/j.eurpsy.2013.06.004 [DOI] [PubMed] [Google Scholar]
- DuPaul, G. J., Anastopoulos, A. D., Power, T. J., Reid, R., Ikeda, M. J., & McGoey, K. E. (1998). Parent ratings of attention-deficit/hyperactivity disorder symptoms: Factor structure and normative data. Journal of Psychopathology and Behavioral Assessment, 20, 83–102. doi: 10.1177/07342829980160010 [DOI] [Google Scholar]
- DuPaul, G. J., Schaughency, E. A., Weyandt, L. L., Tripp, G., Kiesner, J., Ota, K., & Stanish, H. (2001). Self-report of ADHD symptoms in university students: Cross-gender and cross-national prevalence. Journal of Learning Disabilities, 34, 370–379. doi: 10.1177/002221940103400412 [DOI] [PubMed] [Google Scholar]
- DuPaul, G.J., Reid, R., Anastopoulos, A.D., Lambert, M.C., Watkins, M.W., & Power, T.J. (2016). Parent and teacher ratings of attention-deficit/hyperactivity disorder symptoms: Factor structure and normative data. Psychological Assessment, 28(2), 214–225. Retrieved from https://psycnet.apa.org/doi/10.1037/pas0000166 . [DOI] [PubMed] [Google Scholar]
- Du Rietz, E., Cheung, C. H., McLoughlin, G., Brandeis, D., Banaschewski, T., Asherson, P., & Kuntsi, J. (2016). Self-report of ADHD shows limited agreement with objective markers of persistence and remittance. Journal of Psychiatric Research, 82, 91–99. doi: 10.1016/j.jpsychires.2016.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebejer, J. L., Duffy, D. L., van der Werf, J., Wright, M. J., Montgomery, G., Gillespie, N. A., & Medland, S. E. (2013). Genome-wide association study of inattention and hyperactivity–impulsivity measured as quantitative traits. Twin Research and Human Genetics, 16, 560–574. doi: 10.1017/thg.2013.12 [DOI] [PubMed] [Google Scholar]
- Edebol, H., Helldin, L., & Norlander, T. (2013). The weighed core symptom scale and prediction of ADHD in adults – Objective measures of remission and response to treatment with methylphenidate. Clinical Practice and Epidemiology in Mental Health: CP EMH, 9, 171. doi: 10.2174/1745017901309010171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315, 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hamrawy, L. G., El Sayed, S. M., Soltan, M. R., & Abd El-Gwad, O. M. (2017). Psychiatric comorbidities in a sample of attention deficit hyperactivity disorder children in pediatric psychiatric clinics of El-Dakahlia Hospital of Mental Health. Middle East Current Psychiatry, 24, 134–141. doi: 10.1097/01.XME.0000520064.84110.c0 [DOI] [Google Scholar]
- Fedele, D. A., Hartung, C. M., Canu, W. H., & Wilkowski, B. M. (2010). Potential symptoms of ADHD for emerging adults. Journal of Psychopathology and Behavioral Assessment, 32, 385–396. Retrieved from https://psycnet.apa.org/doi/10.1007/s10862-009-9173-x . [Google Scholar]
- Fliers, E. A., Buitelaar, J. K., Maras, A., Bul, K., Höhle, E., Faraone, S. V., … Rommelse, N. N. (2013). ADHD is a risk factor for overweight and obesity in children. Journal of Developmental and Behavioral Pediatrics, 34(8), 566–574. doi: 10.1097/2FDBP.0b013e3182a50a67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksen, M., Dahl, A. A., Martinsen, E. W., Klungsoyr, O., Faraone, S. V., & Peleikis, D. E. (2014). Childhood and persistent ADHD symptoms associated with educational failure and long-term occupational disability in adult ADHD. ADHD Attention Deficit and Hyperactivity Disorders, 6, 87–99. doi: 10.1007/s12402-014-0126-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel, S., Schmitz, S., & Fulker, D. W. (1996). Comorbidity in hyperactive children: Issues related to selection bias, gender, severity, and internalizing symptoms. Child Psychiatry and Human Development, 27, 15–28. doi: 10.1007/BF02353443 [DOI] [PubMed] [Google Scholar]
- Gadow, K. D., Sprafkin, J., & Nolan, E. E. (2001). DSM-IV symptoms in community and clinic preschool children. Journal of the American Academy of Child and Adolescent Psychiatry, 40, 1383–1392. doi: 10.1097/00004583-200112000-00008 [DOI] [PubMed] [Google Scholar]
- Gaub, M., & Carlson, C. L. (1997). Gender differences in ADHD: A meta-analysis and critical review. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 1036–1045. doi: 10.1097/00004583-199708000-00011 [DOI] [PubMed] [Google Scholar]
- Gershon, J. (2002). A meta-analytic review of gender differences in ADHD. Journal of Attention Disorders, 5, 143–154. doi: 10.1177/108705470200500302 [DOI] [PubMed] [Google Scholar]
- Gershon, J. (2016). Gender differences in ADHD. ADHD Report, 10, 8–16. Retrieved from https://guilfordjournals.com/doi/pdf/10.1521/adhd.10.4.8.22991
- Ghanizadeh, A., Mohammadi, M. R., & Moini, R. (2008). Comorbidity of psychiatric disorders and parental psychiatric disorder of ADHD children. Journal of Attention Disorders, 2016, 149–155. doi: 10.1177/1087054708314601 [DOI] [PubMed] [Google Scholar]
- Gomez, R. (2016). ADHD and hyperkinetic disorder symptoms in Australian adults: Descriptive scores, incidence rates, factor structure, and gender invariance. Journal of Attention Disorders, 20, 325–334. doi: 10.1177/1087054713485206 [DOI] [PubMed] [Google Scholar]
- Graetz, B. W., Sawyer, M. G., Baghurst, P., & Ettridge, K. (2006). Are ADHD gender patterns moderated by sample source? Journal of Attention Disorders, 10, 36–43. doi: 10.1177/1087054705286055 [DOI] [PubMed] [Google Scholar]
- Greven, C. U., Rijsdijk, F. V., & Plomin, R. (2011). A twin study of ADHD symptoms in early adolescence: Hyperactivity–impulsivity and inattentiveness show substantial genetic overlap but also genetic specificity. Journal of Abnormal Child Psychology, 39, 265–275. doi: 10.1007/s10802-010-9451-9 [DOI] [PubMed] [Google Scholar]
- Greven, C. U., Richards, J. S., & Buitelaar, J. K. (2018). Sex differences in ADHD. In Banaschewski T., Coghill D., & Zuddas A. (Eds.), Oxford textbook of attention deficit hyperactivity disorder (pp. 154–160). New York, NY: Oxford University Press. doi: 10.1093/med/9780198739258.003.0016 [DOI] [Google Scholar]
- Gudjonsson, G. H., Sigurdsson, J. F., Adalsteinsson, T. F., & Young, S. (2013). The relationship between ADHD symptoms, mood instability, and self-reported offending. Journal of Attention Disorders, 17, 339–346. doi: 10.1177/1087054711429791 [DOI] [PubMed] [Google Scholar]
- Hartung, C. M., Willcutt, E. G., Lahey, B. B., Pelham, W. E., Loney, J., Stein, M. A., & Keenan, K. (2002). Sex differences in young children who meet criteria for attention deficit hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology, 31, 453–464. Retrieved from https://psycnet.apa.org/doi/10.1207/153744202320802133 . [DOI] [PubMed] [Google Scholar]
- Hellström, C., Wagner, P., Nilsson, K. W., Leppert, J., & Åslund, C. (2017). Gambling frequency and symptoms of attention-deficit hyperactivity disorder in relation to problem gambling among Swedish adolescents: A population-based study. Upsala Journal of Medical Sciences, 122, 119–126. doi: 10.1080/03009734.2017.1294636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw, S. P., Owens, E. B., Sami, N., & Fargeon, S. (2006). Prospective follow-up of girls with attention-deficit/hyperactivity disorder into adolescence: Evidence for continuing cross-domain impairment. Journal of Consulting and Clinical Psychology, 74, 489–499. Retrieved from https://psycnet.apa.org/doi/10.1037/a0029451 [DOI] [PubMed] [Google Scholar]
- Hodgkins, P., Arnold, L. E., Shaw, M., Caci, H., Kahle, J., Woods, A. G., & Young, S. (2012). A systematic review of global publication trends regarding long-term outcomes of ADHD. Frontiers in Psychiatry, 2, 84. doi: 10.3389/fpsyt.2011.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogue, A., Dauber, S., Lichvar, E., & Spiewak, G. (2014). Adolescent and caregiver reports of ADHD symptoms among inner-city youth: Agreement, perceived need for treatment, and behavioral correlates. Journal of Attention Disorders, 18, 212–225. doi: 10.1177/1087054712443160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaconis, M., Boyd, S. J., Hartung, C. M., McCrea, S. M., Lefler, E. K., & Canu, W. H. (2016). Sex differences in claimed and behavioral self-handicapping and ADHD symptomatology in emerging adults. ADHD Attention Deficit and Hyperactivity Disorders, 8, 205–214. doi: 10.1007/s12402-016-0200-y [DOI] [PubMed] [Google Scholar]
- Kean, J. D., Sarris, J., Scholey, A., Silberstein, R., Downey, L. A., & Stough, C. (2017). Reduced inattention and hyperactivity and improved cognition after marine oil extract (PCSO-524®) supplementation in children and adolescents with clinical and sub-clinical symptoms of attention-deficit hyperactivity disorder (ADHD): A randomized, double-blind, placebo-controlled trial. Psychopharmacology, 234, 403–420. doi: 10.1007/s00213-016-4471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. M., Ha, M., Lim, M. H., Kwon, H. J., Yoo, S. J., Kim, E., & Paik, K. C. (2018). The symptom trajectory of attention-deficit hyperactivity disorder in Korean school-age children. Psychiatry Investigation, 15, 470. doi: 10.30773/pi.2017.11.01.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij, S. J., Bejerot, S., Blackwell, A., Caci, H., Casas-Brugué, M., Carpentier, P. J., … Asherson, P. (2010). European consensus statement on diagnosis and treatment of adult ADHD: The European network adult ADHD. BMC Psychiatry, 10, 1–24. doi: 10.1186/1471-244X-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer, H. C., Noda, A., & O'Hara, R. (2004). Categorical versus dimensional approaches to diagnosis: Methodological challenges. Journal of Psychiatric Research, 38, 17–25. doi: 10.1016/S0022-3956(03)00097-9 [DOI] [PubMed] [Google Scholar]
- Lahey, B. B., Hartung, C. M., Loney, J., Pelham, W. E., Chronis, A. M., & Lee, S. S. (2007). Are there sex differences in the predictive validity of DSM-IV ADHD among younger children? Journal of Clinical Child and Adolescent Psychology, 36, 113–126. doi: 10.1080/15374410701274066 [DOI] [PubMed] [Google Scholar]
- Larsson, H., Dilshad, R., Lichtenstein, P., & Barker, E. D. (2011). Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: Genetic effects, family risk, and associated psychopathology. Journal of Child Psychology and Psychiatry, 52, 954–963. doi: 10.1111/j.1469-7610.2011.02379.x [DOI] [PubMed] [Google Scholar]
- Lefler, E. K., Hartung, C. M., Bartgis, J., & Thomas, D. G. (2015). ADHD symptoms in American Indian/Alaska native boys and girls. American Indian and Alaska Native Mental Health Research, 22, 23–40. doi: 10.5820/aian.2202.2015.23 [DOI] [PubMed] [Google Scholar]
- Levitan, R. D., Jain, U. R., & Katzman, M. A. (1999). Seasonal affective symptoms in adults with residual attention-deficit hyperactivity disorder. Comprehensive Psychiatry, 40, 261–267. doi: 10.1016/S0010-440X(99)90125-6 [DOI] [PubMed] [Google Scholar]
- Major, A., Martinussen, R., & Wiener, J. (2013). Self-efficacy for self-regulated learning in adolescents with and without attention deficit hyperactivity disorder (ADHD). Learning and Individual Differences, 27, 149–156. doi: 10.1016/j.lindif.2013.06.009 [DOI] [Google Scholar]
- Michielsen, M., Semeijn, E., Comijs, H. C., van de Ven, P., Beekman, A. T., Deeg, D. J., & Kooij, J. S. (2012). Prevalence of attention-deficit hyperactivity disorder in older adults in the Netherlands. British Journal of Psychiatry, 201, 298–305. doi: 10.1192/bjp.bp.111.101196 [DOI] [PubMed] [Google Scholar]
- Millenet, S., Laucht, M., Hohm, E., Jennen-Steinmetz, C., Hohmann, S., Schmidt, M. H., & Zohsel, K. (2018). Sex-specific trajectories of ADHD symptoms from adolescence to young adulthood. European Child & Adolescent Psychiatry, 27, 1067–1075. doi: 10.1007/s00787-018-1129-9 [DOI] [PubMed] [Google Scholar]
- Mosalanejad, M., Mosalanejad, L., & Lashkarpour, K. (2013). Prevalence of ADHD among students of Zahedan University of Medical Science in Iran. Iranian Journal of Psychiatry and Behavioral Sciences, 7, 83. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3939996/ [PMC free article] [PubMed] [Google Scholar]
- Mowlem, F., Agnew-Blais, J., Taylor, E., & Asherson, P. (2019a). Do different factors influence whether girls versus boys meet ADHD diagnostic criteria? Sex differences among children with high ADHD symptoms. Psychiatry Research, 272, 765–773. doi: 10.1016/j.psychres.2018.12.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowlem, F. D., Rosenqvist, M. A., Martin, J., Lichtenstein, P., Asherson, P., & Larsson, H. (2019b). Sex differences in predicting ADHD clinical diagnosis and pharmacological treatment. European Child & Adolescent Psychiatry, 28, 481–489. doi: 10.1007/s00787-018-1211-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, K., & Barkley, R. A. (1996). Prevalence of DSM-IV symptoms of ADHD in adult licensed drivers: Implications for clinical diagnosis. Journal of Attention Disorders, 1, 147–161. doi: 10.1177/108705479600100303 [DOI] [Google Scholar]
- Øie, M., Hovik, K. T., Andersen, P. N., Czajkowski, N. O., & Skogli, E. W. (2018). Gender differences in the relationship between changes in ADHD symptoms, executive functions, and self- and parent-report depression symptoms in boys and girls with ADHD: A 2-year follow-up study. Journal of Attention Disorders, 22, 446–459. doi: 10.1177/1087054716664407 [DOI] [PubMed] [Google Scholar]
- Onnink, A. M. H., Zwiers, M. P., Hoogman, M., Mostert, J. C., Kan, C. C., Buitelaar, J., & Franke, B. (2014). Brain alterations in adult ADHD: Effects of gender, treatment, and comorbid depression. European Neuropsychopharmacology, 24(3), 397–409. doi: 10.1016/j.euroneuro.2013.11.011 [DOI] [PubMed] [Google Scholar]
- Paavonen, E. J., Raikkonen, K., Lahti, J., Komsi, N., Heinonen, K., Pesonen, A. K., & Porkka-Heiskanen, T. (2009). Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7-to 8-year-old children. Pediatrics, 123, e857–e864. doi: 10.1542/peds.2008-2164 [DOI] [PubMed] [Google Scholar]
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., & Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Systematic Reviews, 10, 1–11. doi: 10.1016/j.ijsu.2021.105906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, B. Y., & Park, H. (2016). Connectivity differences between adult male and female patients with attention deficit hyperactivity disorder according to resting-state functional MRI. Neural Regeneration Research, 11, 119. doi: 10.4103/1673-5374.175056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisecco, S., Huzinec, C., & Curtis, D. (2001). The effect of child characteristics on teachers' acceptability of classroom-based behavioral strategies and psychostimulant medication for the treatment of ADHD. Journal of Clinical Child Psychology, 30, 413–421. doi: 10.1207/S15374424JCCP3003_12 [DOI] [PubMed] [Google Scholar]
- Quinn, P. O., & Madhoo, M. (2014). A review of attention-deficit/hyperactivity disorder in women and girls: Uncovering this hidden diagnosis. Primary Care Companion for CNS Disorders, 16, 27250. doi: 10.4088/PCC.13r01596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retz-Junginger, P., Rösler, M., Jacob, C., Alm, B., & Retz, W. (2010). Gender differences in self- and investigator-rated psychopathology in adult attention-deficit/hyperactivity disorder. ADHD Attention Deficit and Hyperactivity Disorders, 2, 93–101. doi: 10.1007/s12402-010-0024-0 [DOI] [PubMed] [Google Scholar]
- Rhee, S. H., & Waldman, I. D. (2016). Etiology of sex differences in the prevalence of ADHD: An examination of inattention and hyperactivity–impulsivity. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171B, 60–64. doi: 10.1002/ajmg.b.20131 [DOI] [PubMed] [Google Scholar]
- Riddle, M. A., Yershova, K., Lazzaretto, D., Paykina, N., Yenokyan, G., Greenhill, L., & Posner, K. (2013). The preschool attention-deficit/hyperactivity disorder treatment study (PATS) 6-year follow-up. Journal of the American Academy of Child & Adolescent Psychiatry, 52, 264–278. doi: 10.1016/j.jaac.2012.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison, R. J., Reimherr, F. W., Marchant, B. K., Faraone, S. V., Adler, L. A., & West, S. A. (2008). Gender differences in 2 clinical trials of adults with attention-deficit/hyperactivity disorder: A retrospective data analysis. Journal of Clinical Psychiatry, 69, 764–770. doi: 10.4088/jcp.v69n0207 [DOI] [PubMed] [Google Scholar]
- Rosch, K. S., Dirlikov, B., & Mostofsky, S. H. (2015). Reduced intrasubject variability with reinforcement in boys, but not girls, with ADHD: Associations with prefrontal anatomy. Biological Psychology, 110, 12–23. doi: 10.1016/j.biopsycho.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciutto, M. J., Nolfi, C. J., & Bluhm, C. (2004). Effects of child gender and symptom type on referrals for ADHD by elementary school teachers. Journal of Emotional and Behavioural Disorders, 12, 247–253. doi: 10.1177/10634266040120040501 [DOI] [Google Scholar]
- Serra-Pinheiro, M. A., Mattos, P., & Angélica Regalla, M. (2008). Inattention, hyperactivity, and oppositional-defiant symptoms in Brazilian adolescents: Gender prevalence and agreement between teachers and parents in a non-English speaking population. Journal of Attention Disorders, 12, 135–140. doi: 10.1177/1087054708314620 [DOI] [PubMed] [Google Scholar]
- Seymour, K. E., Mostofsky, S. H., & Rosch, K. S. (2016). Cognitive load differentially impacts response control in girls and boys with ADHD. Journal of Abnormal Child Psychology, 44, 141–154. doi: 10.1007/s10802-015-9976-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, M., Hodgkins, P., Caci, H., Young, S., Kahle, J., Woods, A., & Arnold, L. G. (2012). A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: Effects of treatment and non-treatment. BMC Medicine, 10, 99. doi: 10.1186/1741-7015-10-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihvola, E., Rose, R. J., Dick, D. M., Korhonen, T., Pulkkinen, L., Raevuori, A., & Kaprio, J. (2011). Prospective relationships of ADHD symptoms with developing substance use in a population-derived sample. Psychological Medicine, 41, 2615–2623. doi: 10.1017/S0033291711000791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, V., Czobor, P., Bálint, S., Mészáros, A., & Bitter, I. (2009). Prevalence and correlates of adult attention-deficit hyperactivity disorder: Meta-analysis. British Journal of Psychiatry, 194, 204–211. doi: 10.1192/bjp.bp.107.048827 [DOI] [PubMed] [Google Scholar]
- Skogli, E. W., Teicher, M. H., Andersen, P. N., Hovik, K. T., & Øie, M. (2013). ADHD in girls and boys – Gender differences in co-existing symptoms and executive function measures. BMC Psychiatry, 13, 298. doi: 10.1186/1471-244x-13-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., … Thacker, S. B. (2000). Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA, 283(15), 2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- Taylor, M. J., Lichtenstein, P., Larsson, H., Anckarsäter, H., Greven, C. U., & Ronald, A. (2016). Is there a female protective effect against attention-deficit/hyperactivity disorder? Evidence from two representative twin samples. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 504–512e2. doi: 10.1016/j.jaac.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Jamovi Project. (2022). Jamovi (version 1.8). Retrieved from https://www.jamovi.org
- Thomas, R., Sanders, S., Doust, J., Beller, E., & Glasziou, P. (2015). Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics, 135, e994–e1001. doi: 10.1542/peds.2014-3482 [DOI] [PubMed] [Google Scholar]
- Thorell, L. B., & Rydell, A. M. (2008). Behaviour problems and social competence deficits associated with symptoms of attention-deficit/hyperactivity disorder: Effects of age and gender. Child: Care, Health & Development, 34, 584–595. doi: 10.1111/j.1365-2214.2008.00869.x [DOI] [PubMed] [Google Scholar]
- Tseng, W. L., Kawabata, Y., Gau, S. S. F., Banny, A. M., Lingras, K. A., & Crick, N. R. (2012). Relations of inattention and hyperactivity/impulsivity to preadolescent peer functioning: The mediating roles of aggressive and prosocial behaviors. Journal of Clinical Child and Adolescent Psychology, 41, 275–287. doi: 10.1080/15374416.2012.656556 [DOI] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Waddell, J., & McCarthy, M. M. (2012). Sexual differentiation of the brain and ADHD: What is a sex difference in prevalence telling us? Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment, 9, 341–360. doi: 10.1007/7854_2010_114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschbusch, D. A., & King, S. (2006). Should sex-specific norms be used to assess attention-deficit/hyperactivity disorder or oppositional defiant disorder? Journal of Consulting and Clinical Psychology, 74, 179. Retrieved from https://psycnet.apa.org/doi/10.1037/0022-006X.74.1.179 [DOI] [PubMed] [Google Scholar]
- Wells, G., Shea, B., O'Connell, D., Peterson, J., Welch, V., Losos, M., & Tugwell, P. (1997). The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Retrieved from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Willcutt, E. G. (2012). The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics, 9, 490–499. doi: 10.1007/s13311-012-0135-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt, E. G., & Pennington, B. F. (2000). Comorbidity of reading disability and attention-deficit/hyperactivity disorder: Differences by gender and subtype. Journal of Learning Disabilities, 33, 179–191. doi: 10.1177/002221940003300206 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2019). International Statistical Classification of Diseases and Related Health Problems 11th Revision (ICD-11): Version 2019. Retrieved from https://icd.who.int/
- Yoo, H. J., Cho, S. C., Ha, J., Yune, S. K., Kim, S. J., Hwang, J., & Lyoo, I. K. (2004). Attention deficit hyperactivity symptoms and internet addiction. Psychiatry and Clinical Neurosciences, 58, 487–494. doi: 10.1111/j.1440-1819.2004.01290.x [DOI] [PubMed] [Google Scholar]
- Young, S., Gudjonsson, G. H., Wells, J., Asherson, P., Theobald, D., Oliver, B., & Mooney, A. (2009). Attention deficit hyperactivity disorder and critical incidents in a Scottish prison population. Personality and Individual Differences, 46, 265–269. doi: 10.1016/j.paid.2008.10.003 [DOI] [Google Scholar]
- Young, S., Adamo, N., Asgeirsdottir, B. B., Branney, P., Beckett, M., Colley, W., … Woodhouse, E. (2020). Females with ADHD: An expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention deficit hyperactivity disorder in girls and women. BMC Psychiatry, 20, 404. doi: 10.1186/s12888-020-02707-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Young et al. supplementary material
Young et al. supplementary material
Young et al. supplementary material
Young et al. supplementary material
Young et al. supplementary material
Data Availability Statement
The template data collection forms, data extracted from included studies, and data used for all analyses are not publicly available. Requests for accessing the datasets should be directed to O.K., drozgekilic@gmail.com.